ABSTRACT
The aim of the present study is to evaluate the possible curative effect of erythropoietin (EPO) on radiation-induced damage to the lung and its renin angiotensin system. EPO (200 U/100g) was i.p. injected to male rats one hour post 6 Gy whole body gamma irradiation. The animals were sacrificed after 14 days post irradiation. Irradiation induced significant drop of haematological values, bone marrow (BM) count, lung oxidative stress markers, glutathione (GSH) and superoxide dismutase(SOD) associated with significant elevation of malondialdehyde (MDA), advanced oxidation protein product (AOPP) and nitric oxide (NO) besides serum inflammatory markers, tumor necrosis factor alpha (TNF-α) and lactate dehydrogenase (LDH). Also serum and lung renin angiotensin system markers, sodium (Na) and potassium (K) were elevated whereas calcium (Ca) was decreased. EPO treatment post irradiation has significantly ameliorated blood parameters and BM count also lung oxidative stress markers were improved associated with decreased serum Na, TNF-α, and LDH levels. Lung K and Ca showed no change compared to irradiated group. The findings of the present study suggest that EPO might contribute to enhance recovery of the lungs from radiation-induced damage due to its erythropoietic, anti-oxidative and anti-inflammatory effects.
Key words:
erythropoietin; radiation; inflammation; oxidative stress
INTRODUCTION
Human and animal cells permanently react with oxygen. As a consequence of these reactions highly reactive molecules including free radicals are formed which leads to oxidative stress in cells. Oxidative stress is considered to be imbalance between the production of reactive oxygen species (ROS) and the biological ability to remove reactive intermediates [1]1 Yin J, Ren W, Wu X, Yang G, Wang J, Li T, et al. Oxidative stress mediated signaling pathways. A review. J Food Agric Environ. 2013;11:132-139..
Acute effects of radiation include hematopoietic cell loss, immune suppression, and potential injury to other sites such as the lung, kidney and central nervous system [2]2 Augustine A D, Gondre´-Lewis T, McBride W, Miller L, Pellmarc TC, Rockwelld S. Animal Models for Radiation Injury, Protection and Therapy. Rad Res. 2005;164: 100-109.. Acute radiation syndrome involves pathophysiological processes of organ dysfunction where the gut and the lung play a pivotal role. Radiation-induced lung fibrosis is a limiting complication in the treatment of haematological disorders involved in total body exposure to radiation [3]3 Molteni A, Wolfe LF, Ward WF, Ts'ao CH, Molteni LB, Veno P, et al. Effect of angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007; 13: 1307-1316.. Inflammation is the predominant early finding within irradiated lungs. This is followed by a second wave of inflammatory response that takes place 1-4 weeks after exposure, with inflammatory cell recruitment in the lungs. After irradiation, central inflammation is caused by increased expression of pro-inflammatory cytokines such as TNF-α [44 Spitz DR, Hauer-Jensen M. Ionizing radiation-induced responses: where free radical chemistry meets redox biology and medicine. Antioxid Redox Signal. 2014;20: 1407-1409. ]. The enzyme i-nitric oxide synthase (i-NOS) is very well known to be a significant part of the fibrotic pathway [55 Tsavlis D, Kokaraki G, Koliakos-Kouzi K, Tzoumaka A, Afroditi P, Angomachalelis I, et al. Erythropoietin Inhibits the Bleomycin-Induced Pulmonary Fibrosis in Rats. Chest. 2010;138 (4 _MeetingAbstracts). ] and oxidative stress depletes alveolar epithelial glutathione levels. Irradiation is known to stimulate renin angiotensin system (RAS) [66 Nistala R, Wei Y, Sowers J, Whaley-Connell A. RAAS-mediated redox effects in chronic kidney disease . Translat Res. 2009;5 : 1 - 12 . ].
Erythropoietin (EPO) has been widely used for treatment of anemia in chronic kidney disease and cancer chemotherapy-associated anemia [77 Johnson DW, Pollock CA, Macdougall IC. Erythropoeisis-stimulating agent hyporesponsiveness. Nephrology (Carlton). 2007;12,321-330. ] and for reduction of allogenic blood transfusion in surgery patients. Researches in the last decade have shown that EPO and its receptor are expressed in tissues other than those concerned in erythropoiesis including the brain, the reproductive tract, the heart, the spleen, and the lung [88 Rabie T, Marti HH. Brain Protection by Erythropoietin: A Manifold Task. Physiol. 2008; 23:263-274. ]. There is a growing body of evidence that EPO may have important therapeutic roles in preventing or ameliorating ischemic or toxic damage to critical organs through anti-inflammatory actions [99 Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264: 405-324. ]. EPO may exert the anti-inflammatory actions either directly by antagonism of pro-inflammatory cytokines (such as TNF-α) or indirectly by mitigation of tissue injury [1010 Johnson DW, Vesey DA, Gobe GC. Erythropoietin protects against acute kidney injury and failure. The Open Drug Discovery J. 2010;2: 8-17. ].
