Abstracts
Twenty-two stream segments, representing the diverse types of environments in the mid-western region of the Paraná State, southern Brazil, were sampled for occurrence of macroalgal communities from May to October in 2002. Twenty-seven macroalgal taxa were found, with Chlorophyta as the dominant algal group, followed by Cyanobacteria, Rhodophyta and Chrysophyta. The most widespread species was Phormidium retzii. Distribution was patchy, with species number per sampling sites ranging from zero to six and correlated positively with the abundance. On the other hand, no significant correlation was found among the species number and abundance with environmental variables. Results indicated wide and random variation among the streams. Thus, it seemed that the distribution of macroalgal communities in the study area responded more closely to the local-scale variation than the regional characteristics.
local-scale variation; Paraná State; stream macroalgae; restricted distribution; Brazil
Durante o período de maio a outubro de 2002, vinte e dois segmentos de riachos, representando os diversos tipos de ambientes da região centro-oeste do estado do Paraná, sul do Brasil, foram amostrados quanto à comunidade de macroalgas. Foram encontradas vinte e sete macroalgas. A divisão Chlorophyta foi o grupo algal predominante, seguido por Cyanobacteria, Rhodophyta e Chrysophyta. A espécie com melhor distribuição foi Phormidium retzii. Foi observado um padrão de distribuição em mosaico. O número de espécies por ponto de amostragem variou de zero a seis e correlacionou-se positivamente com abundância de espécies. Por outro lado, nenhuma outra correlação significativa foi encontrada entre número de espécies e abundância com as variáveis ambientais. Os resultados, suportados por dados de literatura, indicaram uma variação ampla e aleatória entre os riachos, sugerindo que a distribuição da comunidade de macroalgas na área de estudos parece responder mais fortemente a variações nas condições em escala local do que em escalas regionais maiores.
BIOLOGICAL AND APPLIED SCIENCE
Distribution of stream macroalgal communities from the mid-western region of Paraná state, southern Brazil: importance of local scale variation
Ciro Cesar Zanini Branco* * Author for correspondence: czbranco@assis.unesp.br ; Rogério Antônio Krupek and Cleto Kaveski Peres
Departamento de Ciências Biológicas; Universidade Estadual Paulista; Av. Dom Antônio, 2.100; 19806-900; Assis - SP - Brasil
ABSTRACT
Twenty-two stream segments, representing the diverse types of environments in the mid-western region of the Paraná State, southern Brazil, were sampled for occurrence of macroalgal communities from May to October in 2002. Twenty-seven macroalgal taxa were found, with Chlorophyta as the dominant algal group, followed by Cyanobacteria, Rhodophyta and Chrysophyta. The most widespread species was Phormidium retzii. Distribution was patchy, with species number per sampling sites ranging from zero to six and correlated positively with the abundance. On the other hand, no significant correlation was found among the species number and abundance with environmental variables. Results indicated wide and random variation among the streams. Thus, it seemed that the distribution of macroalgal communities in the study area responded more closely to the local-scale variation than the regional characteristics.
Key words: local-scale variation, Paraná State, stream macroalgae, restricted distribution, Brazil
RESUMO
Durante o período de maio a outubro de 2002, vinte e dois segmentos de riachos, representando os diversos tipos de ambientes da região centro-oeste do estado do Paraná, sul do Brasil, foram amostrados quanto à comunidade de macroalgas. Foram encontradas vinte e sete macroalgas. A divisão Chlorophyta foi o grupo algal predominante, seguido por Cyanobacteria, Rhodophyta e Chrysophyta. A espécie com melhor distribuição foi Phormidium retzii. Foi observado um padrão de distribuição em mosaico. O número de espécies por ponto de amostragem variou de zero a seis e correlacionou-se positivamente com abundância de espécies. Por outro lado, nenhuma outra correlação significativa foi encontrada entre número de espécies e abundância com as variáveis ambientais. Os resultados, suportados por dados de literatura, indicaram uma variação ampla e aleatória entre os riachos, sugerindo que a distribuição da comunidade de macroalgas na área de estudos parece responder mais fortemente a variações nas condições em escala local do que em escalas regionais maiores.
INTRODUCTION
Primary production in lotic ecosystems is strongly influenced by the local aquatic community (Biggs, 1990). In this context, stream algae (particularly those with macroscopic growth - macroalgae) represent, beside the aquatic macrophytes (Thomaz et al., 1999; Pezzato and Camargo, 2004), one of the most important components of these communities (Holmes and Whitton, 1981). Thus, studies relating the establishment and maintenance of stream macroalgal communities with specific biotic and abiotic factors are desirable.
