Abstract
Shea tree sawdust delignification kinetic data during alkaline peroxide pretreatment were investigated at temperatures of 120 °C, 135 °C, and 150 °C. The activation energy during delignification was 76.4 kJ/mol and the Arrhenius constant was calculated as 8.4 x 106 per min. The reducing sugar yield for the treated to the untreated biomass was about 22-fold. Enzymatic hydrolysis conditions studied were; time (72 h and 96 h), substrate concentration (20, 30, 40, and 50 g/L), and enzyme loadings (10, 25, 40, 50 FPU/g dry biomass), which showed the optimum conditions of 96 h, 40 g/L, and 25 FPU/g dry biomass at 45 °C hydrolysis temperature. At the optimized enzymatic hydrolysis conditions, the reducing sugar yield was 416.32 mg equivalent glucose/g treated dry biomass. After 96 h fermentation of treated biomass, the ethanol obtained at 2% effective cellulose loading was 12.73 g/L. Alkaline peroxide oxidation pretreatment and subsequent enzymatic hydrolysis improved the ethanol yield of the biomass.
Keywords:
Alkaline peroxide oxidation; Fermentation; Vitellaria paradoxa; Optimization; Pretreatment; Enzymatic hydrolysis.
INTRODUCTION
Bioenergy is a renewable resource because of the short life cycle compared to the fossil fuel alternative. Plants and plant-derived materials (biomass) are important feed-stocks for lignocellulosic biofuels. The biomass cell wall structure is rigid and hard to break down. Pretreatment is an important tool for lignocelluloses bioconversion processes and is required to alter the biomass structure to make cellulose more accessible to the enzymatic complex (Mosier et al., 2005Mosier, N., Wyman, C. E., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M. T. and Ladish, M., Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673-686 (2005).). Pretreatments release cellulose from amorphous lignin and hemicellulose complexes. Wood and other lignocellulosic biomass con sist of four major chemical components: cellulose, lignin, hemicellulose, and extractives. In ethanol production from lignocellulose, it is necessary to remove the lignin and extractives from the wood, leaving primarily the wood polymer carbohydrates, cellulose and hemicellulose. There are bottlenecks for efficient ethanol production from lignocellulosic materials, among these the presence of lignin sur rounding the cellulose acts as a physical barrier, reducing the available sites for enzymatic hydrolysis (Krishna and Chowdary, 2000Krishna, S. H. and Chowdary, G. V., Optimization of simultaneous saccharification and fermentation for the production of ethanol from lignocellulosic biomass. Journal of Agricultural and Food Chemistry, 48, 1971-1976 (2000).; Kim and Lee, 2006Kim, T. H. and Lee, Y. Y., Fractionation of corn stover by hot-water and aqueous ammonia treatment. Bioresource Technology, 97, 224-232 (2006).). Lignin is responsible for the integrity, structural rigidity, and prevention of the swelling of lignocelluloses. Lignin constitutes the most recognized factor that is responsible for the resistance of lignocellulosic materials to enzymatic degradation by limiting the enzyme accessibility. Alkaline peroxide oxidation pretreatment is one method that has been discovered to improve the accessibility of lignocellulosic bio mass to cellulolytic enzymes (Kumar et al., 2009Kumar, P., Barrett, D. M., Delwiche, M. J. and Stroeve, P., Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and bio fuel production. Industrial Engineering Chemistry Research, 48, 3713-3729 (2009).). This pretreatment approach has been studied extensively (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).; Wei and Cheng, 1985Wei, C. J. and Cheng, C. Y., Effect of hydrogen peroxide pretreatment on the structural features and the enzymatic hydrolysis of rice straw. Biotechnology and Bioengineering, 27, 1418-1426 (1985).; Gould, 1984Gould, J. M., Alkaline peroxide delignification of agricultural residues to enhance enzymatic saccharification. Biotechnology and Bioengineering, 26, 46-52 (1984).; Gould et al., 1989Gould, J. M., Jasberg, B. K., Fahey, G. C. and Berger, L. L., Treatment of wheat straw with alkaline hydrogen peroxide in a modified extruder. Biotechnology and Bioengineering, 33, 233-236 (1989).; Qi et al., 2009Qi, B., Chen, X., Shen, F., Su, Y. and Wan, Y., Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Industrial and Engineering Chemistry Research, 48, 7346-7353 (2009).; Li et al., 2013Li, S., Xu, S., Liu, S., Yang, C. and Lu, Q., Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technology, 85, 1201-1211 (2004).). Studies on cellulosic biomass delig nification (lignin removal) usually involve two different approaches, assuming that the lignocellulosic material contains one type of lignin, and that the material is composed of several species (usually initial, bulk, and residual lignin) dissolving at different rates (Kleinert, 1975Kleinert, T. N., Ethanol-water delignification of wood. Rate constants and activation energy. Tappi Journal, 58, 170-171 (1975).; Springer, 1963Springer, E. L., Harris, J. F. and Neil, W. K., Rate studies of the hydrotropic delignification of aspen wood. Tappi Journal, 46, 551-555 (1963).). The most usual approach is to assume that the different lignin species react consecutively according to first-order kinetics. In the several species of lignin approach, the different phases observed during delignification correspond to changes in the mechanism that controls the reaction rate of the overall process (Parajó et al., 1995Parajó, J. C., Alonso, J. L. and Santos, V., Kinetics of catalysed organosolv processing of pine wood. Industrial and Engineering Chemistry Research, 34, 4333-4341 (1995).; Vázquez et al., 1995Vázquez, G., Antorrena, G. and Gonzalez, J., Kinetics of acid-catalysed delignification of Eucalyptus globulus wood by acetic acid. Wood Science and Technology, 29, 267-275 (1995).). The chemical kinetics of alkaline pretreatment processes have been demonstrated in the scientific literature (Kleinert, 1966; Kim and Holtzapple, 2006Kim, S. and Holtzapple, M. T., Delignification kinetics of corn stover in lime pretreatment. Bioresource Technology, 97, 778-785 (2006).). The Shea tree, Vitellaria paradoxa, is rich in carbohydrate and lig nin contents (Ayeni et al., 2014Ayeni, A. O., Omoleye, J. A., Mudliar, S. N., Hymore, F. K. and Pandey, R. A., Utilization of lignocellulosic waste for ethanol production: Enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean Journal of Chemical Engineering, 31(7), 1180-1186 (2014).). The Shea tree, which furnishes a woody (hardwood) lignocellulosic material, is typically a savannah woodland tree species. This tree is small to medium-sized, 10-15 m tall with a diameter ranging from 0.3 to 1 m. It is native to African countries. It occurs on an estimated 1 million km2 between western Senegal and north western Uganda. The exotic species is found in the Dominican Republic and Honduras (Sallé, 1991Sallé, J., Boussim, A., Raynal-Roques, A. and Brunck, F., Potential wealth of the Shea nut tree. Research perspective for improving yield. Bois et Foréts des Tropiques228, 11-23 (1991).). The trunk of the Shea tree makes excellent charcoal and is favoured as a source of high quality wood fuel once the tree has passed the fruit bearing age (Kristensen and Lykke, 2003Kristensen, M. and Lykke, A. M., Informant-based evaluation of use and conservation preferences of savanna trees in Burkina Faso. Economic Botany, 57(2), 203-217 (2003).). The carbohydrates (cellulose and hemicellulose) are suitable precursors for enzymatic hydrolysis conversion to fermentable sugars. The high lignin content also makes the Shea tree wood a suitable material to produce other fuels and chemicals. Lignin can also be burnt for its high heating value (Ladisch, et al. 1979Ladisch, M. R, Flickinger, M. C. and Tsao, G. T., Fuels and Chemicals from biomass. Energy, 4(2), 263-275 (1979).).
Previously (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).), we achieved up to 60% cellulose conversion, 80% hemicellulose solubilization, and 17% lignin removal with an alkaline peroxide assisted wet air oxidation (APAWAO) pre treatment of Shea tree sawdust (under optimized conditions: reaction temperature of 150 °C, 45 min reaction time, 1% H2O2 (v/v), and 1.0 MPa air pres sure). This was compared to alkaline peroxide oxida tion (APO) pretreatment (reaction temperature of 150 °C, 45 min reaction time, 1% H2O2 (v/v)) with 53.86% cellulose conversion, 70% hemicellulose solubilization and 11% lignin removal. We also re ported that the APAWAO conditions enhanced the enzymatic convertibility of treated samples to reduc ing sugars from an initial 177.89 mg equivalent glucose/g dry biomass (APO conditions) to 263.49 mg equivalent glucose/g dry biomass. In this study, the kinetics of delignification were evaluated during the pretreatment APO process at three operating tem peratures. The enzymatic convertibility (using cellulase and β-glucosidase enzymes and a 4-day hydroly sis period) under the optimized pretreatment condi tions (150 °C, 45 min, 1% H2O2 (v/v) and 1.0 MPa air pressure) was investigated at varying dry biomass loadings of 2, 3, 4, and 5% with corresponding in creases in enzyme loadings. Enzymatic conversions without β-glucosidase supplements were also con sidered for hydrolysis at 45 °C for 4 days. Further more, the ferment ability of the treated solids using simultaneous saccharification and fermentation meth ods was evaluated.
