Abstract
Bioprocess development studies concerning the production of cellulases are of crucial importance due to the significant impact of these enzymes on the economics of biomass conversion into fuels and chemicals. This work evaluates the effects of solid-state fermentation (SSF) operational conditions on cellulase production by a novel strain of Aspergillus oryzae using an instrumented lab-scale bioreactor equipped with an on-line automated monitoring and control system. The use of SSF cultivation under controlled conditions substantially improved cellulase production. Highest production of FPase (0.40 IU g-1), endoglucanase (123.64 IU g-1), and β-glucosidase (18.32 IU g-1) was achieved at 28 °C, using an initial substrate moisture content of 70%, with an inlet air humidity of 80% and an airflow rate of 20 mL min-1. Further studies of kinetic profiles and respirometric analyses were performed. The results showed that these data could be very useful for bioprocess development of cellulase production and scale-up.
Keywords:
Aspergillus oryzae; Cellulase; Solid-state fermentation; Bioreactor; Respirometric analysis; Instrumentation; Amazon Forest
INTRODUCTION
Cellulase is a complex of enzymes whose components have a synergistic action during degradation of the polymeric chains of cellulose. Studies of cellulase production are of critical importance due to the influence of these enzymes on the economics of bio mass bioconversion into fuels and other chemicals (Klein-Marcuschamer et al., 2012Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A., Blanch, H. W., The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnology and Bioengineering, 109(4), 1083-1087 (2012).). The cellulolytic enzymatic complex is produced by a wide variety of microorganisms (bacteria and fungi); however, the aerobic fungi are known for their high growth and protein secretion rates (Lynd et al., 2002Lynd, L., Weimer, P., van Zyl, W., Pretorius, I., Microbial cellulose utilization: Fundamentals and biotechnology. Microbiology and Molecular Biol ogy Reviews, 66(3), 506 (2002).). Most commercial cellulases are produced by filamentous fungi of the genera Trichoderma and Aspergillus (Bhat, 2000Bhat, M. K., Cellulases and related enzymes in biotechnology. Biotechnology Advances, 18(5), 355-383 (2000).; Gusakov, 2011Gusakov, A. V., Alternatives to Trichoderma reesei in biofuel production. Trends in Biotechnology, 29(9), 419-425 (2011).). Among the Aspergillus genera, A. niger along with A. oryzae are the two most important fungi worldwide for biotechnological applications (Hu et al., 2011Hu, H., van den Brink, J., Gruben, B., Wosten, H., Gu, J., de Vries, R., Improved enzyme production by co-cultivation of Aspergillus niger and As pergillus oryzae and with other fungi. International Biodeterioration and Biodegradation, 65(1), 248-252 (2011).). Nevertheless, recent findings on the genomics of A. oryzae have revealed that it is highly enriched with genes involved in biomass degradation (Kobayashi et al., 2007Kobayashi, T., Abe, K., Asai, K., Gomi, K., Juvvadi, P., Kato, M., Kitamoto, K., Takeuchi, M., Machida, M., Genomics of Aspergillus oryzae. Bioscience Biotechnology and Biochemistry, 71(3), 646-670 (2007).). Besides the well-established applications of A. oryzae in the food industry, this fungus has great potential for the production of industrial enzymes, which needs to be further investigated.
The use of solid-state fermentation (SSF) for the development of industrial bioprocesses has been re ceiving significant attention over the past 20 years (Barrios-Gonzalez, 2012Barrios-Gonzalez, J., Solid-state fermentation: Physi ology of solid medium, its molecular basis and applications. Process Biochemistry, 47(2), 175-185 (2012).; Thomas et al., 2013Thomas, L., Larroche, C., Pandey, A., Current de velopments in solid-state fermentation. Biochemi cal Engineering Journal, 81, 146-161 (2013).). SSF is particularly advantageous for enzyme production by filamentous fungi, since it simulates the natural habitat of these microorganisms (Holker and Lenz, 2005Holker, U., Lenz, J., Solid-state fermentation - are there any biotechnological advantages? Current Opinion in Microbiology, 8(3), 301-306 (2005).). However, the characterization of individual microorganisms in terms of the influence of temperature and moisture content of the substrate on the kinetics of growth and product formation is essential for SSF process scale-up. Previous studies have shown the importance of evaluating the influence of process operational parameters on cellulase production by Aspergillus niger cultivated under SSF using controlled conditions of forced aeration and inlet air relative humidity (Farinas et al., 2011Farinas, C., Vitcosque, G., Fonseca, R., Neto, V., Couri, S., Modeling the effects of solid state fer mentation operating conditions on endoglucanase production using an instrumented bioreactor. Industrial Crops and Products, 34(1), 1186-1192 (2011).; Vitcosque et al., 2012Vitcosque, G. L., Fonseca, R. F., Rodríguez-Zuniga, U. F., Bertucci Neto, V., Couri, S., Farinas, C. S., Production of Biomass-degrading multienzyme complexes under solid-state fermentation of soy bean meal using a bioreactor. Enzyme Research, 9 (2012).).