Many studies have been carried out in several parts of the world, including boreal, temperate and tropical regions, and a large quantity of important information about the influence of selected environmental variables under lotic macroalgae have been produced (Kawecka, 1980; 1982; Holmes and Whitton, 1981; Johansson, 1982; Whitton, 1984; John and Moore, 1985; Sheath et al., 1986; 1989; 1996; Biggs and Price, 1987; Entwisle, 1989; Sheath and Cole, 1992; Necchi et al., 1995; 2000; Branco and Necchi, 1996a; Branco and Necchi, 1998). Information like these has allowed a better comprehension of the functional mechanisms involved in the determination of the ecological distribution pattern of the stream macroalgal communities worldwide.
Investigations concerning the ecological distribution of stream macroalgal communities have also been conducted in Brazil (Branco and Necchi, 1996b; 1998; Necchi et al., 2000). However, the majority of those studies are concentrated in a single tropical region of São Paulo State, southeastern Brazil. Considering that Brazil is a big country with variety of tropical and subtropical regions, more ecological information from other Brazilian areas might be important to bring new insights about the stream macroalgal communities.
The present study was carried out to analyze the species richness and abundance of macroalgal communities from lotic ecosystems of the mid-western region of Paraná State, southern Brazil. Simultaneously, species occurrence was investigated in accordance with the stream environmental data in order to evaluate possible ecological tendencies.
MATERIAL AND METHODS
Study Area
The occurrence of stream macroalgal communities was investigated in the mid-western region of Paraná State, southern Brazil (23º58'-26º13'S; 50º53'-54º35'W). The study area is circumscribed by the following geographic accidents: Esperança Scarp (east border), Piquiri River (north border), Iguaçu River (south border) and Paraná River (west border) (Fig. 1). The topography of the sampling area is variable along its extension and the relief is characterized as a plain (Maack, 1981). There is an important declivity from east to west border and total slope is relatively high with the highest altitude reaching 1,250 m and the lowest 550 m (Wons, 1982). The original vegetation is relatively well-preserved, showing highest disturbance by human activity near the biggest cities. About 80% of the sampling sites were well-preserved as to the original surround vegetation. Three main vegetational types are described as predominant in regional landscape: a) Clean Grassland with predominance of grass; b) Subtropical Rainforest with predominance of the pine species Araucaria angustifolia (Bertol.) Kuntze; c) Tropical Rainforest with highest vegetational diversity and restricted to lowest altitudes (Wons, 1982). The regional climate corresponds to a variation of the humid subtropical (Cfa), according to Köppen classification (Wons, 1982).
Methodological approach
Twenty-two stream segments were sampled from late fall through early spring (May to October) in 2002, period considered as having the highest diversity and abundance of macroalgae in Brazilian streams (Necchi and Pascoaloto, 1993; Necchi et al., 1995; Branco and Necchi, 1996b).
Sites were visited once and were 10-30 km apart from each other. The estimation of the required number of the sampling sites was based on an adaptation of the Michaelis-Menten equation for reaction kinetics, using accumulated number of species and of streams during the sampling program (Necchi et al., 1995). According to this estimation, twenty stream segments would be enough to yield a representative species richness in the study region.
Each sampling site consisted of 10-m reaches in streams of variable sizes (1st to 4th order), which were thoroughly examined by means of cross-transect technique (Sheath and Burkholder, 1985) for the presence of macroalgae. In addition, abundance of each species was estimated in terms of percentage cover as described by Sheath and Burkholder (1985), adopting the concept of macroalgae as defined by Sheath and Cole (1992).
The following stream variables were measured: water temperature, specific conductance, turbidity, pH, and oxygen saturation. All measurements were made directly in the field with the help of a Consort C535 water quality checker. Current velocity was measured using a 2030R General Oceanics mechanical flow meter, at five equally spaced points along the stream reaches, and averaged. Besides, substratum type (according to particle size classes given by Gordon et al., 1992) and shading (on the basis of a four-class scale: open, partly shaded, shaded and heavily shaded, modified from DeNicola et al., 1992) were also annotated.