Erythropoietin, and other haematopoeitic cytokines, use Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway that is used by various components of renin angiotensin system (RAS) which have been identified in the lung [1111 Horiuchi M, Akishita M, Dzau VJ. Molecular and cellular mechanism of angiotensin ?? mediated apoptosis. Endocr Res. 1998;24:307-314. ]. The present study aims to evaluate the outcome of EPO on hematopoietic reconstitution, lung oxidative stress and RAS in irradiated rats.
MATERIALS AND METHODS
Mature male albino rats of pure strain ranging from 110-150 g body weight; 8 weeks old were obtained from the Egyptian Holding Company for Biological Products and Vaccine (Cairo, Egypt). The animals were maintained under standard conditions of ventilation, temperature, light and humidity and allowed free access to standard pellet diet and tap water. All animal treatments were conducted according to the Ethics Committee of the National Research Centre and in accordance with the recommendations for the proper care and use of laboratory animals (HIN publication No. 85-23, revised 1985) in accordance with international ethical considerations.
Radiation facility
Whole body γ- irradiation was performed with a Canadian 137Cs Gamma Cell-40 biological irradiator located at the National Centre for Radiation Research and Technology, Cairo, Egypt; at a dose rate of 0.49 Gy/min. Rats were exposed to whole body γ- radiations delivered as an acute single dose of 6Gy to induce lung damage [1212 Mansoub N H and Sarvestani AH. Effects of gamma irradiation on histomorphology of different organs in rats. Annals of Biological Research. 2011;2: 431-436. ].
EPO treatment:
Erythropoietin was purchased as ampoules (Epoetin) from SEDICO, Egypt. Rats received a single i.p. injection at a dose of 200 U/100g [55 Tsavlis D, Kokaraki G, Koliakos-Kouzi K, Tzoumaka A, Afroditi P, Angomachalelis I, et al. Erythropoietin Inhibits the Bleomycin-Induced Pulmonary Fibrosis in Rats. Chest. 2010;138 (4 _MeetingAbstracts). ] and were sacrificed after 14 days to detect radiation damage.
Groups of Animals
Animals used in the present experiments included 4 groups of 6 males categorized into: 1- Control untreated rats (C). 2- Group of rats injected i.p. with 200 U/100g of (EPO). 3- Group of rats exposed to 6Gy whole body gamma irradiation (R). 4- Group of rats exposed to 6Gy gamma irradiation and injected i.p. with a single dose of 200 U/100g EPO, one hour post irradiation (R +EPO). All animals groups were sacrificed after 14 days.
Samples collection
After 14 days, animals were sacrificed and blood was immediately collected by heart puncture. Part of the blood was placed on ethylene diamine tetra acetic acid (EDTA) from Sigma Aldrich Chemical Co. St Louis, MO, USA, for haematological analysis and the rest was separated by centrifugation and stored as serum at -20°C until analyzed.
Peripheral blood cell counts were analyzed in a MASCOT Multi-species Hematology System (CDC Technologies, Oxford, CT, USA).
Bone marrow suspension count: Femur bones were cleaned and chipped. The bone marrow was blow out into 5 ml saline solution, pooled and mixed by drawing and expelling it several times from the syringe without needle. Total number of bone marrow nucleated cells were counted using trypan blue on a haemocytometer [1313 Goldberg ED, Dygal AM, Shakhov V P. Methods for tissue culture in hematology; TGU Publishing House, Tomsk.1992; 256-257. ].