MATERIALS AND METHODS
Raw Material
Raw material preparation from the field to the la boratory before compositional analysis and the pre treatment steps have been described extensively else where (Ayeni et al., 2013aAyeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).). The tree was harvested (in early April, 2010) from the forest around Idanre town (6o51'N 5o06'E) and the sawdust was collected from the central processing unit of the local sawmill (Ilepa, Ifo town, south west, Nigeria) in late June, 2010. Samples used in all the experiments were sieved to pass through mesh 14 (British Standard Sieve specifications) and be retained by mesh 80. The average particle sizes therefore varied between 0.09 to 0.51 mm, which made up 73% of the har vested raw material. The screening of dried raw ma terial was carried out to minimize the effects of very fine particles (which appeared in the form of dust) on pretreatment. The dried (using a convection oven at 105 °C for 3 h to a dry matter content of 88%) and sieved materials were stored in plastic bottles capped tightly and kept at 26 °C. The materials were used shortly after. The chemicals used in this study were acetone, calcium hydroxide, D-glucose, and ethanol, and were purchased from ThermoFisher Scientific, India, hydrogen peroxide from E-Merch, India, and β-glucosidase from Himedia Laboratories, India. Trichoderma reesei cellulases (in the form of a brown liquid) was kindly provided by M/s Zytex, Mumbai, India. Deionized water was used in all experiments.
Experimental Set Up
Response surface methodology (RSM) was adopted for the optimization of the process variables in the alkaline peroxide oxidation (APO) pretreatment of Vitellaria paradoxa sawdust based on central compo site design (CCD) experiments. A 23 five level CCD with central and axial points was used to develop the statistical model for the optimization of process vari ables (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).). The CCD design was made up of 20 base runs (8 cube points, 4 center points in the cube, 6 axial points, and 2 center points on the axial). Figure 1 shows the schematic diagram of the pretreatment experimental set up.
The pretreatment process experimental set up consisted of a 1.8 L volume batch Parr reactor (Model-4578, Floor stand HP/HT, Parr Instruments, IL., USA) equipped with double six-blade turbine impellers with external heating embedded in a jacket. An independent controller maintained the temperature in the reactor regulated to within ±2 °C of the set point values with constant stirring at 21 rad/s. Oxidative pretreatments were achieved by the use of Ca(OH)2-H2O2 solution. Lime loadings varied be tween 9.0 g to 30.0 g with corresponding volumes of H2O2 based on the central composite design. The combination of Ca(OH)2-H2O2-air pressure was achieved at 1.0 MPa. 30 g of dry substrate were mixed with 500 mL of distilled water containing H2O2. The slurry was adjusted to pH 11.5 with Ca(OH)2 accordingly. Each reaction was terminated by running cold water through the internal loops. After the specified reaction time, the reactor and slurry were allowed to cool to ambient temperature. The pretreated slurry was separated into the solid and liquid fractions by vacuum filtration. The solid fraction was washed with water until the pH approached 7. A portion of the wet pretreated biomass was weighed and dried to a constant weight at 105 °C in a convection oven in order to determine the total solid left after pretreatment and the amount of wet treated biomass in terms of dry biomass required for the enzymatic hydrolysis and fermentation steps (Dowe and McMillian, 2008Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).). The remaining portion of the wet pretreated biomass was used in the enzy matic digestibility and ferment ability of biomass, as well as the delignification of the raw biomass.
Delignification Parameters
Kinetic data for the pretreatment were evaluated at different temperatures (120 °C, 135 °C, and 150 °C) and a kinetic model for the wood waste delignification with lime as a function of temperature was de termined. For each of these isothermals, the com position of pretreated sawdust was determined at reaction times 20, 25, 30, 35, 40, and 45 min. Pre treatment reaction kinetics is an important tool for the economics of a process since reactor volume, and hence the capital equipment requirement, is said to be proportional to the residence time in the reactor (Li et al., 2013Li, Z., Chen, C. H., Hegg, E. L. and Hodge, D. B., Rapid and effective oxidative pretreatment of woody biomass at mild reaction conditions and low oxidant loadings. Biotechnology for Biofuels, 6, 19 (2013).). The kinetics of delignification were studied by following the amount of lignin removed from the pretreated solids (Table 1) and analyzed on the basis of the proportion of the total lignin re moved with time (Esteves et al., 2005Esteves, B., Gominho, J., Rodrigues, J. C., Miranda, I. and Pereira, H., Pulping yield and delignification kinetics of heartwood and sapwood of maritime pine. Journal of Wood Chemistry and Technology, 25, 217-230 (2005).).
The reaction was considered to be irreversible and to follow a second order as:
The reaction rate, rL could be expressed as:
If oxygen is in excess, the concentration of oxygen can be considered to be constant, giving the equation:
The reaction is now pseudo first-order and the re lationship can be expressed as:
where dL/dt = lignin removal rate; k = chemical reaction rate constant; L = residual lignin after pre treatment.
It has also been established that alkaline pulping is a first order, pseudo homogenous reaction and that the rate of change of lignin removed per unit mass of solid is related to the product of the amount of lignin remaining in the solid mass and the concentration of the alkali (Correira et al., 2001Correira, F., Roy, D. N. and Goel, K., Chemistry and delignification kinetics of Canadian industrial hemp. Journal of Wood Chemistry and Technology, 21, 97-111 (2001).). Integrating Equation (4) shows it is a straight line relationship:
where L = total lignin content at time t, L0 = total lignin content at time t = 0
The rate constant, k, is obtained from the linear regression coefficient. The temperature dependence of the rate constant is characterized by the value of the energy of activation (Nelson, et al., 1987Nelson, P. J. and Gniel, G. M., Delignification of Eucalyptus woods during alkali pulping. 4th International Symposium on Wood and Pulping Chemistry, 125-129 (1987).). The energy of activation is the minimum energy that must be possessed by reacting molecules before the reaction can occur. k is related to temperature by the Arrhenius Law:
where A = Arrhenius constant; Ea = activation energy; R = gas constant = 8.314 kJ/mole∙K; T = absolute tem perature (K).
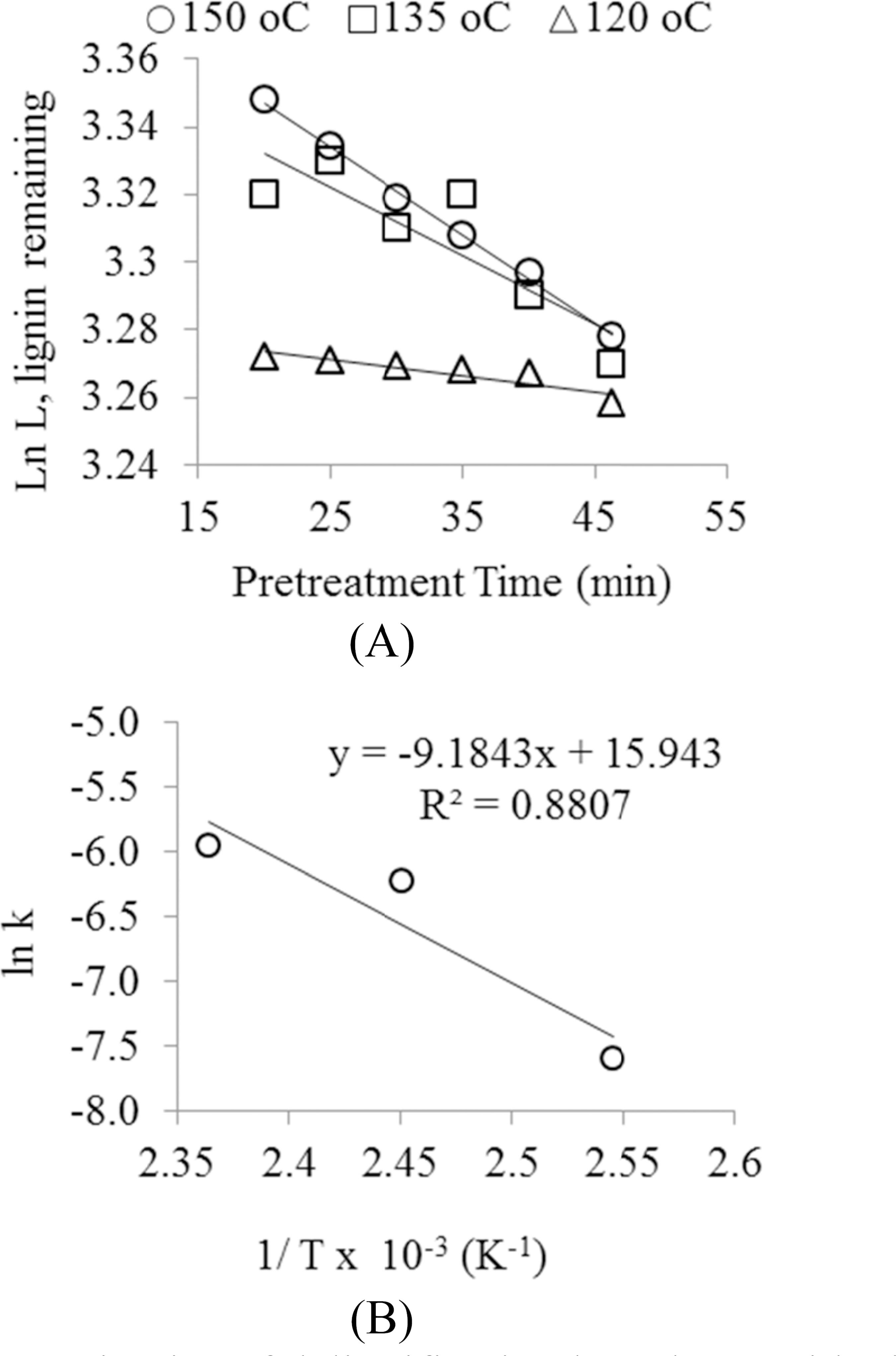
The activation energy (Ea) of delignification was calculated for each kinetically homogeneous lignin fraction from the logarithmic form of the Arrhenius equation (ln k = ln A - Ea/RT) by plotting ln k against 1/T with the slope equal to Ea/R (Figure 2(B)) (Esteves et al., 2005Esteves, B., Gominho, J., Rodrigues, J. C., Miranda, I. and Pereira, H., Pulping yield and delignification kinetics of heartwood and sapwood of maritime pine. Journal of Wood Chemistry and Technology, 25, 217-230 (2005).), ln A is the intercept from where the Arrhenius constant is calculated. The kinetics of delignification was established by assuming the ma terial contains one type of lignin.