Cluster analysis was performed to identify the associations of sampling sites from a similarity matrix of sampling sites per species occurrence based on Jaccard index. Hypotheses were developed in order to find some relationship among the species composition and stream features. Thus, three model matrices (using separately chemical data, physical data and, finally, the approximate distance among sampling sites each other) were constructed and then Mantel tests were used to assess the statistical significance of the matrix correlation between the Jaccard similarity matrix and model matrices. Statistical significances were tested using 5,000 random permutations. The statistical analyses were carried out using NTSYS (Rohlf , 1998).
RESULTS
Each stream variable showed a particular pattern of variation throughout the study area. Mean temperature during the sampling program was 18.6 + 2.0ºC with minimum value of 15.4ºC and maximum of 22.7ºC. Oxygen saturation ranged widely (43.0-93.0%,  = 57.3 + 14.8%) among the sampling sites. Specific conductance was relatively low in most of the sampling sites with mean value of 39.2 + 24.6 µS.cm.s-1. An exceptionally elevated value was observed in a single sampling site (135.4 µS.cm.s-1), as well as a positive correlation with pH (r = 0.541, p<0.001).
= 57.3 + 14.8%) among the sampling sites. Specific conductance was relatively low in most of the sampling sites with mean value of 39.2 + 24.6 µS.cm.s-1. An exceptionally elevated value was observed in a single sampling site (135.4 µS.cm.s-1), as well as a positive correlation with pH (r = 0.541, p<0.001).
The pH (5.4-8.3,  = 6.8 + 0.7 ) and current velocity (5.0-99.0 cm.s-1,
= 6.8 + 0.7 ) and current velocity (5.0-99.0 cm.s-1,  = 39.6 + 29.9) showed relatively wide variation throughout the study area. Most sampling sites (56%) were dominated by the rock substrates (including continuous bedrock, boulders, pebbles, and gravels). Other important substratum types were: sand-clay (17%), sand (15%) and macrophytes (12%). Depth values ranged from shallow to mid-size stream (5.0-37.1 cm,
= 39.6 + 29.9) showed relatively wide variation throughout the study area. Most sampling sites (56%) were dominated by the rock substrates (including continuous bedrock, boulders, pebbles, and gravels). Other important substratum types were: sand-clay (17%), sand (15%) and macrophytes (12%). Depth values ranged from shallow to mid-size stream (5.0-37.1 cm,  = 17.75 + 7.91) and were positively correlated with current velocity (r = 0.596, p<0.001) and gravel substrate (r = 0.354, p<0.05). In terms of shading, stream reaches showed the following features: Open (32% of all sampling sites), partly shaded (26.5%), shaded (23.5%), and heavily shaded (18%).
= 17.75 + 7.91) and were positively correlated with current velocity (r = 0.596, p<0.001) and gravel substrate (r = 0.354, p<0.05). In terms of shading, stream reaches showed the following features: Open (32% of all sampling sites), partly shaded (26.5%), shaded (23.5%), and heavily shaded (18%).
Twenty-seven macroalgae were found in the study area, including 19 subgeneric taxa and eight vegetative groups with no new record for Brazilian streams. Chlorophyta was the dominant algal group (55.5% of the species), followed by Cyanobacteria (18.5%), Rhodophyta (14.8%) and Heterokontophyta (11.2%).
The most widespread species was Phormidium retzii (C. Agardh) Gomont (Cyanobacteria), occurring in 31.8% of the sampling sites. In addition, "Chantransia" stage of Batrachospermum sp. (45.5%) was well distributed in the streams from the study area also. In contrast, 21 species (77.7%) were found only in one (14) or two (7) sampling sites.
On the other hand, half of the sampling sites (50%) had a macroalgal flora represented by few species (maximum of two species) (Table 1).
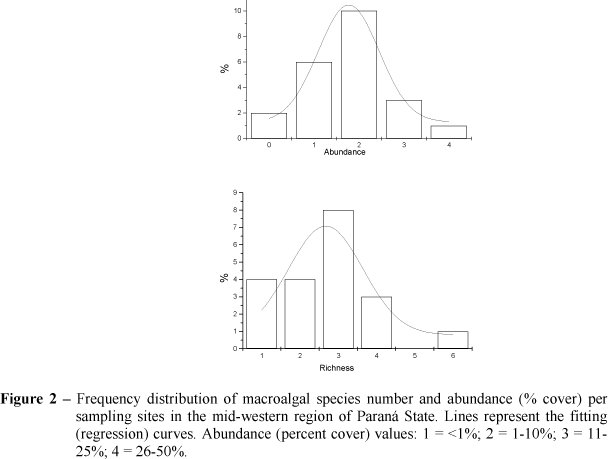
As a general rule, open and partly shaded streams showed higher species number (mean of 3.3 species/stream) than shaded and heavily shaded areas (1.7 species/stream). Species richness per sampling site varied from 0 to 6 (2.7 + 1.7), whereas species abundance ranged from 0 to 23.1% (5.7 + 7.4, Table 1 and Fig. 2).
