Abstract
Purpose:
To investigate the effect of chitosan oligosaccharides (COS) against osteoarthritis (OA) and preliminarily discuss the osteoprotegerin (OPG), receptor activator of NF-κB ligand (RANKL) and RANK expression in a rat OA model.
Methods:
Thirty-six 6-week-old Male SD rats were randomly divided into three groups: sham-operated group(CON), OA-induction group(OA), COS intervention group(n=12/group). At 4 weeks after the operation, COS (50 ul) intervention weekily for consecutive 5 weeks. The OA and CON groups received an injection of 50 ul physiological saline. At death, 11 weeks following surgery, cartilage was harvested and total RNA and protein were extracted. Both the morphological changes of the cartilage were observed and harvested the total RNA and protein. Meanwhile, the expression of OPG, RANKL and RANK in cartilage were determined.
Results:
The expression of OPG and RANKL were both enhanced in the cartilage of the OA model. Compared with the OA group, COS treatment improved the cartilage damage (both extent and grade). Furthermore, the COS group showed highly OPG and lower RANKL. Simultaneously, COS treatment upregulated the ratio of OPG/RANKL and downregulated the RANKL/RANK.
Conclusion:
Chitosan oligosaccharides may be used as a unique biological agent to prevent and treat osteoarthritis, and this effect is associated with modulation of the expression of osteoprotegerin and receptor activator of NF-κB ligand.
Key words:
Chitosan; Osteoarthritis; Osteoprotegerin; RANK Ligand; Rats.
Introduction
Osteoarthritis (OA) is one of the most common joint disorders mainly affecting individuals over 60 years of age11 Neogi T.The epidemiology and impact of pain in osteoarthritis.Osteoarthritis Cartilage. 2013 Sep;21(9):1145-53. PMID: 23973124.. It is a degenerative process in joints, which involve cartilage, synovial membrane, and subchondral bone22 Chojnacki M, Kwapisz A, Synder M, Szemraj J. Osteoarthritis: etiology, risk factors, molecular mechanisms. Postepy Hig Med Dosw (Online). Jan 2;68:640-52. PMID: 24864114.. Patients with OA suffer from pain and functional disability, followed by a significant social and economic burden. Although significant progress has been made in the last few decades, the complete remission of this disease is not yet achieved33 Hochberg MC, Yerges-Armstrong L, Yau M, Mitchell BD. Genetic epidemiology of osteoarthritis: recent developments and future directions. Curr Opin Rheumatol. 2013 Mar;25(2):192-7. PMID: 23249833.. Thus, this condition should be considered as a serious social problem.
Chitosan is a cationic polysaccharide, which is composed of less than 20% β-(1,4)-2-acetamido-D-glucopyranose and more than 80% β-(1,4)-2-amino-D-glucopyranose, which presents in the exoskeleton of crustaceans and in cell walls of fungi and insects44 Narayanan D, Jayakumar R, Chennazhi KP. Versatile carboxymethyl chitin and chitosan nanomaterials: a review. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014 Nov-Dec;6(6):574-98. PMID: 25266740.. And COS is the hydrolysed products of chitosan by chemical and enzymatic hydrolysis that composed of linear polymers of β-1-4-linked D-glucosamine (Figure 1)55 Dutta J, Tripathi S, Dutta PK. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: a systematic study needs for food applications. Food Sci Technol Int. 2012 Feb;18(1):3-34. PMID: 21954316.. COS is a unique nature positively charged cations basic amino oligosaccharide. Traditionally, COS were widely used as functional materials for their biodegradability and adsorption properties. In the past decades, COS were also shown to possess different biological activities including antimicrobial activities, anti-tumor activities, anti-inflammation, anti-oxidative and anti-apoptotic effects and immuno-enhancing effects66 Reighard KP, Hill DB, Dixon GA, Worley BV, Schoenfisch MH. Disruption and eradication of P. aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling. 2015;31(9-10):775-87. PMID: 26610146.
7 Fang I, Yang C, Yang C. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp Eye Res. 2015 Jan;130:38-50. PMID: 25479043.