Material Balances and Biomass Composition
Untreated and treated samples were repeatedly washed with fresh distilled water until the decanted water became colourless. The total dry weight of the sample was measured before and after pretreatment and washing. Dry weight measurement for material balances was described previously based on drying the biomass at 105 °C for 6 h (Ayeni et al., 2013aAyeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).; Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).). Extractives and hemicellulose contents were measured as described previously (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).). Extractives determination was carried out by the means of a Soxhlet extractor. 300 mL of acetone was used as solvent on 5 g of dry raw and treated biomass. The temperature and time during extraction for the boiling and rising stages equalled 70 °C and 25 min, respectively, for a 4h run period. After extraction, the sample was air dried for a few minutes at room temperature and further dried at 105 °C in a convection oven. The extractives con tent was calculated as the difference in weight be tween the raw and extracted material (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).; Blasi et al., 1999Blasi, C. D., Signorelli, G., Di Russo, C. and Rea, G., Product distribution from pyrolysis of wood and agricultural residues. Industrial Engineering and Chemistry Research, 38, 2216-2224 (1999).; Li et al., 2004Li, S., Xu, S., Liu, S., Yang, C. and Lu, Q., Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technology, 85, 1201-1211 (2004)., Lin et al., 2010Lin, L., Yan, R., Liu, Y. and Jiang, W., In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose, and lignin. Bioresource Tech nology, 101, 8217-8223 (2010).). Mineral components were determined by ashing at 575 °C for 6 h. The hemicellulose content was determined by transferring 1 g of dried biomass from the extractive analysis into a 250 mL Erlen meyer flask. 150 mL of 0.5 mol/L NaOH solution was added to the dried biomass. The mixture was boiled for 3 hours and 30 minutes with distilled water. The residue was dried to a constant weight at 105 °C and later cooled in a desiccator and weighed. The hemicellulose content was calculated as the differ ence between the sample weight before and after this treatment (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).; Blasi et al., 1999Blasi, C. D., Signorelli, G., Di Russo, C. and Rea, G., Product distribution from pyrolysis of wood and agricultural residues. Industrial Engineering and Chemistry Research, 38, 2216-2224 (1999).; Li et al., 2004Li, S., Xu, S., Liu, S., Yang, C. and Lu, Q., Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technology, 85, 1201-1211 (2004)., Lin et al., 2010Lin, L., Yan, R., Liu, Y. and Jiang, W., In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose, and lignin. Bioresource Tech nology, 101, 8217-8223 (2010).). Lignin content was de termined as the summation of Klason lignin and acid soluble lignin on the extractive free samples by the National Renewable Energy Laboratory standard pro cedures (Sluiter et al., 2008Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. and Croker, D., Determination of structural carbohydrates and lignin in biomass: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; April. NREL Report No.: TP-510-42618. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).). Cellulose content was determined by difference, assuming only ash, extrac tives, hemicelluloses, lignin were present in the bio mass (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).; Blasi et al., 1999Blasi, C. D., Signorelli, G., Di Russo, C. and Rea, G., Product distribution from pyrolysis of wood and agricultural residues. Industrial Engineering and Chemistry Research, 38, 2216-2224 (1999).; Li et al., 2004Li, S., Xu, S., Liu, S., Yang, C. and Lu, Q., Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technology, 85, 1201-1211 (2004)., Lin et al., 2010Li, Z., Chen, C. H., Hegg, E. L. and Hodge, D. B., Rapid and effective oxidative pretreatment of woody biomass at mild reaction conditions and low oxidant loadings. Biotechnology for Biofuels, 6, 19 (2013).). The raw biomass compo sitional analysis, percent weight by weight, estimated cellulose content of 45.90%, hemicellu lose: 20.31%, lignin: 29.90%, extractives: 1.89%, and ash: 2.04%.
Enzymatic Conversion of Treated and Untreated Biomass
The action of cellulase and β-glucosidase en zymes on the treated and untreated biomass was evaluated. In 30 mL culture tubes (arranged in parallel for sampling different time points) were added 20, 30, 40, and 50 g/L wet treated and washed-only untreated biomass samples. A commercial prepara tion of Trichoderma reesei cellulases (activity of 57.8 FPU/mL) and β-glucosidase (Extra pure, CAS No.: 9001-22-3, with an activity of 10 IU/mg solid) were added at loadings which corresponded to the increased substrate concentrations (Table 2).
4-D effect of substrate concentration with corresponding increase in enzyme concentration and incubation period on the enzymatic saccharification of pretreated sawdust; conditions of 150°C, 1% H2O2, 1.0 MPa, and 45 min.
The activity of the cellulase enzymes was deter mined in terms of filter paper units. A linear glucose standard curve using the absolute amounts of glucose standards (mg/0.5 mL) was plotted against absorb ance at 540 nm. This graph was used to determine the concentration of reducing sugars in the sample tubes, which had to be incubated with cellulase en zyme solutions of varying dilutions at 50 °C for 60 min. The value of 2.0 mg of reducing sugar as glu cose from 50 mg of filter paper (4% conversion) in 60 min was used for calculating filter paper cellulase units (FPU). (Ghose, 1987Ghose, T. K., Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257-268 (1987).). β-glucosidase activity was determined by the method described by Bailey and Linko (1990)Bailey, M. J. and Linko, M., Production of β-glucosidase by Aspergillus oryzae in submerged bioreactor cultivation. Journal of Biotechnology, 16, 57-66 (1990)..
An appropriate volume of distilled water was added until the 10 mL final volume including 0.05 M sodium acetate buffer, pH 4.8. The reaction mixture was placed in an orbital shaking incubator (Scigenics Biotech, Chennai, India) maintained at a temperature of 45 °C and at 14 rad/s. At specified periods, culture tubes were removed and 1 mL aliquot samples were taken for sugar analysis (Dowe and McMillan, 2008Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).). Hydrolyzed samples were centrifuged at 2254 gravities for 5 min to remove residual solids and total reducing sugars was quantified with DNS (3,5-dinitrosalicylic acid) assay (Ayeni et al., 2013aAyeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).; Miller, 1959Miller, G. L., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426-428 (1959).).
Calibration curves (using glucose as a standard) were used to determine the reducing sugar concen trations (Chang et al., 1998Chang, V. S., Nagwani, M. and Holtzapple, M. T., Lime pretreatment of crop residues bagasse and wheat straw. Applied Biochemistry Biotechnology, 74, 135-159 (1998).). The sample was diluted properly so that the measured absorbance was in the linear range. Reducing sugar yields were expressed as mg equivalent glucose/g dry substrate (treated substrate as cellulose and hemicellulose). Further more, hydrolysis results (% digestibility; as substrate conversion), were expressed in terms of the theoretical or total polysaccharide in the treated substrate (Yoo et al., 2011Yoo, C. G., Lee, C. W. and Kim, T. H., Optimization of two stage fractionation process for lignocellulosic biomass using response surface methodology (RSM). Biomass and Bioenergy, 35, 4901-4909 (2011).).
Wet treated biomass was used for the enzymatic hydrolysis and fermentation steps. Sugar yields were also expressed as dry biomass based on equivalent material balance of wet treated sample (Dowe and McMillan, 2008Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).).