Abundance showed a positive correlation with species richness (r=0.397, p<0.05), however, no significant correlation was found with any of the stream variables analyzed. Species abundance was positively correlated with rock substrate (r=0.371, p<0.05) and negatively with gravel (r=-0.318, p<0.05) and sand substrate (r=-0.348, p<0.05).
Cluster analyses (Fig. 3) and ordination procedures failed to produce an adequate representation of the sampling sites based on floristic characteristics. The cophenetic correlation was 0.741, considered by Rodrigues et al. (2002) as a relatively low value by standard criterion (usually only values above 0.8 provided a reasonable representation of similarity). The following correlations between Jaccard similarity matrix and model matrices were found: 1) chemical characteristics matrix - r=-0.024, p=0.551; 2) physical characteristics matrix - r=0.159, p=0.063 and 3) distance among sampling sites matrix - r=-0.058, p=0.253.
DISCUSSION
Despite the sampling program was carried out during the period considered as having the highest diversity and abundance of macroalgae in Brazilian streams (Necchi and Pascoaloto, 1993; Necchi et al., 1995; Branco and Necchi, 1996b), the macroalgal species diversity found in the mid-western region of Paraná State (27 species/22 sampling sites) was considered lower when compared with other similar studies (Sheath et al., 1986; 1989; Necchi et al., 1995; Branco and Necchi, 1996a; Filkin et al., 2003). All macroalgal species identified were previously known for Brazilian streams. The species diversity found in the study area was lower than those observed in other biomes, including the regions with very severe climate, such as south-central Alaska (Sheath et al., 1986), as well as those strongly impacted, such as the northwest region of São Paulo State (Necchi et al., 1995). Values of species diversity in the mid-western region of Paraná State were similar to those found in eastern Atlantic Rainforest of São Paulo State (Branco and Necchi, 1996b). Mean species richness per sampling sites was the lowest ever reported for the macroalgal communities from other regions (Sheath et al., 1986 - 3.8; Sheath et al., 1989 - 4.9; Sheath and Cole, 1992 - 2.7-3.6; Necchi et al., 1995 - 3.1). Similar to as described for the Atlantic Forest streams (Branco and Necchi, 1996a), a higher species number observed in the unshaded streams segments in comparison to the shaded ones suggested that the quality and quantity of light available to the macroalgal communities could be an important factor to explain the ecological distribution of these communities.
All clustering procedures failed to produce an adequate representation of the similarity among the floristic composition and stream sampled in the mid-western region of the Paraná State. These results indicated wide and random variation among the streams. Thus, it seemed that the distribution of macroalgal communities in the study area responded more closely to the local-scale variation than to the regional characteristics. Therefore, specific combinations of the characteristics in each stream interval (e.g., microhabitat combinations among a set of physical, chemical and biotic characteristics, such as, shading, substrate type, hydrodynamic features, resource supply, presence and type of herbivorous, among others) had more influence on the macroalgal communities diversity and distribution than any other global variable (e.g., climate features, land use, geology, and others).
It was observed that the macroalgal flora found in the majority of the sampling sites was represented by few species and most of the macroalgal species identified showed restricted distribution with presence in few sampling sites. The wide distribution of Phormidium retzii in the study area (also reported to other region around the world) represented an evident exception to this assumption. However, there are several uncertainties about taxonomy circumscription of this species that, probably, represents a collective type in which several taxa are included under the same denomination (Geitler, 1932; Branco et. al., 1999).
Other taxonomic and ecological studies on stream macroalgal communities developed in tropical, boreal and temperate regions from the American Continent showed that occurrence of stream macroalgal species with restricted distribution was very common. Sheath AND Burkholder (1985) showed that 17 of the 39 species (44% of all species identified) found in the stream from Rhode Island had their distribution restricted, at most, to the 5% of all sampling sites sampled. Still in North America, Sheath et al. (1989) observed about 45% of all species (30 of the 67 species) occurring in less than 5% of stream segments analyzed in the Boreal Forest. Similarly, Sheath et al. (1986) found 22 of the 40 species (55%) in up to 5% sampling sites from center-south region of Alaska.