8 Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015 Jan 14;6(1):33-49. PMID: 25594943.-99 Chung MJ, Park JK, Il Park Y. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int Immunopharmacol. 2012 Feb;12(2):453-9. PMID: 22266066.. For example, COS can effectively protect HUVECs (human umbilical vein endothelial cells) against oxidative stress by H2O2 through regulation of p38 MAPK and PI3K/Akt signaling pathways, which might be of importance in the treatment of cardiovascular diseases1010 Liu H, Li W, Xu G, Li X, Bai X, Wei P, Yu C, Du YG. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol Res. 2009 Mar;59(3):167-75. PMID: 19121394.. COS was also reported to suppress hepatic CYP (cytochrome P450 proteins) enzymes and induce phase II detoxifying reactions in the liver and kidneys of rabbits, which promoted drug metabolism and detoxification1111 Yao H, Luo M, Hung L, Chiang M, Lin J, Lii C, Huang C. Effects of chitosan oligosaccharides on drug-metabolizing enzymes in rat liver and kidneys. Food Chem Toxicol. 2012 May;50(5):1171-7. PMID: 22386817.. In our previous study, carboxymethyl chitosan (39 kDa) was found to inhibit interleukin-1β-induced apoptosis in rabbit chondrocytes. And COS was depolymerized with a special enzyme technology product resulting oligosaccharides of chitosan. Therefore, we assumed that COS has the protective effects in OA model. However, no direct evidence was provided about the protective effects of COS on OA. It is hoped that the question will be resolved with our proposed approach.
The OPG/RANKL/RANK system is important in the balance between bone formation and resorption. Although it is well-known that bone, and particularly the subchondral bone remodeling process is tightly controlled by a molecular triad composed of osteoprotegerin (OPG), receptor activator NF-kB ligand (RANK) and RANK ligand (RANKL)1212 Tat SK, Pelletier J, Velasco CR, Padrines M, Martel-Pelletier J. New perspective in osteoarthritis: the OPG and RANKL system as a potential therapeutic target? Keio J Med. 2009 Mar;58(1):29-40. PMID: 19398882.. Earlier studies suggest the involvement of OPG, RANK and RANKL in OA subchondral bone metabolism. RANKL mediates bone resorption through the regulation of osteoclastogenesis and the activation of mature osteoclasts. RANKL binds to the cell surface receptor RANK, which is located on precursor and mature osteoclasts. OPG is secreted by the stromal cells and other cell types including osteoblasts. By binding to RANKL, OPG inhibits the interaction of RANKL-RANK, thereby preventing RANK activation and subsequent osteoclastogenesis1313 Sousa BL, Barroso-Neto IL, Oliveira EF, Fonseca E, Lima-Neto P, Ladeira LO, Freire VN. Explaining RANKL inhibition by OPG through quantum biochemistry computations and insights into peptide-design for the treatment of osteoporosis. RSC Advances. 2016 Aug;88(6):84926-42. doi: 10.1039/C6RA16712H.
https://doi.org/10.1039/C6RA16712H...
. This system has also been reported to play roles in several cellular functions, which include apoptotic effect, immune response maintainance, and carcinogenesis1414 Wang J, Chen T, Qin S, Duan Y, Wang G. Inhibitory effect of metformin on bone metastasis of cancer via OPG/RANKL/RANK system. Med Hypotheses. 2013 Nov;81(5):805-6. PMID: 24074896.,1515 Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014 Oct 20;5:511. PMID: 25368616.. Recently, studies showed that human OA chondrocytes also express and produce OPG, RANKL and RANK, and the molecular triad appears to be involved in OA progression1616 Kwan Tat S, Amiable N, Pelletier J, Boileau C, Lajeunesse D, Duval N, Martel-Pelletier J. Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology (Oxford). 2009 Dec;48(12):1482-90. PMID: 19762475.. Howerer, very little data exists regarding the expression/production of these factors by OA rats. Thus, in this study, we focus on the influences of COS on the changes of OPG, RANK and RANKL expression in experimentally induced OA rats. The aim of this study was to investigate the relative effects of COS on OPG, RANKL and RANK mRNA and protein expression in OA. Also, further studies on the effects of COS on OA will be summarized in our next study.
Methods
Chemicals and reagents
COS (MW<1KDa, degree of deacetylation≥93%) was supplied by Zhaoqing Longline Biotechnology Co., Ltd. (Guangdong, China). TRIzol were purchased from Gibco BRL (Paisley, UK). RT-PCR kit was purchased from Invitrogen (Paisley, UK). OPG, RANKL, RANK and glyceraldehyde-3-phosphate dehydrogenase(GAPDH) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Formaldehyde and EDTA were purchased from Boster Biological Company (Wuhan, China). BCA protein quantitative assay kit was purchased from Beyotime Institute of Biotechnology (China). All the other common chemicals and reagents were of the high purity commercially available.