Biochemical analysis
All chemicals and reagents were purchased of pure chemical materials from Sigma Aldrich, St Louis, MO, USA. Measurements in the lungs: For assessment of oxidative stress biomarkers a portion of the lung was weighed and 10% weight/volume (W/V) tissue homogenates were prepared in 0.1M phosphate buffer (pH 7.4) using Teflon homogenizer (Glas-Col, Terre Haute, Ind., USA). The homogenates were centrifuged at 10,000g for 15 min. Aliquots of supernatants were separated for use. Lung tissues malondialdehyde (MDA), an end product of lipid peroxidation, was determined according to Yoshioka et al. [1414 Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal cord blood and protective mechanism against activated oxygen toxicity in blood. Am J Obstet Gynecol. 1979;135(3): 372. ], and nitric oxide (NO) was estimated according to Cortas and Wakid [1515 Cortas N, Wakid N. Determination of inorganic nitrate inserum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36(8 Pt1): 1440 - 1443. ], advanced oxidation protein product (AOPP) was determined according to Witko-Sarsat et al. [1616 Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen A T, Zingraff J, Jungers P, Deschamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uraemia. Kidney Int. 1996; 49: 1304 - 1313. ], Superoxide dismutase (SOD) activity was assayed by the method of Minami and Yoshikawa ENT#091;1717 Minami M, Yoshikawa H. A simplified assay method of super oxide dismutase. Clinica Chimica Acta. 1979; 29: 337-342.ENT#093;, wehereas (GSH) was determined according to Beutler, et al. [1818 Beutler E, Duran O, Kelly BM. Improved method of blood glutathione. J Lab Clin Med. 1963;61(51):852. ]. Sodium and potassium were determined according to Kim et al. [1919 Kim EK, Waddell LD, Logan JE. Evaluation for four reagent kits and two flame photometers used to determine sodium and potassium in serum. J Clin Chem. 1972;18: 124 - 128. ], calcium was determined according to Janssen and Helbing [2020 Janssen JW, Helbing AR. An improvement of the routine calcium determination in serum. Eur J Clin Chem Clin Biochem. 1991;29: 197 - 201. ] using Quimica Clinica Aplicada, S.A. Co., kits. Serum LDH concentration was determined according to Kachmar and Moss [2121 Kachmar JF, Moss DW. In Fundamentals of Clinical Chemistry, 2nd ed. NW Tietz, Editor. WB Saunders, Philadelphia. 1976; p 682. ] and TNF-α concentrationwas assayed by commercially available ELISA kit (Quantikine, R & D Systems, Minneapolis, MN) and measured according to Aramachi [2222 Aramachi T. Japan's Bioventures Today. Immuno-Biological Laboratories Company, Ltd., Japan, 1989; 370: 831. ]. All determinations were done using T60 UV/VIS spectrophotometer (PG Instruments, London, UK).
Statistical analysis:
Comparisons among groups (n= 6) were performed by computer program SPSS (Chicago, IL, USA) version 15. Statistical significance was determined using Student t-test. Differences between means were considered significant at P <0.05. The values are expressed as means ± SD (standard deviation).
RESULTS
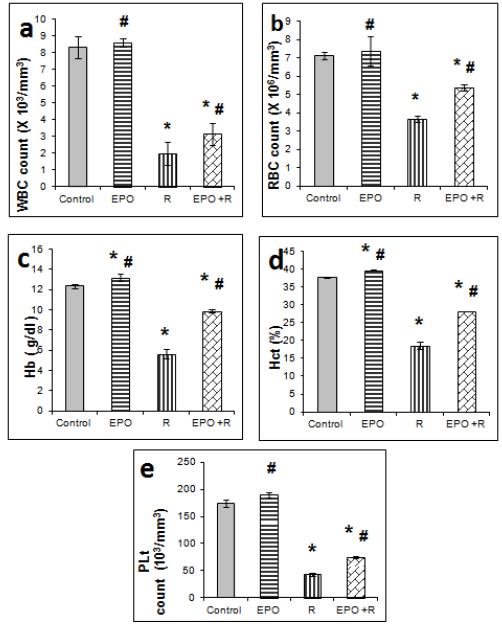
EPO injection (200 U/100g) resulted in elevated Hb, Hct and platelets and no change was observed in leucocytes and erythrocytes as well as viable bone marrow cells in non irradiated rats compared to the control 14 days after treatment. All blood parameters and BM cells investigated 14 days post irradiation showed significant (p ≤ 0.05) decreases compared to the control animals. The decrease in RBC, WBC, Hb, Hct and platelets by irradiation was significantly elevated in rats treated with EPO (Figure 1) compared to irradiated group. Accelerated bone marrow reconstitution was also observed by EPO treatment to irradiated animals compared to irradiated group (Figure 2).
Effect of 6 Gy gamma irradiation and erythropoietin on blood parameters: *: significantly different from that of the control. #: significantly different from that of irradiated rats.
Effect of 6 Gy gamma irradiation and erythropoietin on total bone marrow count X 103. *: significantly different from that of the control. #: significantly different from that of irradiated rats.