In Brazil, there are several studies showing similar results. Necchi et al. (1995) observed 55% of all species identified in the northwest region of São Paulo State as occurring in less than 5% of the streams investigated. Branco AND Necchi (1996b) found 61% of all species in less than 5% of the stream segments of the eastern region of the Atlantic Forest from São Paulo State. In the same study, approximately 49% of the species occurred in only one of the 52 sampling sites analyzed.
Similar results were found in studies carried out in smaller geographic regions or in particular taxonomic groups. Necchi et al. (1994) investigated the macroalgal community of a tropical drainage basin and reported 32 macroalgal species, from which 15 (47%) were found in less than 10% of streams investigated. Finally, Branco et al. (1999) surveyed Cyanobacteria from 172 stream segments from São Paulo State, Brazil, and found 77% of the species occurring in, at most, 2% of sampling sites. Species observed exclusively in only one sampling site represented 65% of all species.
These data suggested that the spatial heterogeneity of the running water systems was, in fact, the most important factor to define the diversity and distribution of the macroalgal community in these habitats. Thus, the way the spatial heterogeneity in stream was produced could be determined by a combination of several factors that was particular to each small stream fragment (i.e., patch) at a specific time. These dynamic combinations of spatial and temporal variability could be so particular that the community diversity in each small stream fragment couldn't be completely explained by any contemporary models of biodiversity, but by a random distributional model. It might explain the typical patch distribution frequently observed in the stream macroalgal communities in several regions of the world (Sheath et al., 1986; Sheath et al., 1989; Necchi et al., 1995).
In summary, these results as well as other reports from literature suggested that the diversity and distribution of a stream macroalgal community, depended primarily on the local-scale heterogeneity in smaller levels (i.e., microhabitat) and such specific combinations of chemical, physical and biological factors might be determinant of a random distributional model to stream macroalgal communities. However, more experimental studies are strongly recommended in order to test whether the random distribution would represent a general pattern in lotic macroalgae or would be more applicable to particular biome/region. Besides, other studies are required to understand the major mechanisms by which the environmental heterogeneity operates in local scale.
ACKNOWLEDGMENTS
This research was supported by a CNPq research grant to CCZB (520257/01-4) and a CNPq grant to RAK and FRB (520257/01-4).
Received: August 30, 2006;
Revised: March 30, 2007;
Accepted: June 16, 2008.
- Biggs, B. J. F. (1990), Periphyton communities and their enviroments in New Zealand rivers. N. Z. J. Mar. Freshw. Res., 24, 367-386.
- Biggs, B. J. F. and Price, G. M. A. (1987), A survey of filamentous algal proliferation in New Zealand rivers. N. Z. J. Mar. Freshw. Res., 21, 175-191.
- Branco, C. C. Z. and Necchi, O. JR. (1996a), Survey of stream macroalgae of eastern Atlantic Rainforest of São Paulo State, southeastern Brazil. Algol. Stud, 80, 35-57.
- Branco, C. C. Z. and Necchi, O. JR. (1996b), Distribution of stream macroalgae in the eastern Atlantic Rainforest of São Paulo State, southeastern Brazil. Hydrobiologia, 333, 139-150.
- Branco, L. H. Z. and Necchi, O. JR. (1998), Distribution of macroalgae in three tropical drainage basins of southeastern Brazil. Arch. Hydrobiol., 142, 241-256.
- Branco, L. H. Z.; Necchi, O. JR. and Branco, C. C. Z. (1999), Cyanophyceae from lotic ecosystems of São Paulo State, southeastern Brazil. Algol. Stud, 94, 63-87.
- DeNicola, D. M.; Hogland, K. D. and Roemer, S. C. (1992), Influence of canopy cover on spectral irradiance and periphyton assemblages in a prairie stream. J. N. Am. Benthol. Soc., 11, 391-404.
- Entwisle, T. J. (1989), Macroalgae in Yarra River basin: flora and distribution. Proc. R. Soc. Vic, 101, 1-76.
- Filkin, N. R.; Sherwood, A. R. and Vis, M. L. (2003), Stream macroalgae of the Hawaiian Islands: 23 addittional collection sites. Pac. Sci., 57,421-431.
- Geitler, L. (1932), Cyanophyceae. In - Kryptogamen-flora von Deutschland Österrich und der Schweiz, ed. L. Rabenhorst. Akademische Verlagsgesell-schaft, Leipzig, pp. 1-1196.