Ethanol Fermentation Process
The sugar from the pretreatment process solid fraction was fermented to ethanol through simultane ous saccharification and fermentation (SSF) by Sac charomyces cerevisiae kindly provided by Purti Power and Sugar Ltd., Distillery Division, Nagpur, India. The S. cerevisiae inoculum culture medium was pre pared aseptically in a 250 mL shaking flask covered with a cotton stopper with 100 mL of medium con taining 3 g/L malt extract, 3 g/L yeast extract, 5 g/L peptone, and 10 g/L glucose, and incubated on a rotary shaker at 130 revolution per minute and 32 ± 2.0 °C for 24 h. All media were sterilized by auto claving at 121 °C for 30 min. The cells were harvested for SSF fermentation to a final optical density (OD) of 0.6 measured at 600 nm (Dowe and McMillan, 2008Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).). SSF was performed under anaerobic condition, made possible by making the rubber stoppered flasks air-tight with Teflon tape and thereafter covered with aluminum foil, in sterile 250 mL shaking flasks with the incubator shaker at 30 ± 2.0 °C for 96 h. 5 mL of inoculum culture were added into the 50 mL working volume, sterilized for the fermentation of the pre treated solids. The pH of the medium was adjusted to 4.8 ± 0.2 with 0.05 M citrate buffer. With a total sam ple size of 50 g (total working volume for fermenta tion was 50 mL), the desired cellulose effective load ings of 2% (w/w) and 3% (w/w) were considered, which were equivalent to 1.0 g and 1.5 g of cellulose, respectively, in treated substrate. The cellulase load ing (filter paper activity = 57.8 FPU/mL) was 25 FPU/g cellulose and the concentration of yeast (Sac charomyces cerevisiae) inoculum was 10% (v/v). At the end of fermentation period, 5 mL of mixture were removed and centrifugation was performed at 4,500 revolution per minute for 5 min (Dowe and McMillan, 2008Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).). The supernatant after centrifuga tion was used for the ethanol estimation.
The ferment ability of the pretreated solids was characterized by the equation (Dowe and McMillan, 2008Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).):
where [EtOH]f = ethanol concentration at the end of the fermentation (g/L) minus any ethanol produced from the enzyme and medium,
[EtOH]0 = ethanol concentration at the beginning of the fermentation (g/L), which should be zero, [Biomass] = dry biomass concentration at the begin ning of the SSF (g/L), f = Cellulose fraction of the dry biomass (g/g), 0.51 = Conversion factor for glucose to ethanol based on the stoichiometric biochemistry of the etha nol fermentation, 1.111 = factor that converts cellulose to equiva lent glucose.
The ethanol yield was calculated as a percentage of the theoretical yield on the basis of the total effec tive cellulose in the pretreated material, in other words, the % theoretical ethanol yield can also be given as % cellulose conversion. The control fermen tation (without biomass samples) was performed and the result was subtracted from the test fermentations for each biomass loading.
Ethanol analysis was carried out from the absorb ance of the sample using the dichromate assay method (Ayeni et al., 2014Ayeni, A. O., Omoleye, J. A., Mudliar, S. N., Hymore, F. K. and Pandey, R. A., Utilization of lignocellulosic waste for ethanol production: Enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean Journal of Chemical Engineering, 31(7), 1180-1186 (2014).; Bennet, 1971Bennet, C., Spectrophotometric acid dichromate method for the determination of ethyl alcohol. American Journal of Medical Technology, 37, 217-220 (1971).); acid di chromate solution (0.1 M Cr2O72- in 5 M H2SO4) was prepared by dissolving 7.5 g of potassium dichro mate in 70 mL of dilute sulfuric acid and the final volume was adjusted to 250 mL with deionized wa ter. The standard curve was made by adding 300 μL of ethanol solution to beaker containing 3 mL of acid dichromate. The beakers were covered and sealed with parafilm and kept at room temperature for 30 min. The absorbance was recorded at 590 nm on spectrophotometer (UV-1800 Shimadzu, Japan).
RESULTS AND DISCUSSION
Determination of the Kinetic Parameters for Delig nification
During the pretreatment process, lignin removal between 8.1 and 12.3% was observed under the ex perimental conditions. The delignification did not exceed 17% under enhanced pretreatment conditions (Ayeni, et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).). The proportions of lignin re moved calculated for the three different process tem peratures suggested high compositional stability within the tested values. The hindrance posed to the ease of delignification during pretreatment may be connected with the high lignin content (29.9%) in the raw biomass (Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).). High delignifica tion may also have been hindered because of the type of catalyst used (Ca(OH)2). The first-order kinetic be haviour of delignification process is shown in Figure 2(A) and the values of rate constants obtained from the slope of the straight lines are presented in the same figure. The results indicated that the rate constants increased with increasing temperature. At increasing temperatures there were more collisions of the reactants, which further increased the rate of reaction.
Lignin remaining at the three operating tem peratures (calculated on dry weight of treated bio mass) were estimated based on the linear regres sions; 150 °C, y = -0.0026X + 3.39991, R2 = 0.9971; 135 °C, y = -0.002X + 3.3724, R2 = 0.7525; 120 °C, y = -0.0005X + 3.2828, R2 = 0.8177. The activation energy of the delignification of the pretreated saw dust waste was derived using Equation (5) from the slope of ln k against 1/T. The activation energy value was calculated to be 76.4 kJ/mole and the Arrhenius constant was 8.4 x 106/min (Figure 2(B)). Activation energies for some lignocelluloses have been reported in the scientific literature (Kim and Holtzapple, 2006Kim, T. H. and Lee, Y. Y., Fractionation of corn stover by hot-water and aqueous ammonia treatment. Bioresource Technology, 97, 224-232 (2006).). In alkaline conditions, corn stover and sugar cane bagasse may have more favourable structures than wood and, as a result, lower activation energies for delignification (Kim and Hotlzapple, 2006Kim, S. and Holtzapple, M. T., Delignification kinetics of corn stover in lime pretreatment. Bioresource Technology, 97, 778-785 (2006).). Corn stover and sugarcane bagasse under alkaline condi tions were reported to have activation energies of 54.21 kJ/mol and 31.47 kJ/mol, respectively (Kim and Holtzapple, 2006Kim, S. and Holtzapple, M. T., Delignification kinetics of corn stover in lime pretreatment. Bioresource Technology, 97, 778-785 (2006).; Fuentes et al., 2011Fuentes, L. G., Rabelo, S. C., Filho, R. M. and Costa, A. C., Kinetics of lime pretreatment of sugarcane bagasse to enhance enzymatic hydrolysis. Applied Biochemistry and Biotechnology, 163, 612-625 (2011).). With oxidative pretreatment, the activation energies for kraft delignification of wood were reported to vary between 110-130 kJ/mol (Kim and Holtzapple, 2006Kim, S. and Holtzapple, M. T., Delignification kinetics of corn stover in lime pretreatment. Bioresource Technology, 97, 778-785 (2006)., Dolk et al. 1989Dolk, M., Yan, J. F. and McCarthy, J. L., Lignin 25. Kinetics of delignification of western hemlock in flow through reactors under alkaline conditions. Holzforschung, 43(2), 91-98 (1989).). Therefore, for delignifica tion in alkaline conditions, most non-woody ligno celluloses have lower delignification activation ener gies than woody biomass. In this study, the activation energy, 74.6 kJ/mol, for the Shea tree sawdust is higher than values for some non-woody lignocellu loses. As a result, in the light of the prevailing pre treatment conditions, the activation energy of Shea tree sawdust has been established. However, com pared to some other woody biomass, the value of 74.6 kJ /mol is lower. The value of the rate constant, k, is said to be affected by the chemical concentra tion during pretreatments and the structural features of the biomass (Miguel et al., 1999Miguel, A. G., Rodriguez, F., Santos, A., Oliet, M., Garcia-Ochoa, F. and Tijero, J., Kinetics of Eucalyptus globulus delignification in methanol-water medium. Industrial Engineering and Chemistry Research, 38, 3324-3322 (1999).). At a tempera ture between 110-200 °C, the activation energies during delignification of Eucalyptus globulus varied between 31.8 kJ/mol and 98.4 kJ/mol, which were below the values reported by Kim and Holtzapple (2006)Kim, S. and Holtzapple, M. T., Delignification kinetics of corn stover in lime pretreatment. Bioresource Technology, 97, 778-785 (2006). and Dolk et al., (1989)Dolk, M., Yan, J. F. and McCarthy, J. L., Lignin 25. Kinetics of delignification of western hemlock in flow through reactors under alkaline conditions. Holzforschung, 43(2), 91-98 (1989).. Several other authors also reported that wood particle size and diffusion reactions have effects on alkaline penetration during pretreatments (Dang and Nguyen, 2008Dang V. O. and Nguyen K. L., A universal kinetic model for characterization of the effect of chip thickness on kraft pulping. Bioresource Technology, 99, 1486-1490 (2008).). The discrepancy in delignification activation energy of the Shea tree sawdust relative to other woody biomass under the prevailing pretreatment conditions, could be due to the variations in pretreatment conditions. Furthermore, the very stable conditions of lignin during pretreatments may have accounted for the differences.
Kinetics of delignification based on residual lignin content. (A) logarithmic plots for lignin re maining at the operating temperatures after pretreat ment versus time; (B) Arrhenius plot of the treated biomass.