Whole body exposure of rats to gamma radiation (6Gy ) provoked oxidative stress demonstrated by significant ( p ≤ 0.05) increase of MDA, NO, AOPP levels of lung tissues associated with significant decrease of GSH content and SOD activity as compared to their respective values in the control group (Table 1) . Administration of EPO 1 hour post irradiation induced significant decrease (p ≤ 0.05) of oxidative biomarkers levels of lung tissues and significant increases of antioxidants compared to the irradiated group.
The present results (Table 2) demonstrated that EPO treatment decreased serum Na and Ca values compared to the control. A significant increase of TNFα and LDH values were induced by irradiation, whereas after 14 days from EPO treatment to irradiated rats, a significant decrease was detected in these values compared to the irradiated group. The irradiated animals showed significant elevations of Na and K accompanied by a significant decrease of Ca in both serum and lung (Tables 2 and 3). EPO treatment to irradiated animals significantly decreased serum Na and K and elevated Ca values compared to the irradiated group, whereas, no significant change was noted in lung K and Ca values.
DISCUSSION
Exposure to ionizing radiation produces ROS (hydroxyl radicals, superoxide anion radicals and hydrogen peroxide) which causes antioxidant /oxidant imbalance and cause cellular damage [2323 Konopacka M, Rogolinski J. Thiamine prevents X-ray induction of genetic changes in human lymphocytes in vitro. Acta Biochem Pol. 2004;51: 839 - 843. ]. Antioxidant/oxidant balance is necessary to maintain redox homeostasis especially during oxidative stress conditions [2424 Thangasamy T, Jeyakumar P, Sittadjody S, Joyee AG, Chinnakannu P. L-carnitine mediates protection against DNA damage in lymphocytes of aged rats. Biogerontol. 2009;10: 163-172. ].
Irradiation of the animals at 6 Gy resulted in a significant decrease in WBCs, RBCs, platelets, Hct value, Hb content and bone marrow viable cells. The hematological values decrease is attributed to a significant reduction of hematopoietic stem cells (HSC) and impairment of its self-renewal via activation of the specific cellular pathways by irradiation [2525 Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107(1): 358-366. ]. Nevertheless, the decrease in RBCs count and thus Hb content might also be attributed to increased permeability of cell membrane, leading to erythrocyte haemolysis [2626 Nikishkin IA, Sukolinskij VN, Kovaleva OV, Raspopova , NI, Naumenko VK . Enzyme of erythrocyte membrane protection under the combined effect of an antioxidant complex and acute irradiation. Radiobiologia. 1992;32: 738 - 745. ]. The decrease in WBCs is the consequence of radiation-induced lipid peroxidation and damage of their cell membranes rich in polyunsaturated fatty acids which coincides with elevated MDA content. Irradiation induced leucopenia has likewise been reported in gamma irradiated mice as a direct concequence of lymphopenia and neutropenia following irradiation [2727 Mishima S, Saito K,Maruyama H,Inoue M,Yamashita T, Ishida T,Gu Y. Antioxidant and immune-enhancing effects of Echinacea purpura . Biol Pharm Bull. 2004;27:1004-1009. ]. The radiation induced decrease in Hb content is attributed to the decline in the observed number of red blood cells and to decreased Hb affinity for oxygen [2828 Thiriot C, Kergonou JF, Allary M, Saint-Blancard J, Rocquet G. Effect of whole body gamma irradiation on oxygen transport by rat erythrocytes. Biochimie. 1982;64(1): 79 - 83. ]. Hct decrease can be attributed to the failure of erythropoiesis, destruction of mature cells, or increased plasma volume [2929 Malhotra N, Srivastava PN. Haematological eff ects after administration of radiophosphorus in mice: Fractionated irradiation and late effects. J Radiobiol Radiother. 1978;19: 347- 350. ].
EPO treatment attenuated the decrease in blood parameters and BM cells in irradiated animals. This is attributed to the supportive effect of cytokines to bone marrow cells in reconstitution of haematopoietic organs [3030 Webb DS, Shimizu Y, Van Seventer GA, Shaw S, Gerrard TL. LFA-3, CD44 and CD45, physiologic triggers of human monocyte TNF andIL-1 release. Science. 1990;249: 1295 - 1297. ] and to EPO role in treatment of anemia and stimulation of erythropoiesis [77 Johnson DW, Pollock CA, Macdougall IC. Erythropoeisis-stimulating agent hyporesponsiveness. Nephrology (Carlton). 2007;12,321-330. ]. Erythropoietin binds to EPO receptors on RBCs surface and activates JAK2 cascade [3131 Hazendaroglu IC, Özturk MA. Towards the understanding of the local hematopoeitic bone marrow renin-angiotensin system. Int J Biochem Cell Biol. 2003;35:868-880. ]. This pathway can serve as a point of cross talk between the components of locally present RAS in the bone marrow and haematopoiesis.