- Gordon, N. D.; McMahon, T. A. and Finlayson, B. L. (1992), Stream hydrology, an introduction for ecologists John Wiley and Sons, Chichester.
- Holmes, N. T. H. and Whitton, B. A. (1981), Phytobenthos of River Tees and its tributairies. Freshw. Biol, 11, 43-60.
- John, D. M. and Moore, J. A. (1985), Observation on phytobenthos of freshwater Thames I. The environment, floristic composition and distribution of macrophytes (principally macroalgae). Arch. Hydrobiol., 102, 435-459.
- Johansson, C. (1982), Attached algal vegetation in running waters of Jämtland, Sweden. Acta Phytogeogr. Suec., 71, 1-83.
- Kawecka, B. (1980), Sessile alge in European mountain stream. 1: the ecological characteristic of communities. Acta Hydrobiol., 22, 361-420.
- Kawecka, B. (1982), Stream ecossystem in mountain grassland (West Carpanthians). 6: Sessile algae communities. Acta Hydrobiol., 24, 357-365.
- Maack, R. (1981), Geografia física do Estado do Paraná Livraria José Olympio, Rio de Janeiro.
- Necchi, O. JR.; Branco, C. C. Z.; Simões, R. C. G. and Branco, L. H. Z. (1995), Distribution of stream macroalgae in northwest region of São Paulo State, southeastern Brazil. Hydrobiologia, 299, 219-230.
- Necchi, O. JR.; Branco, C. C. Z. and Branco, L. H. Z. (2000), Distribution of stream macroalgae in São Paulo State, southeastern Brazil. Algo.l Stud., 97, 43-57.
- Necchi, O. JR. and Pascoaloto, D. (1993), Seasonal dynamics of macroalgal communities in the Preto River basin, São Paulo, southeastern Brazil. Arch. Hydrobiol., 129, 231-252.
- Necchi, O. JR.; Pascoaloto, D. and Branco, L. H. Z. (1994), Distribution of macroalgae in a tropical river basin from southeastern Brazil. Arch. Hydrobiol., 129, 459-471.
- Pezzato, M. M. and Camargo, A. F. M. (2004), Photosynthetic rate of the aquatic macrophyte Egeria densa Planch. (Hydrocharitaceae) in two rivers from the Itanhaém River Basin in São Paulo State, Brazil. Braz. arch. biol. technol, 47, 153-162.
- Rodrigues, F. M. R.; Diniz-Filho, J. A. F.; Bataus, L. A. M. and Bastos, R. P. (2002), Hyposthesis testing of genetic similarity base don RAPD data using Mantel tests and model matrices. Genet. Mol. Biol., 25, 435-439.
- Rohlf, F. J. (1998), NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System, Version 2.0, User's Guide Exeter Software, New York.
- Sheath, R. G. and Burkholder, J. (1985), Characteristics of softwater stream in Rhode Island. II: Composition and seasonal dynamics of macroalgae communities. Hydrobiologia, 128, 109-118.
- Sheath, R. G. and Cole, K. M. (1992), Biogeography of stream macroalgae in North America. J. Phycol., 28, 448-460.
- Sheath, R. G.; Hamilton, P. B.; Hambrook, J. A. and Cole, K. M. (1989), Stream macroalgae of eastern boreal forest region of North America. Can. J. Bot., 67, 3553-3562.
- Sheath, R. G.; Morison, M. O.; Korch, J. E.; Kaczmarczyk, D. and Cole, K. M. (1986), Distribution of stream macroalgae in south-central Alaska. Hydrobiologia, 135, 259-269.
- Sheath, R. G.; VIS, M. L., Hambrook, J. A. and Cole, K. M. (1996), Tundra stream macroalgae of North America: composition, distribution and physiological adaptations. Hydrobiologia, 336, 67-82.
- Thomaz, S. M.; Bini, L. M.; Souza, M. C.; Kita, K. K.and Camargo, A. F. M. (1999), Aquatic Macrophytes of Itaipu Reservoir, Brazil: Survey of Species and Ecological Considerations. Braz. arch. biol. technol, 42, 15-22.
- Whitton, B. A. (1984), Ecology of European Rivers Blackwell Scitific Publishers, Oxford.
- Wons, I. (1982), Geografia do Paraná Editora Ensino Renovado, Curitiba.
Publication Dates
-
Publication in this collection
06 May 2009 -
Date of issue
Apr 2009
History
-
Received
30 Aug 2006 -
Reviewed
30 Mar 2007 -
Accepted
16 June 2008