Establishment of a rat knee OA model
A total of 36 male SD rats, weighing 200-220 g, were obtained from the Animal Centre of Wuhan University were used in this experiment. The animals were accommodated to standard laboratory conditions (12 h light and dark cycle at: 21±2°C, and humidity: 55-60%) and allowed free access to food and water. The OA model was established in the right knee joint by anterior cruciate ligament transection and medial meniscectomy (ACLT+MM). After one week of acclimation, the following experimental groups were developed: (1) 12 rats with sham surgery (CON group), (2) 12 rats with ACLT+MM (OA group) and (3) 12 rats with ACLT+MM+COS intra-articular injection (COS group). The OA model was established in the right knee joint by anterior cruciate ligament transection combined with medial menisci resection (ACLT+MM) as previously described1717 Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006 Feb;38(2):234-43. PMID: 16185945.,1818 Kim JL, Moon CW, Son YS, Kim SJ. Combined effect of bilateral ovariectomy and anterior cruciate ligament transection with medial meniscectomy on the development of osteoarthritis model. Ann Rehabil Med. 2016 Aug;40(4):583-91. PMID: 27606264.. In brief, animals were anaesthetized intraperitoneally with trichloroacetaldehyde hydrate (10%, 0.3 ml/100 g) in sterile saline. Vertically incised in the midline was made in the skin, and a medial arthrotomy was performed. The patella was dislocated and the knee placed in full flexion to expose the articular cavity. The synovial membrane was excised and the knee joint was bent to expose the anterior cruciate ligament. Then, the anterior cruciate ligament was transected and the medial meniscus was completely removed with a surgical scissor. After surgery, the patella was then relocated back to its original position. Then the knee was irrigated with physiological saline, after which the capsule and skin were closed and the fascia and skin were closed with 4-0 nylon suture. The same procedure was performed for both OA and COS rats. For the sham-operated group, the wounds were sutured after exposing the knee joint cartilage surface. The animals were injected intramuscularly with antibiotics (1.0-1.3 mg/cefotiam hydrochloride) for 3 days after surgery. Postoperatively, the animals were permitted freedom of the cage without immobilization, the cage was maintained under consistent environmental conditions. 4 weeks after surgery, the COS group rats received 50 ul of intra-articular COS (1 mg/mL) by injection once a week for 5 weeks. Meanwhile, the sham-operated and OA-induction groups received an injection of 50 ul physiological saline into the right knee joint. All animals were not sacrificed until the 11th week after surgery. All animal studies were conducted with approval from the Animal Care and Use Committee of Medical School, Wuhan University.
Articular cartilage assessment
At the time of harvest, the rat articular cartilage were evaluated from the right knee joints. The samples were harvested, divided into thirds, and stored at -80°C for further studies.
Histologic study
For the histologic study (n=4, each group), knee joint tissues with synovial membrane were extracted by trimming the muscles. The right knee joints were immediately fixed in 4% paraformaldehyde after dissection for 24 h, decalcified in Calci-Clear slow solution [10% (w/v) EDTA, pH 7.4] for at least 3 weeks and embedded in paraffin and then sectioned in the sagittal plane under the midline at 6 μm thickness. To observe the nucleus and cytoplasm, hematoxylin and eosin (H&E) and Safranin O staining was also performed. The light microscopic photographs of stained slides (at×200) were taken.
Semi-quantitative histopathological grading was performed by two blinded observers according to a modified Mankin’s scoring system used to quantify the degree of OA1919 Kuroki H, Nakagawa Y, Mori K, Ohba M, Suzuki T, Mizuno Y, Ando K, Takenaka M, Ikeuchi K, Nakamura T. Acoustic stiffness and change in plug cartilage over time after autologous osteochondral grafting: correlation between ultrasound signal intensity and histological score in a rabbit model. Arthritis Res Ther. 2004;6(6):R492-504. PMID: 15535827.. Items for scoring were (i) cartilage structure (0-6), (ii) cartilage cells (0-3), (iii) Safranin O staining (0-4), and (iv) tidemark integrity (0-1).The modified Mankin’s score range is from 0 to 14 and the higher the score, the more severe is the extent of osteoarthritis2020 Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP, Matyas J, Pritzker KP, D'Lima DD, Lotz MK. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis Cartilage. 2012 Jun;20(6):476-85. PMID: 22353747..