The function of the pulmonary RAS seems to be of particular importance since it plays a role in the pathogenesis of lung diseases related to lung injury and fluid homeostasis [3232 Königshoff M, Wilhelm A, Jahn A, Sedding D, Amarie, O V, Eul B, et al. Angiotensin ?? receptor 2 is Expressed and mediates Angiotensin ?? signaling in lung fibrosis. Am J Respirat Cell Molec Biol. 2007; 37: 640-650. ]. Irradiation is known to stimulate RAS [66 Nistala R, Wei Y, Sowers J, Whaley-Connell A. RAAS-mediated redox effects in chronic kidney disease . Translat Res. 2009;5 : 1 - 12 . ]. ANG ІІ is a hormone that causes vasoconstriction and tubular reabsorption of sodium. It is a growth promoting substance that is implicated in lung fibrosis [3232 Königshoff M, Wilhelm A, Jahn A, Sedding D, Amarie, O V, Eul B, et al. Angiotensin ?? receptor 2 is Expressed and mediates Angiotensin ?? signaling in lung fibrosis. Am J Respirat Cell Molec Biol. 2007; 37: 640-650. ]. The significant increase of Na and K values in serum and lung of irradiated animals besides the decrease of Ca in the present results is an indication of activation of RAS inducing salt retention [3333 Thomas MM, Tikellis C, Burns WM, Bialkowski K, Cao Z, Coughlan MT, et al. Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol. 2005;16: 2976 - 2984. ]. It is also attributed to destruction of mature erythrocytes due to partial damage of the natural barriers for Na and K movements by gamma radiolysis [3434 Klimenko VI, Iukhimuk LM. The morpho-functional indices of the erythrocytic link in hemopoiesis in persons constantly working in an area of intensified radio-ecological control. Lik Sprava. 1993 ;(2-3):31-36. ] and the inhibitory effect of ionizing radiation on Ca ion channels [3535 Nunia V, Sncheti G, Goyal PK. Protection of Swiss albino mice against whole-body gamma irradiation by diltiazem. Br J Radiol. 2007;80: 77- 84. ], or radiation induced elevation of aldosterone which contributes to the loss of Ca [3636 Ashry OM, Moustafa M, Abd el Baset A , Abu Sinna G , Farouk H. Outcome of venom bradykinin potentiating factor on renin angiotensin system in irradiated rats. Int J Radiat Biol. 2012;88: 840 - 845. ]. In the present study treatment of irradiated rats with EPO exerted significant drop of Na and K and elevation of Ca in the serum and significant decrease of lung Na which could be attributed to EPO anti-apoptotic and anti-oxidative properties preventing damage to critical organs [3737 Tsavlis D, Tzoumaka A, Kokaraki G, Koliakos-Kouzi K, Koutsonikolas D, Tektonidou A, et al. Erythropoietin Inhibits the Expression of Erythropoietin Receptor (EPO-R) in Bleomycin (BLM)-Induced Pulmonary Fibrosis in Rats . Chest. 2012;142 (4_MeetingAbstracts). ].
Antioxidant enzymes are considered to be the first line of cellular defense against oxidative damage [3838 Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2009; 16;85(23-26):830-4 ]. The current study showed significant inhibition of GSH content and SOD in parallel to increment of MDA, NO, AOPP in γ-irradiated rats. GSH present in the lung lining plays a crucial role in protecting the lung from oxidative stress by detoxifying exogenous toxicants and quenching ROS [3939 Doelman CJ, Blast A. Oxygen radicals in lung pathology. Free Radic Biol Med. 1990;9: 381. ]. Radiation-induced oxidative stress depletes alveolar epithelial GSH and SOD.
Inflammation is the predominant early histological and physiological finding within irradiated lungs. This is followed by a second wave of inflammatory response that takes place 1-4 weeks after exposure, with inflammatory cell recruitment in the lungs [4040 Hallahan DE and Virudachalam S .Intercellular adhesion molecule I knockout abrogates radiation induced pulmonary inflammation. Proc. Natl. Acad. Sci. USA. 1997;94:6432-6437 . ]. It is well known that NO modulates cell radiosensetivity and immunological response [4141 Verovski NN, Vanden Berg DL, Soete GA, Bols BL, Storm GA. Intrinsic radiosensetivity of human pancreatic tumor cells and the radiosensitivity potency of the nitric oxide donor sodium nitropruside. Br J Cancer. 1996;74:1734. ] and ionizing radiation increase NO production by inducing NOS expression and stimulating constitutive NOS [4242 Leach JK, Black SM, Schmidt-Ullrich RK, Mikkelson RB. Activation of consecutive nitric-oxide synthase in early signaling event induced by ionizing radiation. J. Biol. Chem. 2002;277 (18):15400 - 15406. ]. i-NOS is very well known to be a significant part of the fibrotic pathway [5] which coincides with the present results. In the same way, the present increase of AOPP levels might be attributed to the interaction of proteins with ROS [4343 Eskiocak S, Tutunculer F, Basaran U, Taskiran A, Cakir E. The effect of melatonin on protein oxidation and nitric oxide in the brain tissue of hypoxic neonatal rats. Brain Dev. J. 2007;29: 19 - 24. ].