Effect of Enzymatic Hydrolysis on Treated and Untreated Biomass
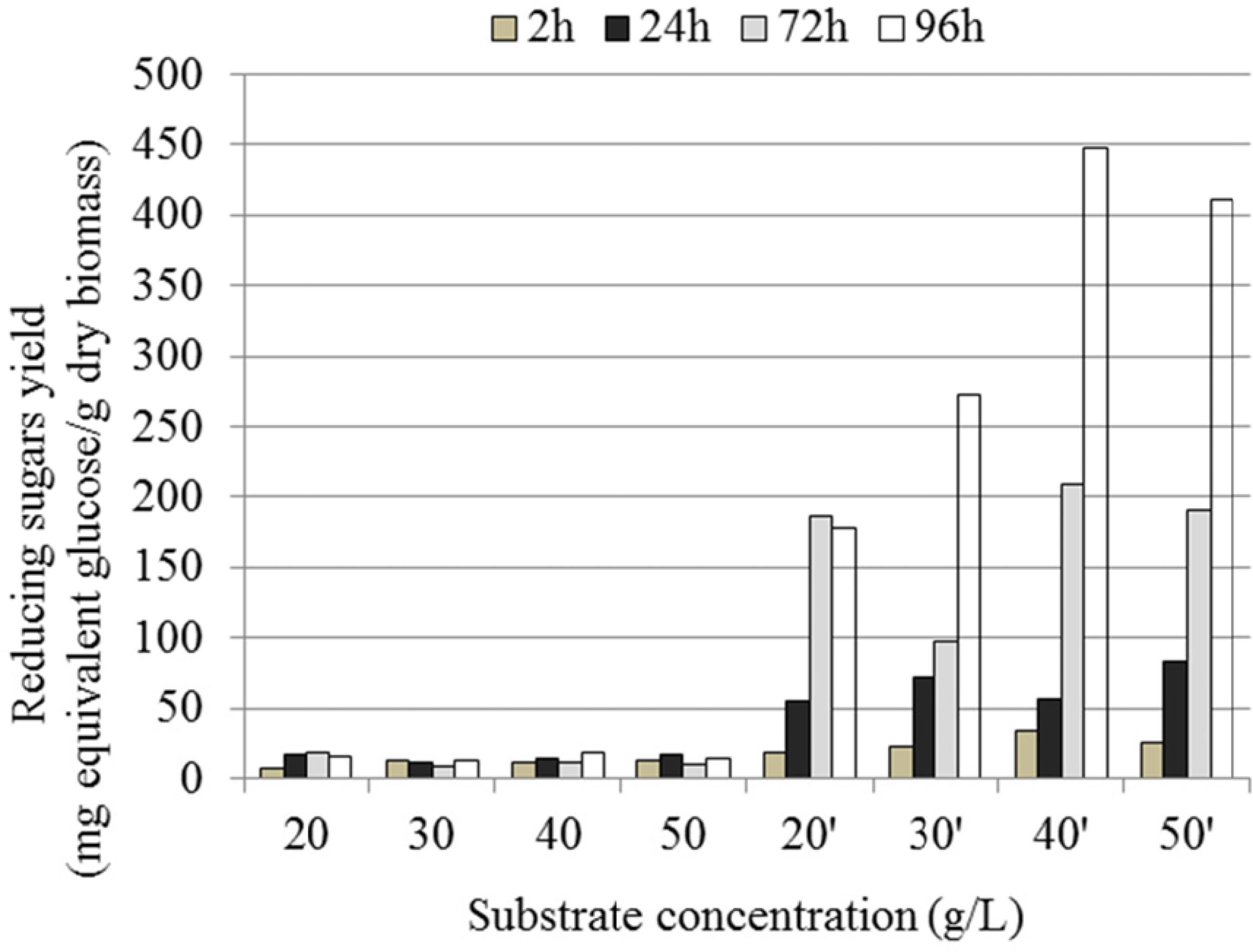
Our earlier studies established that a combination of air and hydrogen peroxide as oxidizing agents increased the delignification of Vitellaria paradoxa sawdust leading to increased enzymatic hydrolysis of treated biomass (Ayeni et al., 2013aAyeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).; Ayeni et al., 2013bAyeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).; Ayeni et al., 2014Ayeni, A. O., Omoleye, J. A., Mudliar, S. N., Hymore, F. K. and Pandey, R. A., Utilization of lignocellulosic waste for ethanol production: Enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean Journal of Chemical Engineering, 31(7), 1180-1186 (2014).). It was pointed out that, under the optimized pretreatment conditions (150 °C, 45 min, 1%H2O2, and 1.0 MPa air pressure), at 2% pretreated biomass loading, enzymatic hydrolysis temperature of 50 °C, and hydrolysis period of 72 h, the total reducing sugars obtained were 263.49 mg equivalent glucose/g dry biomass. Defining a single optimum for enzymatic hydrolysis is impossible. End products of the hydrolysis (mainly cellobiose and glucose, which may also include the furan com pounds as furan and hydroxylmethyl furfural (HMF)) (Andrić et al., 2010Andrić, P., Meyer, A. S., Jensen, P. A. and Dam-Johansen, K., Reactor design for minimizing product inhibition during enzymatic lignocellulosic hydrolysis II. Quantification of inhibition and suitability of membrane reactors. Biotechnology Advances, 28, 407-425 (2010).) may also inhibit the enzyme activity, the build-up of any of these products nega tively affects cellulose hydrolysis. The concentration of the cellulase enzyme complex has a high impact on the conversion of the cellulose. The maximum cellulase activity for most fungal-derived cellulases and β-glucosidase was reported to occur at 50 ± 5 °C and a pH between 4.0 and 5.0 (Gregg et al., 1998Gregg, D. J., Boussaid, A. and Saddler, J. N., Techno-economic evaluation of a generic wood to-ethanol process: Effect of increased cellulose yields and enzyme recycle. Bioresource Technology, 63, 7-12 (1998).). At lower temperatures, the hydrolysis rate per unit of active enzyme is slower, but so is enzyme denaturation (Kaar and Holtzapple, 2000Kaar, W. E. and Holtzapple, M. T., Using lime pretreatment to facilitate the enzyme hydrolysis of corn stover. Biomass and Bioenergy, 18, 189-199 (2000).). The optimum tem perature and pH is not only a function of the raw material and the enzyme source, but is also highly dependent on the hydrolysis time. The optimal con ditions change with the hydrolysis residence time (Tengborg et al., 2001Tengborg, C., Galbe, M. and Zacchi, G., Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam pretreated softwood. Biotechnology Progress, 17, 110-117 (2001).), and are also dependent on the source of the enzymes. In order to improve on our previous studies (Ayeni et al., 2013aAyeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).; Ayeni et al., 2014Ayeni, A. O., Omoleye, J. A., Mudliar, S. N., Hymore, F. K. and Pandey, R. A., Utilization of lignocellulosic waste for ethanol production: Enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean Journal of Chemical Engineering, 31(7), 1180-1186 (2014).), enzymatic digestibility was evaluated considering increasing substrate concentrations, hy drolysis time, and hydrolysis temperature, as well as increasing enzyme loadings. The effects of substrate concentration variation (20, 30, 40, and 50 g/L) with and without supplemental β-glucosidase, hydrolysis temperature (45 °C), enzyme loadings, as well as hydrolysis time (96 h) on digestibility were investi gated as pretreatment conditions. The results for the pretreated sawdust material after saccharification for the pretreatment conditions 150oC, 1% H2O2, 1.0 MPa air pressure are given in Table 2. The effects of substrate concentration variation (20, 30, 40, and 50 g/L) with and without supplemental β-glucosidase, hydrolysis temperature (45 °C), and enzyme load ings, as well as hydrolysis time (96 h), on digestibil ity were investigated in the pretreatment conditions. With a cellulase enzyme loading of 25 FPU/g treated substrate, the reducing sugar yield was 187.03 mg/g dry biomass in 72 h. A higher reducing sugar yield (274 mg/g dry biomass) was obtained with the same enzymatic hydrolysis but different pretreatment con ditions; 170 °C, 1.0 MPa, and 10 min (Ayeni et al., 2013aAyeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).). The effect of temperature on biomass pre treatment and enzymatic hydrolysis may have ac counted for this variation. In this study, the results showed that reducing sugar yields increased with 20, 30, 40 g/L substrate concentrations considered up to the fourth day of hydrolysis. It showed that more sugar is likely to be produced if the hydrolysis time was increased beyond 96 h. The optimum tempera ture and pH are not only a function of the raw mate rial and enzyme source, but are also highly depend ent on the hydrolysis time (Martín, et al., 1988Martín, C., Negro, M. J., Alfonsel, M. and Sáez, R., Enzymatic hydrolysis of lignocellulosic biomass from. Biotechnology and Bioengineering, 32, 341-344 (1988).). This also showed that the enzymes were still active to cause more hydrolysis at the reduced hydrolysis temperature of 45 °C. However, for 50 g/L substrate concentration and 62.5 FPU/g dry biomass loading, the sugar yield was 411.07 mg glucose equivalent/g dry biomass, which was lower than the sugar yield at 40 g/L and 50 FPU/g dry biomass loading (448.06 mg/g dry biomass). Increasing the substrate concen tration to 50 g/L caused decreased hydrolysis, de spite increased enzyme loadings. This is probably due to end product inhibition, other inhibitors or inefficient mixing. Furthermore, reducing sugar yields without supplemental β-glucosidase were comparable to when the enzyme was added. For example, at 40 g/L substrate concentration and a 50 FPU/g treated substrate enzyme loading, the reduc ing sugars yield after 96 h with β-glucosidase was 448.06 mg/g dry biomass, while under the same con dition but without β-glucosidase supplement the reducing sugars yield was 399.28 mg/g dry biomass. This is just about 11% lower. Addition of cellobiase (β-glucosidase) has been reported to greatly increase pretreated biomass conversion. However, increasing cellobiase loading beyond a limit may not enhance biomass digestibility. Zhu (2005)Zhu, L., Fundamental Study of Structural Features Affecting Enzymatic Hydrolysis of Lignocellulosic Biomass. Ph.D. Dissertation, Texas A&M University, College Station (2005). reported that be yond 28.4 CBU/g dry biomass cellobiase loading, the digestibility of pretreated corn stover did not improve. Crude enzymes with high cellobiase activ ity are less affected by supplemental cellobiase and commercial enzyme sources may also differ in resi dent cellobiase (Zhu, 2005Zhu, L., Fundamental Study of Structural Features Affecting Enzymatic Hydrolysis of Lignocellulosic Biomass. Ph.D. Dissertation, Texas A&M University, College Station (2005).). The discrepancy could also be attributed to the amount of β-glucosidase added and the extent of hydrolysis, which depends highly on structural features and cellulase loading (Zhu, 2005Zhu, L., Fundamental Study of Structural Features Affecting Enzymatic Hydrolysis of Lignocellulosic Biomass. Ph.D. Dissertation, Texas A&M University, College Station (2005).; Pu et al., 2013Pu, Y., Hu, F., Huang, F., Davidson, B. H. and Ragauskas, A. J., Accessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnology for biofuels, 6, 15, (2013).). Therefore, for the econ omy of the studied process, the absence of β-gluco sidase generally gave better results of digestibility than adding β-glucosidase supplements. However, finding an optimum level of addition offers a chal lenge for future study. The yields of reducing sugar with no β-glucosidase addition may be due to the reduction in the inhibitive effects of glucose on the enzyme.