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from rat knee articular cartilage with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The purity and quantity of the RNA preparation were assessed by measuring the absorbance at 260 and 280 nm. Total RNA was reverse-transcribed with the PrimeScript RT Reagent kit (TaKaRa, Dalian, China). Real-time PCR was then performed using an ABI 7900 System in the presence of SYBR- Green (TaKaRa, Dalian, China) following the manufacturer’s instructions in an Eco Real Time PCR System (Illumina China, Shanghai, China). The mRNA level of individual genes was normalized and presented as a ratio to GAPDH. Quantitative RT-PCR data were calculated using the 2−ΔΔCT method. The primer sequences are listed in Table 1.
Western blot analysis
The relative levels of target proteins were detected by western blot analysis. Briefly, protein samples were extracted from rat knee articular cartilage for immunoblotting analysis of OPG, RANKL and RANK. The cartilage specimens previously stored in liquid nitrogen were milled in 200 μl of radioimmune precipitation assay lysis buffer supplemented with protease inhibitor cocktail and phenylmethanesulfonyl fluoride with a homogenizer. Cell lysates were extracted using RIPA lysis buffer containing protease inhibitor cocktail. The mixture was incubated on ice for 30 min and centrifuged at 10.000×g for 10 min at 4°C, and the supernatant was then collected. Protein concentrations were determined using BCA assay. Cell lysates containing 40 μg of protein were loaded and separated on 10% SDS-PAGE gels and subsequently transferred to polyvinylidene difluoride membranes (PVDF). After briefly washing in Tris-buffer saline Tween-20 (TBST), the membranes were blocked with 5% (w/v) nonfat drymilk in TBST at roomtemperature for 1 h. The membranes were incubated at 4°C overnight with following primary antibodies: a goat polyclonal OPG (Santa Cruz; sc-8468; 1:800), goat polyclonal RANKL (Santa Cruz; sc-7628; 1:1000), goat polyclonal RANK (Santa Cruz; sc-6248; 1:1000), a mouse monoclonal GAPDH (Santa Cruz; sc-47778; 1: 1,000). They were then washed, and incubated with horseradish peroxidase conjugated secondary antibody at dilution 1:5.000 for 1 h at room temperature (Amersham Biosciences, Piscataway, NJ, USA). Immunoblot bands were analyzed using Odyssey infrared imaging system (LI-COR, NE, USA). The expression levels of target proteins were normalized to GAPDH.
Statistical analysis
All values were expressed as the mean ± standard deviation (SD). The differences between each group were compared for statistical significance using one-way ANOVA analysis and Student’s t-test with SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). P < 0.05 was regarded as statistical significant. All statistical tests were performed using GraphPad Prism software, version 5.0 (San Diego, CA, USA).
Results
Effects of COS on histopathology in OA cartilage
Histopathological changes in each group were concentrated on the cartilage surface and matrix layer (Figure 2). In the CON group, articular cartilage possessed regular morphological structure. In contrast, in the OA induction group, the surface of articular cartilage was irregular and the articular cartilage was badly eroded and the cartilage matrix was thinner. Rats treated with intra-articular injection of COS, the right knee articular cartilage thickness had increased significantly, and the severity of lesions and osteophytes were apparently ameliorated. Meanwhile, cartilage proteoglycan content assay performed using Safranin-O staining showed that the cartilage matrix was well preserved in the COS groups, whereas a remarkable loss of proteoglycan was observed in the OA induction group.
Effect of COS on histological changes in articular cartilage of the rat model (x200). Gross morphology, histological analyses of rat articular cartilage by H&E and Safranin O staining in each experimental group.
The modified Mankin scores of three groups are shown in Table 2. Modified Mankin’s scores were 1.5±0.4 in the CON group, 9.6±1.6 in the OA group and 3.8±1.8 in the OA group. There were significant differences among all groups. The severity of the OA group was much higher than that of the CON group (*** P<0.001). Specifically, the severity of the COS group was much lower than that of the OA-induction group (## P<0.01 and ###P<0.001).
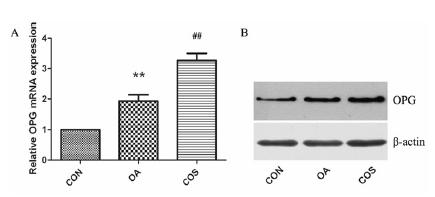
Effects of COS on the expression of OPG in rat OA model
As shown in Figure 3, we further examined the expression of OPG, the OPG mRNA was increased in OA compared to CON (**P < 0.01). COS treatment further promoted the OPG mRNA level in OA cartilage (##P < 0.01). The protein level of OPG was further detected by western blot, in accordance with the mRNA data, OPG was expressed in normal and OA cartilage, and OA group showed an increased expression of OPG in cartilage. When treated with COS, expression of OPG was even higher than OA group.