In the current study, oxidative stress parameters in the lungs were significantly improved by EPO post-administration. EPO is regarded as a general tissue-protective cytokine, a strong antioxidant and increases the activity of antioxidant enzymes, such as SOD, and has been reported to decrease MDA levels in hypoxic-ischemic organ injuries [99 Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264: 405-324. ]. It is also shown that EPO has protective effects associated with acute lung injury model by inhibiting leukocyte accumulation and reducing oxidative stress-associated lipid peroxidation [4444 Tascilar O, Cakmak GK, Tekin IO, Emre AU, Ucan BH, Bahadir B, et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol. 2007; 46:6172-6182. ]. Hypoxia- induced EPO production regulated by hypoxia- inducible factor-1(HIF-1) has been observed in astrocytes in the brain and endothelial cells [4545 Chikuma M, Nasuda S, Kobayashi T, Nagao M, Sasaki R. Tissue specific regulation of erythropoietin production in the murine kidney, brain and uterus. Am J Physiol Endocrinol Metab. 2000;279: E1242-E1248. ] suggesting that EPO may mediate a number of organ responses to low oxygen tension, beyond simple erythropoiesis.
Elevated TNFα in the present study is explained by [4646 Van der Meeren A, Vandamme M, Squiban C, Gaugler MH, Mouthon MA. . Inflammatory Reaction and Changes in Expression of Coagulation Proteins on Lung Endothelial Cells after Total-Body Irradiation in Mice. Radiat Res. 2003;160 :637- 646. ] on the basis that immunologically mediated inflammation undoubtedly plays a central role in airway inflammation. In the same way LDH elevation, as an indication of tissue and cellular damage, was attributed to irradiation induced leakage of cytosolic enzymes such as LDH [4747 Cai L, Iskander S, Cherian MG, Hammond RR. Zinc- or cadmium-pre-induced metallothionein protects human central nervous system cells and astrocytes from radiation-induced apoptosis. Toxicol Lett. 2004;146(3): 217. ]. Both acute and chronic inflammation may be involved in radiation-induced late organ damage, as anti-inflammatory treatments have been demonstrated to be beneficial regarding late organ damage/dysfunction [4848 Michalowski AS. On radiation damage to normal tissues and its treatment II. Anti-inflammatory drugs. Acta Oncol. 1994; 33: 139-157. ]. The improvement of TNFα and LDH exerted by EPO seemed to be related to its anti-apoptotic, anti-oxidative, and anti-inflammatory properties as well as its angiogenic effect [4949 Hardee ME, Aricasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332-339. ]. EPO may also exert anti-inflammatory actions either directly by antagonism of pro-inflammatory cytokines such as TNFα or indirectly by mitigation of tissue injury [99 Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264: 405-324. ].
CONCLUSION
Based on the results obtained in the current study, it appears that EPO contributes to attenuation of lung injury via its antioxidant and anti-inflammatory effects.
References
-
1Yin J, Ren W, Wu X, Yang G, Wang J, Li T, et al. Oxidative stress mediated signaling pathways. A review. J Food Agric Environ. 2013;11:132-139.
-
2Augustine A D, Gondre´-Lewis T, McBride W, Miller L, Pellmarc TC, Rockwelld S. Animal Models for Radiation Injury, Protection and Therapy. Rad Res. 2005;164: 100-109.
-
3Molteni A, Wolfe LF, Ward WF, Ts'ao CH, Molteni LB, Veno P, et al. Effect of angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007; 13: 1307-1316.
-
4Spitz DR, Hauer-Jensen M. Ionizing radiation-induced responses: where free radical chemistry meets redox biology and medicine. Antioxid Redox Signal. 2014;20: 1407-1409.
-
5Tsavlis D, Kokaraki G, Koliakos-Kouzi K, Tzoumaka A, Afroditi P, Angomachalelis I, et al. Erythropoietin Inhibits the Bleomycin-Induced Pulmonary Fibrosis in Rats. Chest. 2010;138 (4 _MeetingAbstracts).