Figure 3 shows the enzymatic hydrolysis results for untreated biomass samples and treated samples with no supplemental β-glucosidase. The untreated and washed raw biomass was used as the control for comparing the enzymatic digestibility of the treated sawdust. Concentrations in prime notation indicate enzymatic hydrolysis of treated samples.
4-d Effect of time and substrate concentra tion on the sugar yield for untreated and treated bio mass. Concentrations in prime notation indicate en zymatic hydrolysis of pretreated samples. Pretreat ment conditions: 150 °C, 1% H2O2, 1.0 MPa, 45 min. Enzyme hydrolysis conditions: same conditions as indicated in Table 2.
The substrate concentration variation (20, 30, 40, and 50 g/L) with corresponding increase in enzyme loadings and supplemental β-glucosidase on digest ibility were investigated. Chang et al., (2001)Chang, V. S., Nagwani, M., Kim, C. and Holtzapple, M. T., Oxidative lime pretreatment of highlignin biomass. Applied Biochemistry and Biotechnology, 94, 1-28 (2001). inves tigated the effects of pretreatment time and tempera ture of poplar wood (lignin content of about 25%) on enzymatic hydrolysis and concluded that oxidative lime pretreatment enhanced the reducing sugar yields from 62 mg equivalent glucose/g dry biomass (un treated) to as much as 622 mg equivalent glucose/g dry biomass (treated at 150 °C and 6 h). In this study, using the substrate concentration variations with corresponding increases in enzymes loadings, the treated biomass reducing sugars concentration was 399.28 mg/g dry biomass as compared to untreated material of 19.08 mg/g dry biomass (Figure 3). The maximum reducing sugar yield in this study under the conditions specified was 42.4% lower than that reported for poplar wood (Chang et al., 2001Chang, V. S., Nagwani, M., Kim, C. and Holtzapple, M. T., Oxidative lime pretreatment of highlignin biomass. Applied Biochemistry and Biotechnology, 94, 1-28 (2001).). The difference in values may have been caused by raw material composition, pretreatment conditions, efficiency of the different enzymes used, enzyme concentration, and reaction period.
Enzyme Loading Studies
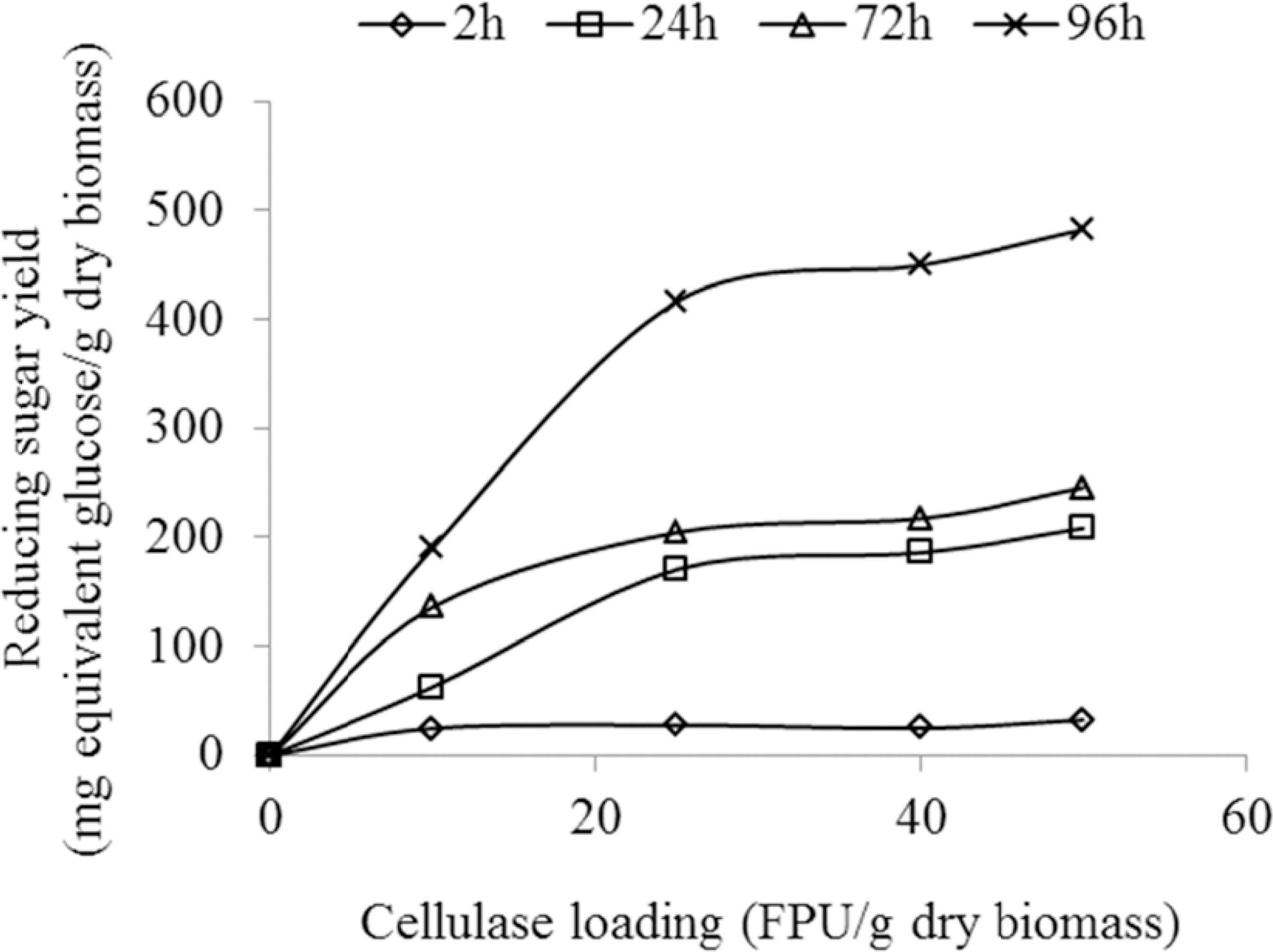
Whether there are significant yield benefits from loadings higher than 25 FPU/g dry biomass or whether cellulase loadings less than 25 FPU/g biomass were sufficient was also investigated (Figure 4).
4-d Effect of enzyme loading on sugar yields. Pretreatment conditions: 150 °C, 1% H2O2, 1.0 MPa, 45 min. Enzyme hydrolysis conditions: 5UI β-gluco sidase/g dry biomass, 45 °C hydrolysis temperature, pH 4.8, 40 g/L substrate concentration.