COS increased the expression of OPG in OA. (A) OPG mRNA was examined by real-time PCR, there was a significantly increased expression in OA cartilage, while COS treatment further promoted the OPG mRNA level in OA cartilage. (B) The protein level of OPG was in accordance with the mRNA data. ** P < 0.01 vs CON, ## P < 0.01 vs OA.
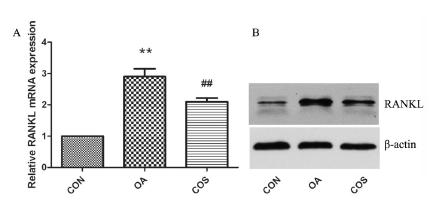
Effects of COS on the expression of RANKL in rat OA model
As shown in Figure 4, we further examined the expression of RANKL, the RANKL mRNA was increased in OA cartilage compared to normal cartilage (**P < 0.01), however, COS treatment decreased the RANKL mRNA in OA cartilage (##P < 0.01). Consistent with mRNA data, RANKL expression was upregulated in OA cartilage, and the effect was reversed by COS treatment.
COS decreased the expression of RANKL in OA. (A) RANKL mRNA was examined by real-time PCR, there was a significantly increased expression in OA cartilage, while COS treatment inhibit the RANKL mRNA level in OA cartilage. (B) The protein level of RANKL was in accordance with the mRNA data. ** P < 0.01 vs CON, ## P < 0.01 vs OA.
Effects of COS on the ratio of OPG/RANKL and RANKL/RANK in rat OA model
We also investigated the expression of RANK expression. There was expression on both CON and OA cartilage, and the difference was not significant (P > 0.05). The effect of COS treatment on RANK expression was also insignificant (P > 0.05) (Figure 5A). We then asked whether the ratio of OPG/RANKL and RANKL/RANK was changed. We found that the ratio of OPG/RANKL was decreased in OA cartilage (*P < 0.05), while COS treatment increased the ratio of OPG/RANKL (**P < 0.01) (Figure 5B). The ratio of RANKL/RANK was upregulated in OA cartilage (##P < 0.01), and COS treatment downregulated the ratio in OA cartilage (#P < 0.05) (Figure 5C).
Effects of COS on the ratio of OPG/RANKL and RANKL/RANK. (A) RANK expression was not affected either in OA model or by COS. (B) The ratio of OPG/RANKL was decreased in OA cartilage, while COS treatment increased the ratio of OPG/RANKL. (C) The ratio of RANKL/RANK was upregulated in OA cartilage and COS treatment downregulated the ratio in OA cartilage. * P < 0.05 and ** P < 0.01 vs CON, # P < 0.05 and ## P < 0.01 vs OA.
Discussion
Osteoarthritis (OA) is thought to be the most prevalent chronic joint disease, which characterized by cartilage degeneration and an imbalance between the synthesis and degradation, leading to an impairment of joint functions and quality of life2121 Sugahara K, Pothacharoen P, Najarus S, Settakorn J, Mizumoto S, Kongtawelert P. Effects of sesamin on the biosynthesis of chondroitin sulfate proteoglycans in human articular chondrocytes in primary culture. Glycoconj J. 2014 Apr;31(3):221-30. PMID: 24338203.. Numerous animal models of OA have been developed to mimic pathologic human cartilage2222 Cohen-Solal M, Funck-Brentano T, Hay E. Animal models of osteoarthritis for the understanding of the bone contribution. Bonekey Rep. 2013 Oct 2;2:422. PMID: 24422124.. Among these different models, transection of the ACLT+MM has been one of the most widely used to induce OA, The joint instability caused by ACLT damage further leads to biological mechanics changes resemble the morphologic and biochemical changes seen in human OA2323 Kim JL, Moon CW, Son YS, Kim SJ. Combined effect of bilateral ovariectomy and anterior cruciate ligament transection with medial meniscectomy on the development of osteoarthritis model. Ann Rehabil Med. 2016 Aug;40(4):583-91. PMID: 27606264.. ACLT+MM model provides progressive lesions that develop over time which is useful to study early stages of the OA2424 Pritzker KPH. Osteoarthritis joint instability and OA: do animal models provide insights? Nat Rev Rheumatol. 2011 Jul 12;7(8):444-5. PMID: 21750530.. In the present study, ACLT+MM was done in rat. Overall, we obtained a reproducible OA model with gradual and progressive articular cartilage degeneration.