-
6Nistala R, Wei Y, Sowers J, Whaley-Connell A. RAAS-mediated redox effects in chronic kidney disease . Translat Res. 2009;5 : 1 - 12 .
-
7Johnson DW, Pollock CA, Macdougall IC. Erythropoeisis-stimulating agent hyporesponsiveness. Nephrology (Carlton). 2007;12,321-330.
-
8Rabie T, Marti HH. Brain Protection by Erythropoietin: A Manifold Task. Physiol. 2008; 23:263-274.
-
9Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264: 405-324.
-
10Johnson DW, Vesey DA, Gobe GC. Erythropoietin protects against acute kidney injury and failure. The Open Drug Discovery J. 2010;2: 8-17.
-
11Horiuchi M, Akishita M, Dzau VJ. Molecular and cellular mechanism of angiotensin ?? mediated apoptosis. Endocr Res. 1998;24:307-314.
-
12Mansoub N H and Sarvestani AH. Effects of gamma irradiation on histomorphology of different organs in rats. Annals of Biological Research. 2011;2: 431-436.
-
13Goldberg ED, Dygal AM, Shakhov V P. Methods for tissue culture in hematology; TGU Publishing House, Tomsk.1992; 256-257.
-
14Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal cord blood and protective mechanism against activated oxygen toxicity in blood. Am J Obstet Gynecol. 1979;135(3): 372.
-
15Cortas N, Wakid N. Determination of inorganic nitrate inserum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36(8 Pt1): 1440 - 1443.
-
16Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen A T, Zingraff J, Jungers P, Deschamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uraemia. Kidney Int. 1996; 49: 1304 - 1313.
-
17Minami M, Yoshikawa H. A simplified assay method of super oxide dismutase. Clinica Chimica Acta. 1979; 29: 337-342.
-
18Beutler E, Duran O, Kelly BM. Improved method of blood glutathione. J Lab Clin Med. 1963;61(51):852.
-
19Kim EK, Waddell LD, Logan JE. Evaluation for four reagent kits and two flame photometers used to determine sodium and potassium in serum. J Clin Chem. 1972;18: 124 - 128.
-
20Janssen JW, Helbing AR. An improvement of the routine calcium determination in serum. Eur J Clin Chem Clin Biochem. 1991;29: 197 - 201.
-
21Kachmar JF, Moss DW. In Fundamentals of Clinical Chemistry, 2nd ed. NW Tietz, Editor. WB Saunders, Philadelphia. 1976; p 682.
-
22Aramachi T. Japan's Bioventures Today. Immuno-Biological Laboratories Company, Ltd., Japan, 1989; 370: 831.
-
23Konopacka M, Rogolinski J. Thiamine prevents X-ray induction of genetic changes in human lymphocytes in vitro. Acta Biochem Pol. 2004;51: 839 - 843.
-
24Thangasamy T, Jeyakumar P, Sittadjody S, Joyee AG, Chinnakannu P. L-carnitine mediates protection against DNA damage in lymphocytes of aged rats. Biogerontol. 2009;10: 163-172.
-
25Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107(1): 358-366.
-
26Nikishkin IA, Sukolinskij VN, Kovaleva OV, Raspopova , NI, Naumenko VK . Enzyme of erythrocyte membrane protection under the combined effect of an antioxidant complex and acute irradiation. Radiobiologia. 1992;32: 738 - 745.
-
27Mishima S, Saito K,Maruyama H,Inoue M,Yamashita T, Ishida T,Gu Y. Antioxidant and immune-enhancing effects of Echinacea purpura . Biol Pharm Bull. 2004;27:1004-1009.
-
28Thiriot C, Kergonou JF, Allary M, Saint-Blancard J, Rocquet G. Effect of whole body gamma irradiation on oxygen transport by rat erythrocytes. Biochimie. 1982;64(1): 79 - 83.
-
29Malhotra N, Srivastava PN. Haematological eff ects after administration of radiophosphorus in mice: Fractionated irradiation and late effects. J Radiobiol Radiother. 1978;19: 347- 350.
-
30Webb DS, Shimizu Y, Van Seventer GA, Shaw S, Gerrard TL. LFA-3, CD44 and CD45, physiologic triggers of human monocyte TNF andIL-1 release. Science. 1990;249: 1295 - 1297.
-
31Hazendaroglu IC, Özturk MA. Towards the understanding of the local hematopoeitic bone marrow renin-angiotensin system. Int J Biochem Cell Biol. 2003;35:868-880.