Sugar yields with lower enzyme loadings (1, 3, 5, 7, 10 and 15 FPU/g dry biomass) exist in the scien tific literature (Kaar and Holtzapple, 2000Kaar, W. E. and Holtzapple, M. T., Using lime pretreatment to facilitate the enzyme hydrolysis of corn stover. Biomass and Bioenergy, 18, 189-199 (2000).; Martín et al., 1988Martín, C., Negro, M. J., Alfonsel, M. and Sáez, R., Enzymatic hydrolysis of lignocellulosic biomass from. Biotechnology and Bioengineering, 32, 341-344 (1988).). After lime pretreatment of corn stover, with 10 FPU/g dry biomass enzyme loading at 40 °C incubation temperature and 100 h, Kaar and Holtzapple (2000)Kaar, W. E. and Holtzapple, M. T., Using lime pretreatment to facilitate the enzyme hydrolysis of corn stover. Biomass and Bioenergy, 18, 189-199 (2000). concluded that the optimum reducing sugars yield was about 610 mg/g dry biomass. Palonen et al., (2004)Palonen, H., Thomsen, A. B., Tenkanen, M., Schmidt, A. S. and Viikari, L., Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Applied Biochemistry and Biotechnology, 117, 1-17 (2004). investigated wet air oxidation pre treated (at 200 °C) softwood enzymatic hydrolysis to reducing sugar using two cellulase mixtures with loadings corresponding to 5, 10, 30 FPU/g dry biomass. They discovered that using the highest load of 30 FPU/g dry biomass, under hydrolysis conditions of 40 °C, 20 g/L substrate concentration for 24 h, maximum sugar yield of 257 mg/g dry biomass (55% conversion of polysaccharide) was achieved. The low sugar yield was partly caused by the low lignin removal during pretreatment (between 24-42% lignin in the softwood was removed). In this study, the enzyme loading results shown in Figure 4 contain some important features. Higher reducing sugar yields were obtained at higher enzyme loadings (for example 25 FPU to 50 FPU/g dry biomass in the 4-day hydrolysis time. The 4-day reducing sugar yield at 50 FPU/g dry biomass for 150 °C, 1%H2O2, 1.0 MPa air pressure, 45 min pretreatment conditions was 482.40 mg/g dry biomass, while at 25 FPU/g dry biomass the yield was 416.32 mg/g dry biomass (about a 22-fold increase compared to untreated biomass). The 25 FPU/g dry biomass loading was found to be adequate with this material (Figure 4), but further improvements in pretreatment efficiency would ena ble decreasing to more economical enzyme dosages. Further inspections show that the sugar yield was about 2.5 times higher after 96 h (416.32 mg/g) from the 24 h (170.51 mg/g) hydrolysis period. It is ex pected that, at longer periods and low temperature (in this case 45 °C), hydrolysis should increase until a constant value is attained in which the enzymes were supposed to have been exhausted or their ac tions stopped by inhibitors. Cellulase loadings greater than 25 FPU/g dry biomass may have caused the cellulose sites to be saturated by the enzymes.
Fermentation of Treated Biomass
After 72 h fermentation, the quantity of ethanol obtained (g/L) at 2% effective cellulose loading was 10.69 g/L (% theoretical ethanol yield: 31.03%) for pretreatment at 150 °C, 1%H2O2, 1.0 MPa air pres sure, and 45 min. At an increased effective cellulose loading of 3%, the ethanol obtained did not signifi cantly increase (12.43 g/L with theoretical ethanol yield of 29.56%). The % theoretical ethanol yields (based on cellulose conversion) for the pretreatment conditions were higher at 2% effective cellulose loading than at 3% effective cellulose loading. The ethanol concentration tended to be higher at 3% sub strate loading, but at this loading the % theoretical ethanol yields were much lower than that at 2% sub strate loading. This implies that more of the cellulose was converted at 2% loading than at 3% substrate loading. The ethanol yield at 3% loading showed that more reducing sugar was produced by enzymatic hydrolysis and was probably more quickly assimi lated by yeast for cell growth and ethanol production than at 2% loading. For the 96 h fermentation period, the corresponding ethanol values for the 2% and 3% biomass loadings did not appreciably increase. At 2% effective cellulose loading, the quantity of etha nol obtained was 12.73 g/L (32.75% theoretical etha nol yield). The 3% cellulose loading it corresponded to 13.84 g/L with 33.44% theoretical ethanol yield. The ethanol yield at 2% cellulose loading was compa rable to the 3% cellulose loading. Yeast may have acted faster on the 2% cellulose loading than the 3% cellulose loading. At high cellulose loading, the increased glucose monomers may also be inhibit ing the actions of the microbes. Cellulose conversion at 2% substrate loading should be more appropriate for the SSF under the conditions considered. How ever, more of the substrate will be needed for the fermentation process. Generally, the low ethanol yield in this study is connected to the high lignin content because cellulose accessibility to cellulases is largely limited by the anatomical structure of the plant cell wall (Pu et al., 2013Pu, Y., Hu, F., Huang, F., Davidson, B. H. and Ragauskas, A. J., Accessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnology for biofuels, 6, 15, (2013).). Lignin interferes with enzymatic hydrolysis by blocking access to cellulase and irreversibly binding hydrolytic enzyme. Lignin inhibits the enzymes during hydrolysis. In future studies, to remedy and reduce the recalcitrant nature of the biomass, addition of extra nutrients and minerals [in the form of yeast extract, bacto tryptone (casein extract)] to the fermentation broth will be taken into consideration. Extra nutrients and minerals can increase the ethanol concentration at very short incu bation periods (Lissens et al., 2004Lissens, G., Klinke, H., Verstraete, W., Ahring, B. and Thomsen, A. B., Wet oxidation pretreatment of woody yard waste: Parameter optimization and enzymatic digestibility for ethanol production. Journal of Chemical Technology and Biotechnology, 79, 889-895 (2004).). Furthermore, a combination of both hemicellulase and cellulase enzymes could cause increased enzymatic hydrolysis in the biomass-to-ethanol process.
CONCLUSIONS
This study proved the efficiency of alkaline perox ide oxidation pretreatment on a woody biomass, Vitellaria paradoxa (Shea tree) sawdust. The pre treatment caused appreciable disruption of the bio mass structure making cellulose more accessible to the enzymatic complex. Significant increases in reducing sugar yield were observed for the treated relative to the untreated biomass. For the kinetics of delignification, the results indicated the activation energy to be 76.4 kJ/mole and the Arrhenius constant was calculated as 8.4 x 106/min. After pretreatment (optimized conditions of 150 °C, 1% H2O2, 1.0 MPa air pressure, 45 min) and enzymatic hydrolysis (digest ibility conditions of 45 °C, 96 h, pH 4.8 at 40 g/L substrate loading), the reducing sugar yield for the treated biomass was 347.20 mg/g dry biomass. Optimization of the enzymatic digestibility with the enzyme loading studies for the 4-day incubation period showed that a 25 FPU/g dry biomass enzyme loading yielded 416.32 mg/g dry biomass for 40 g/L substrate concentration, compared to the 19.08 mg/g dry biomass reducing sugar yield of the untreated sample. Therefore, under the optimized conditions more of the substrate will be needed for the enzy matic hydrolysis process. Conversion of the treated woody material to ethanol at 2% effective cellulose loading after 96 h fermentation by Saccharomyces cerevisiae was 12.73 g/L. In all, the Shea tree was found to be challenging due to the high lignin con tent. However, in an integrated bio-refinery, the un-dissolved lignin can be available for energy produc tion by combustion, and for conversion to other fuels and chemicals.
As a result of the recalcitrant nature of the bio mass, further improvements in pretreatment and the enzymatic digestibility are needed.
-
*
To whom correspondence should be addressed
ACKNOWLEDGEMENTS
The author (A.O.A) is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India and The World Academy of Sciences for the Advancement of Science in Developing Countries (TWAS), Italy, for the award of a CSIR-TWAS fellowship for Research and Advanced Training tenable at National Environmental Engineering Research Institute (NEERI), Nagpur, India. The Nigerian Conservation Foundation and Chevron Nigeria Limited are appreciated for the Chief S.L. Edu research grant award. Also appreciated is the management of Covenant University, Ota, Nigeria for granting a one-year leave for this study.
REFERENCES
- Ayeni, A. O., Omoleye, J. A., Mudliar, S. N., Hymore, F. K. and Pandey, R. A., Utilization of lignocellulosic waste for ethanol production: Enzymatic digestibility and fermentation of pretreated shea tree sawdust. Korean Journal of Chemical Engineering, 31(7), 1180-1186 (2014).
- Ayeni, A. O., Banerjee, S., Omoleye, J. A., Hymore, F. K., Giri, B. S., Deskmukh, S. C., Pandey, R. A. and Mudliar, S. N., Optimization of pretreatment conditions using full factorial design and enzymatic convertibility of shea tree sawdust. Biomass and Bioenergy, 48, 130-138 (2013a).
- Ayeni, A. O., Hymore, F. K., Mudliar, S. N., Desk mukh, S. C., Satpute, D. B., Omoleye, J. A. and Pandey, R. A., Hydrogen peroxide and lime based oxidative pretreatment of wood waste to enhance enzymatic hydrolysis for a biorefinery: Process parameters optimization using response surface methodology. Fuel, 106, 187-194 (2013b).
- Andrić, P., Meyer, A. S., Jensen, P. A. and Dam-Johansen, K., Reactor design for minimizing product inhibition during enzymatic lignocellulosic hydrolysis II. Quantification of inhibition and suitability of membrane reactors. Biotechnology Advances, 28, 407-425 (2010).