Bioagents based on glycosaminoglycans has chondroprotective effects, for instance, hyaluronic acid could protect chondrocytes against cytotoxicity2525 Zhou J, Liu S, Qiu B, Hu Q, Ming J, Peng H. Effects of hyaluronan on vascular endothelial growth factor and receptor-2 expression in a rabbit osteoarthritis model. J Orthop Sci. 2009 May;14(3):313-9. PMID: 19499299.. COS has an analogous structure with hyaluronic acid and other glycosaminoglycans of cartilage extracellular matrix, COS thus shares some of their characteristics. In this study, we have demonstrated that COS, a soluble derivative of chitosan, could reduce cartilage destruction in OA. Several explanations may be proposed to account for this protective effect. COS may thus protect the articular cartilage by serving as a shock absorber and stress distributor during knee movements. Our previous study have shown that carboxymethyl-chitosan could protect cartilage through multiple ways, including nitric oxide production inhibition, mitochondrial function modulation and scavenge reactive oxygen species2626 Chen Q, Liu S, Du Y, Peng H, Sun L. Carboxymethyl-chitosan protects rabbit chondrocytes from interleukin-1 beta-induced apoptosis. Eur J Pharmacol. 2006 Jul 10;541(1-2):1-8. PMID: 16740257.. As a derivative of chitosan, COS may share these key properties.
The receptor activator of NF-κB ligand (RANKL) (localized on osteoblasts) enhances osteo¬clastogenesis via interaction with its receptor RANK (localized on osteoclasts), whereas os¬teoprotegerin (OPG, produced by osteoblasts) inhibits this osteoclastogenesis by binding to RANKL. Although OPG, RANK and RANKL gain importance in the bone tissue, several studies has shown that OPG and RANKL are produced by chondrocytes1212 Tat SK, Pelletier J, Velasco CR, Padrines M, Martel-Pelletier J. New perspective in osteoarthritis: the OPG and RANKL system as a potential therapeutic target? Keio J Med. 2009 Mar;58(1):29-40. PMID: 19398882.. Both OPG and RANKL are expressed by osteoblastic cells and bone marrow stromal cells and it is now thought that the final step in the osteoclast regulatory pathway may be determined by the relative ratio of the RANKL/OPG system. Data show that rat OA subchondral bone osteoblasts have abnormal OPG and RANKL levels and consequently an altered OPG/RANKL ratio. Recently, two different groups have studied the gene expression of these molecules from macerate of bone biopsies, which have reported that mRNA levels of OPG were lower, and RANKL and OPG/ RANKL ratio levels were higher in patients with fractures in respect to those with osteoarthrosis2727 Logar DB, Komadina R, Prezelj J, Ostanek B, Trost Z, Marc J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J Bone Miner Metab. 2007;25(4):219-25. PMID: 17593491.. Our results confirmed that these factors are expressed in cartilage and changed during OA progression. In vivo and in vitro studies have confirmed the involvement of apoptosis in OA2828 Zamli Z, Sharif M. Chondrocyte apoptosis: a cause or consequence of osteoarthritis? Int J Rheum Dis. 2011 May;14(2):159-66. PMID: 21518315.. Chondrocyte apoptotic bodies releases degradative enzymes and accelerabbite extracellular matrix degradation, inhibition of chondrocyte apoptosis thus has a therapeutic value in osteoarthritis2929 Thomas CM, Whittles CE, Fuller CJ, Sharif M. Variations in chondrocyte apoptosis may explain the increased prevalence of osteoarthritis in some joints. Rheumatol Int. 2011 Oct;31(10):1341-8. PMID: 20396889. Shimizu et al.3030 Shimizu S, Asou Y, Itoh S, Chung U, Kawaguchi H, Shinomiya K, Muneta T. Prevention of cartilage destruction with intraarticular osteoclastogenesis inhibitory factor/osteoprotegerin in a murine model of osteoarthritis. Arthritis Rheum. 2007 Oct;56(10):3358-65. PMID: 17907189. also found that intra-articular administration of OPG prevent cartilage destruction in a murine model of osteoarthritis, and such effect was also associated with anti-apoptotic effect. In our study, we found that OPG was upregulated in OA model and COS further increased its expression. We speculate that the upregulation in OA is a way of compensation to increasing apoptosis, while COS treatment could enhance the expression of OPG and exert strong anti-apoptotic effect.