-
32Königshoff M, Wilhelm A, Jahn A, Sedding D, Amarie, O V, Eul B, et al. Angiotensin ?? receptor 2 is Expressed and mediates Angiotensin ?? signaling in lung fibrosis. Am J Respirat Cell Molec Biol. 2007; 37: 640-650.
-
33Thomas MM, Tikellis C, Burns WM, Bialkowski K, Cao Z, Coughlan MT, et al. Interactions between renin angiotensin system and advanced glycation in the kidney. J Am Soc Nephrol. 2005;16: 2976 - 2984.
-
34Klimenko VI, Iukhimuk LM. The morpho-functional indices of the erythrocytic link in hemopoiesis in persons constantly working in an area of intensified radio-ecological control. Lik Sprava. 1993 ;(2-3):31-36.
-
35Nunia V, Sncheti G, Goyal PK. Protection of Swiss albino mice against whole-body gamma irradiation by diltiazem. Br J Radiol. 2007;80: 77- 84.
-
36Ashry OM, Moustafa M, Abd el Baset A , Abu Sinna G , Farouk H. Outcome of venom bradykinin potentiating factor on renin angiotensin system in irradiated rats. Int J Radiat Biol. 2012;88: 840 - 845.
-
37Tsavlis D, Tzoumaka A, Kokaraki G, Koliakos-Kouzi K, Koutsonikolas D, Tektonidou A, et al. Erythropoietin Inhibits the Expression of Erythropoietin Receptor (EPO-R) in Bleomycin (BLM)-Induced Pulmonary Fibrosis in Rats . Chest. 2012;142 (4_MeetingAbstracts).
-
38Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2009; 16;85(23-26):830-4
-
39Doelman CJ, Blast A. Oxygen radicals in lung pathology. Free Radic Biol Med. 1990;9: 381.
-
40Hallahan DE and Virudachalam S .Intercellular adhesion molecule I knockout abrogates radiation induced pulmonary inflammation. Proc. Natl. Acad. Sci. USA. 1997;94:6432-6437 .
-
41Verovski NN, Vanden Berg DL, Soete GA, Bols BL, Storm GA. Intrinsic radiosensetivity of human pancreatic tumor cells and the radiosensitivity potency of the nitric oxide donor sodium nitropruside. Br J Cancer. 1996;74:1734.
-
42Leach JK, Black SM, Schmidt-Ullrich RK, Mikkelson RB. Activation of consecutive nitric-oxide synthase in early signaling event induced by ionizing radiation. J. Biol. Chem. 2002;277 (18):15400 - 15406.
-
43Eskiocak S, Tutunculer F, Basaran U, Taskiran A, Cakir E. The effect of melatonin on protein oxidation and nitric oxide in the brain tissue of hypoxic neonatal rats. Brain Dev. J. 2007;29: 19 - 24.
-
44Tascilar O, Cakmak GK, Tekin IO, Emre AU, Ucan BH, Bahadir B, et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol. 2007; 46:6172-6182.
-
45Chikuma M, Nasuda S, Kobayashi T, Nagao M, Sasaki R. Tissue specific regulation of erythropoietin production in the murine kidney, brain and uterus. Am J Physiol Endocrinol Metab. 2000;279: E1242-E1248.
-
46Van der Meeren A, Vandamme M, Squiban C, Gaugler MH, Mouthon MA. . Inflammatory Reaction and Changes in Expression of Coagulation Proteins on Lung Endothelial Cells after Total-Body Irradiation in Mice. Radiat Res. 2003;160 :637- 646.
-
47Cai L, Iskander S, Cherian MG, Hammond RR. Zinc- or cadmium-pre-induced metallothionein protects human central nervous system cells and astrocytes from radiation-induced apoptosis. Toxicol Lett. 2004;146(3): 217.
-
48Michalowski AS. On radiation damage to normal tissues and its treatment II. Anti-inflammatory drugs. Acta Oncol. 1994; 33: 139-157.
-
49Hardee ME, Aricasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332-339.
-
Erratum
In Article “The Evolving Role of Erythropoietin in Lung of Irradiated Rats”, with the number of DOI: http://dx.doi.org/10.1590/1678-4324-2017160800, published in journal Brazilian Archives of Biology and Technology, vol. 60, the 01 page.That read:“http://dx.doi.org/10.190/1678-4324-2017160800”Read:“http://dx.doi.org/10.1590/1678-4324-2017160800”
Publication Dates
-
Publication in this collection
2017
History
-
Received
26 Aug 2016 -
Accepted
28 Nov 2016