- Bailey, M. J. and Linko, M., Production of β-glucosidase by Aspergillus oryzae in submerged bioreactor cultivation. Journal of Biotechnology, 16, 57-66 (1990).
- Bennet, C., Spectrophotometric acid dichromate method for the determination of ethyl alcohol. American Journal of Medical Technology, 37, 217-220 (1971).
- Blasi, C. D., Signorelli, G., Di Russo, C. and Rea, G., Product distribution from pyrolysis of wood and agricultural residues. Industrial Engineering and Chemistry Research, 38, 2216-2224 (1999).
- Chang, V. S., Nagwani, M., Kim, C. and Holtzapple, M. T., Oxidative lime pretreatment of highlignin biomass. Applied Biochemistry and Biotechnology, 94, 1-28 (2001).
- Chang, V. S., Nagwani, M. and Holtzapple, M. T., Lime pretreatment of crop residues bagasse and wheat straw. Applied Biochemistry Biotechnology, 74, 135-159 (1998).
- Correira, F., Roy, D. N. and Goel, K., Chemistry and delignification kinetics of Canadian industrial hemp. Journal of Wood Chemistry and Technology, 21, 97-111 (2001).
- Dang V. O. and Nguyen K. L., A universal kinetic model for characterization of the effect of chip thickness on kraft pulping. Bioresource Technology, 99, 1486-1490 (2008).
- Dolk, M., Yan, J. F. and McCarthy, J. L., Lignin 25. Kinetics of delignification of western hemlock in flow through reactors under alkaline conditions. Holzforschung, 43(2), 91-98 (1989).
- Dowe, N. and McMillan, J., SSF experimental protocols: Lignocellulosic biomass hydrolysis and fermentation: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; Jan. NREL Report No.: TP-510-42630. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).
- Esteves, B., Gominho, J., Rodrigues, J. C., Miranda, I. and Pereira, H., Pulping yield and delignification kinetics of heartwood and sapwood of maritime pine. Journal of Wood Chemistry and Technology, 25, 217-230 (2005).
- Fuentes, L. G., Rabelo, S. C., Filho, R. M. and Costa, A. C., Kinetics of lime pretreatment of sugarcane bagasse to enhance enzymatic hydrolysis. Applied Biochemistry and Biotechnology, 163, 612-625 (2011).
- Ghose, T. K., Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257-268 (1987).
- Gould, J. M., Alkaline peroxide delignification of agricultural residues to enhance enzymatic saccharification. Biotechnology and Bioengineering, 26, 46-52 (1984).
- Gould, J. M., Jasberg, B. K., Fahey, G. C. and Berger, L. L., Treatment of wheat straw with alkaline hydrogen peroxide in a modified extruder. Biotechnology and Bioengineering, 33, 233-236 (1989).
- Gregg, D. J., Boussaid, A. and Saddler, J. N., Techno-economic evaluation of a generic wood to-ethanol process: Effect of increased cellulose yields and enzyme recycle. Bioresource Technology, 63, 7-12 (1998).
- Kaar, W. E. and Holtzapple, M. T., Using lime pretreatment to facilitate the enzyme hydrolysis of corn stover. Biomass and Bioenergy, 18, 189-199 (2000).
- Kim, S. and Holtzapple, M. T., Delignification kinetics of corn stover in lime pretreatment. Bioresource Technology, 97, 778-785 (2006).
- Kim, T. H. and Lee, Y. Y., Fractionation of corn stover by hot-water and aqueous ammonia treatment. Bioresource Technology, 97, 224-232 (2006).
- Kleinert, T. N., Mechanisms of alkaline delignification 1. The overall reaction pattern. Tappi Journal, 49, 53-57 (1996).
- Kleinert, T. N., Ethanol-water delignification of wood. Rate constants and activation energy. Tappi Journal, 58, 170-171 (1975).
- Krishna, S. H. and Chowdary, G. V., Optimization of simultaneous saccharification and fermentation for the production of ethanol from lignocellulosic biomass. Journal of Agricultural and Food Chemistry, 48, 1971-1976 (2000).
- Kristensen, M. and Lykke, A. M., Informant-based evaluation of use and conservation preferences of savanna trees in Burkina Faso. Economic Botany, 57(2), 203-217 (2003).
- Kumar, P., Barrett, D. M., Delwiche, M. J. and Stroeve, P., Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and bio fuel production. Industrial Engineering Chemistry Research, 48, 3713-3729 (2009).
- Ladisch, M. R, Flickinger, M. C. and Tsao, G. T., Fuels and Chemicals from biomass. Energy, 4(2), 263-275 (1979).
- Li, S., Xu, S., Liu, S., Yang, C. and Lu, Q., Fast pyrolysis of biomass in free-fall reactor for hydrogen-rich gas. Fuel Process Technology, 85, 1201-1211 (2004).
- Li, Z., Chen, C. H., Hegg, E. L. and Hodge, D. B., Rapid and effective oxidative pretreatment of woody biomass at mild reaction conditions and low oxidant loadings. Biotechnology for Biofuels, 6, 19 (2013).
- Lin, L., Yan, R., Liu, Y. and Jiang, W., In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose, and lignin. Bioresource Tech nology, 101, 8217-8223 (2010).
- Lissens, G., Klinke, H., Verstraete, W., Ahring, B. and Thomsen, A. B., Wet oxidation pretreatment of woody yard waste: Parameter optimization and enzymatic digestibility for ethanol production. Journal of Chemical Technology and Biotechnology, 79, 889-895 (2004).
- Martín, C., Negro, M. J., Alfonsel, M. and Sáez, R., Enzymatic hydrolysis of lignocellulosic biomass from. Biotechnology and Bioengineering, 32, 341-344 (1988).
- Miguel, A. G., Rodriguez, F., Santos, A., Oliet, M., Garcia-Ochoa, F. and Tijero, J., Kinetics of Eucalyptus globulus delignification in methanol-water medium. Industrial Engineering and Chemistry Research, 38, 3324-3322 (1999).
- Miller, G. L., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426-428 (1959).
- Mosier, N., Wyman, C. E., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M. T. and Ladish, M., Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673-686 (2005).
- Nelson, P. J. and Gniel, G. M., Delignification of Eucalyptus woods during alkali pulping. 4th International Symposium on Wood and Pulping Chemistry, 125-129 (1987).
- Palonen, H., Thomsen, A. B., Tenkanen, M., Schmidt, A. S. and Viikari, L., Evaluation of wet oxidation pretreatment for enzymatic hydrolysis of softwood. Applied Biochemistry and Biotechnology, 117, 1-17 (2004).
- Parajó, J. C., Alonso, J. L. and Santos, V., Kinetics of catalysed organosolv processing of pine wood. Industrial and Engineering Chemistry Research, 34, 4333-4341 (1995).
- Pu, Y., Hu, F., Huang, F., Davidson, B. H. and Ragauskas, A. J., Accessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnology for biofuels, 6, 15, (2013).
- Qi, B., Chen, X., Shen, F., Su, Y. and Wan, Y., Optimization of enzymatic hydrolysis of wheat straw pretreated by alkaline peroxide using response surface methodology. Industrial and Engineering Chemistry Research, 48, 7346-7353 (2009).
- Sallé, J., Boussim, A., Raynal-Roques, A. and Brunck, F., Potential wealth of the Shea nut tree. Research perspective for improving yield. Bois et Foréts des Tropiques228, 11-23 (1991).
- Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D. and Croker, D., Determination of structural carbohydrates and lignin in biomass: Laboratory analytical procedure (LAP). Golden, CO: National Renewable Energy Laboratory; April. NREL Report No.: TP-510-42618. Contract No.: DE-AC36-99-G010337. Sponsored by the U.S. Department of Energy (2008).
- Springer, E. L., Harris, J. F. and Neil, W. K., Rate studies of the hydrotropic delignification of aspen wood. Tappi Journal, 46, 551-555 (1963).
- Tengborg, C., Galbe, M. and Zacchi, G., Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam pretreated softwood. Biotechnology Progress, 17, 110-117 (2001).
- Vázquez, G., Antorrena, G. and Gonzalez, J., Kinetics of acid-catalysed delignification of Eucalyptus globulus wood by acetic acid. Wood Science and Technology, 29, 267-275 (1995).
- Wei, C. J. and Cheng, C. Y., Effect of hydrogen peroxide pretreatment on the structural features and the enzymatic hydrolysis of rice straw. Biotechnology and Bioengineering, 27, 1418-1426 (1985).
- Yoo, C. G., Lee, C. W. and Kim, T. H., Optimization of two stage fractionation process for lignocellulosic biomass using response surface methodology (RSM). Biomass and Bioenergy, 35, 4901-4909 (2011).
- Zhu, L., Fundamental Study of Structural Features Affecting Enzymatic Hydrolysis of Lignocellulosic Biomass. Ph.D. Dissertation, Texas A&M University, College Station (2005).
Publication Dates
-
Publication in this collection
Jan-Mar 2016
History
-
Received
06 Dec 2014 -
Reviewed
19 Mar 2015 -
Accepted
15 Apr 2015