The aim of the present study was to investigate the changes in the RANKL/OPG system as well as evaluating whether the COS that modulate the expression of this system. This study demonstrates, for the first time, that OPG secretion in COS treatment is elevated with respect to OPG secretion from OA, while RANKL was inhibited. In addition, the ratio of OPG/RANKL was significantly increased after treatment with COS, while RNAKL/RANK was decreased. These data indicate a decreased OPG/RANKL interaction, therefore maintaining a normal catabolic status. It was recently demonstrated that during longstanding OA and rheumatoid arthritis, the OPG/RANKL ratio in the synovial fluid is much more elevated in OA compared to rheumatoid arthritis. The OPG-RANKL mechanism is much more complex than previously though. As RANKL controls the bioavailability of OPG and vice versa, the equilibrium between membranous RANKL and soluble OPG will be determinant of a curative application of OPG. This data could have been of great importance in establishing a close relationship between the OPG/RANKL ratio and the subchondral bone changes. Hence, as membranous RANKL controls the bioavailability of exogenous OPG, the equilibrium between RANKL and OPG is crucial for future therapeutic use of OPG.
In summary, for the first time, we have also demonstrated that OPG secretion and OPG/RANKL mRNA ratio are higher in COS than in OA group in all studied conditions. And RANKL/RANK mRNA ratio are lower in COS than in OA group. Nonetheless, further experiments exploring the effects of adding or increasing OPG or inhibiting RANKL on the subchondral bone pathophysiological pathways of OA are required.
Conclusions
OPG and RANKL are involved in the pathogenesis of OA. The present study suggests for the first time that COS can relieve the progression of OA, and this effect is associated with modulation of the expression of OPG and RANKL. However, the underlying mechanisms require further investigation. We speculate that OPG/RANKL/RANK may be novel target for OA therapy.
Acknowledgements
To Renmin Hospital and Central Laboratory of Renmin Hospital, Wuhan University, for assistance of the animal center.
References
-
1Neogi T.The epidemiology and impact of pain in osteoarthritis.Osteoarthritis Cartilage. 2013 Sep;21(9):1145-53. PMID: 23973124.
-
2Chojnacki M, Kwapisz A, Synder M, Szemraj J. Osteoarthritis: etiology, risk factors, molecular mechanisms. Postepy Hig Med Dosw (Online). Jan 2;68:640-52. PMID: 24864114.
-
3Hochberg MC, Yerges-Armstrong L, Yau M, Mitchell BD. Genetic epidemiology of osteoarthritis: recent developments and future directions. Curr Opin Rheumatol. 2013 Mar;25(2):192-7. PMID: 23249833.
-
4Narayanan D, Jayakumar R, Chennazhi KP. Versatile carboxymethyl chitin and chitosan nanomaterials: a review. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014 Nov-Dec;6(6):574-98. PMID: 25266740.
-
5Dutta J, Tripathi S, Dutta PK. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: a systematic study needs for food applications. Food Sci Technol Int. 2012 Feb;18(1):3-34. PMID: 21954316.
-
6Reighard KP, Hill DB, Dixon GA, Worley BV, Schoenfisch MH. Disruption and eradication of P. aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling. 2015;31(9-10):775-87. PMID: 26610146.
-
7Fang I, Yang C, Yang C. Chitosan oligosaccharides prevented retinal ischemia and reperfusion injury via reduced oxidative stress and inflammation in rats. Exp Eye Res. 2015 Jan;130:38-50. PMID: 25479043.
-
8Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015 Jan 14;6(1):33-49. PMID: 25594943.
-
9Chung MJ, Park JK, Il Park Y. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int Immunopharmacol. 2012 Feb;12(2):453-9. PMID: 22266066.
-
10Liu H, Li W, Xu G, Li X, Bai X, Wei P, Yu C, Du YG. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol Res. 2009 Mar;59(3):167-75. PMID: 19121394.
-
11Yao H, Luo M, Hung L, Chiang M, Lin J, Lii C, Huang C. Effects of chitosan oligosaccharides on drug-metabolizing enzymes in rat liver and kidneys. Food Chem Toxicol. 2012 May;50(5):1171-7. PMID: 22386817.
-
12Tat SK, Pelletier J, Velasco CR, Padrines M, Martel-Pelletier J. New perspective in osteoarthritis: the OPG and RANKL system as a potential therapeutic target? Keio J Med. 2009 Mar;58(1):29-40. PMID: 19398882.
-
13Sousa BL, Barroso-Neto IL, Oliveira EF, Fonseca E, Lima-Neto P, Ladeira LO, Freire VN. Explaining RANKL inhibition by OPG through quantum biochemistry computations and insights into peptide-design for the treatment of osteoporosis. RSC Advances. 2016 Aug;88(6):84926-42. doi: 10.1039/C6RA16712H.
» https://doi.org/10.1039/C6RA16712H -
14Wang J, Chen T, Qin S, Duan Y, Wang G. Inhibitory effect of metformin on bone metastasis of cancer via OPG/RANKL/RANK system. Med Hypotheses. 2013 Nov;81(5):805-6. PMID: 24074896.
-
15Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014 Oct 20;5:511. PMID: 25368616.
-
16Kwan Tat S, Amiable N, Pelletier J, Boileau C, Lajeunesse D, Duval N, Martel-Pelletier J. Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology (Oxford). 2009 Dec;48(12):1482-90. PMID: 19762475.
-
17Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006 Feb;38(2):234-43. PMID: 16185945.
-
18Kim JL, Moon CW, Son YS, Kim SJ. Combined effect of bilateral ovariectomy and anterior cruciate ligament transection with medial meniscectomy on the development of osteoarthritis model. Ann Rehabil Med. 2016 Aug;40(4):583-91. PMID: 27606264.
-
19Kuroki H, Nakagawa Y, Mori K, Ohba M, Suzuki T, Mizuno Y, Ando K, Takenaka M, Ikeuchi K, Nakamura T. Acoustic stiffness and change in plug cartilage over time after autologous osteochondral grafting: correlation between ultrasound signal intensity and histological score in a rabbit model. Arthritis Res Ther. 2004;6(6):R492-504. PMID: 15535827.
-
20Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP, Matyas J, Pritzker KP, D'Lima DD, Lotz MK. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis Cartilage. 2012 Jun;20(6):476-85. PMID: 22353747.
-
21Sugahara K, Pothacharoen P, Najarus S, Settakorn J, Mizumoto S, Kongtawelert P. Effects of sesamin on the biosynthesis of chondroitin sulfate proteoglycans in human articular chondrocytes in primary culture. Glycoconj J. 2014 Apr;31(3):221-30. PMID: 24338203.
-
22Cohen-Solal M, Funck-Brentano T, Hay E. Animal models of osteoarthritis for the understanding of the bone contribution. Bonekey Rep. 2013 Oct 2;2:422. PMID: 24422124.
-
23Kim JL, Moon CW, Son YS, Kim SJ. Combined effect of bilateral ovariectomy and anterior cruciate ligament transection with medial meniscectomy on the development of osteoarthritis model. Ann Rehabil Med. 2016 Aug;40(4):583-91. PMID: 27606264.
-
24Pritzker KPH. Osteoarthritis joint instability and OA: do animal models provide insights? Nat Rev Rheumatol. 2011 Jul 12;7(8):444-5. PMID: 21750530.
-
25Zhou J, Liu S, Qiu B, Hu Q, Ming J, Peng H. Effects of hyaluronan on vascular endothelial growth factor and receptor-2 expression in a rabbit osteoarthritis model. J Orthop Sci. 2009 May;14(3):313-9. PMID: 19499299.
-
26Chen Q, Liu S, Du Y, Peng H, Sun L. Carboxymethyl-chitosan protects rabbit chondrocytes from interleukin-1 beta-induced apoptosis. Eur J Pharmacol. 2006 Jul 10;541(1-2):1-8. PMID: 16740257.
-
27Logar DB, Komadina R, Prezelj J, Ostanek B, Trost Z, Marc J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J Bone Miner Metab. 2007;25(4):219-25. PMID: 17593491.
-
28Zamli Z, Sharif M. Chondrocyte apoptosis: a cause or consequence of osteoarthritis? Int J Rheum Dis. 2011 May;14(2):159-66. PMID: 21518315.
-
29Thomas CM, Whittles CE, Fuller CJ, Sharif M. Variations in chondrocyte apoptosis may explain the increased prevalence of osteoarthritis in some joints. Rheumatol Int. 2011 Oct;31(10):1341-8. PMID: 20396889.
-
30Shimizu S, Asou Y, Itoh S, Chung U, Kawaguchi H, Shinomiya K, Muneta T. Prevention of cartilage destruction with intraarticular osteoclastogenesis inhibitory factor/osteoprotegerin in a murine model of osteoarthritis. Arthritis Rheum. 2007 Oct;56(10):3358-65. PMID: 17907189.
-
Financial source:
none
-
1
Research performed at Department of Orthopedics, Renmin Hospital, Wuhan University, China.
Publication Dates
-
Publication in this collection
June 2017
History
-
Received
12 Feb 2017 -
Reviewed
17 Apr 2017 -
Accepted
18 May 2017