Studies concerning the production of industrial enzymes by A. oryzae cultivated under SSF have been described in the literature. The enzymes considered include a-amylase (Bogar et al., 2002Bogar, B., Szakacs, G., Tengerdy, R., Linden, J., Pandey, A., Production of alpha-amylase with As pergillus oryzae on spent brewing grain by solid substrate fermentation. Applied Biochemistry and Biotechnology, 102, 453-461 (2002).; Farid and Shata, 2011Farid, M., Shata, H., Amylase production from As pergillus oryzae LS1 by solid-state fermentation and its use for the hydrolysis of wheat flour. Iranian Journal of Biotechnology, 9(4), 267-274 (2011).; Kareem et al., 2009Kareem, S., Akpan, I., Oduntan, S., Cowpea waste: A novel substrate for solid state production of am ylase by Aspergillus oryzae. African Journal of Microbiology Research, 3(12), 974-977 (2009).; Pengthamkee rati et al., 2012Pengthamkeerati, P., Numsomboon, S., Satapanajaru, T., Chairattanamanokorn, P., Production of alpha-amylase by Aspergillus oryzae from cassava ba gasse and wastewater sludge under solid-state fer mentation. Environmental Progress and Sustaina ble Energy, 31(1), 122-129 (2012).; Sivaramakrishnan et al., 2007Sivaramakrishnan, S., Gangadharan, D., Narnpoothiri, K., Soccol, C., Pandey, A., Alpha amylase pro duction by Aspergillus oryzae employing solid-state fermentation. Journal of Scientific and In dustrial Research, 66(8), 621-626 (2007).; Xu et al., 2008Xu, H., Sun, L., Zhao, D., Zhang, B., Shi, Y., Wu, Y., Production of alpha-amylase by Aspergillus oryzae As 3951 in solid state fermentation using spent brewing grains as substrate. Journal of the Sci ence of Food and Agriculture, 88(3), 529-535 (2008).), protease (Chutmanop et al., 2008Chutmanop, J., Chuichulcherm, S., Chisti, Y., Siri nophakun, P., Protease production by Aspergillus oryzae in solid-state fermentation using agroin dustrial substrates. Journal of Chemical Technol ogy and Biotechnology, 83(7), 1012-1018 (2008).), and xylanase (Pirota et al., 2013Pirota, R., Tonelotto, M., Delabona, P. D., Fonseca, R. F., Paixao, D. A. A., Baleeiro, F. C. F., Neto, V. B., Farinas, C. S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under con trolled operation conditions. Industrial Crops and Products, 45, 465-471 (2013).; Szendefy et al., 2006Szendefy, J., Szakacs, G., Christopher, L., Potential of solid-state fermentation enzymes of Aspergil lus oryzae in biobleaching of paper pulp. Enzyme and Microbial Technology, 39(6), 1354-1360 (2006).), amongst others. However, to the best of our knowledge, there have been only a few studies con cerning the production of cellulase by A. oryzae cultivated under SSF, from the bioprocess development point of view (Begum and Alimon, 2011Begum, M. F., Alimon, A. R., Bioconversion and saccharification of some lignocellulosic wastes by Aspergillus oryzae ITCC-4857.01 for fermentable sugar production. Electronic Journal of Biotech nology, 14(5), 3-3 (2011); Lio and Wang, 2012Lio, J. Y., Wang, T., Solid-state fermentation of soy bean and corn processing coproducts for potential feed improvement. Journal of Agricultural and Food Chemistry, 60(31), 7702-7709 (2012).; Sandhu et al., 2012Sandhu, S. K., Oberoi, H. S., Dhaliwal, S. S., Babbar, N., Kaur, U., Nanda, D., Kumar, D., Ethanol pro duction from Kinnow mandarin (Citrus reticu lata) peels via simultaneous saccharification and fermentation using crude enzyme produced by Aspergillus oryzae and the thermotolerant Pichia kudriavzevii strain. Annals of Microbiology, 6(2)2, 655-666 (2012).). Given the poten tial of A. oryzae and its recognized application for industrial enzyme production, there is great interest in the characterization of this fungus in terms of cellulase production using SSF under controlled operational conditions.
The present work investigates the development of a bioprocess for cellulase production (FPase, endoglu canase, and β-glucosidase) by a new Amazon Forest strain of Aspergillus oryzae cultivated under SSF using an instrumented lab-scale bioreactor. The in fluence of temperature and initial substrate moisture content on the efficiency of cellulase production was evaluated, and comparisons were made with static aeration conditions. The selected conditions were used for characterization of kinetic profiles and in respirometric analyses.
MATERIALS AND METHODS
Instrumented Bioreactor
The bioreactor used was a lab-scale system consisting of 16 columns (2.5 cm diameter, 20 cm length) placed in a water bath. The bioreactor was equipped with an on-line system to control the air flow rate and the inlet air relative humidity, as described pre viously (Farinas et al., 2011Farinas, C., Vitcosque, G., Fonseca, R., Neto, V., Couri, S., Modeling the effects of solid state fer mentation operating conditions on endoglucanase production using an instrumented bioreactor. Industrial Crops and Products, 34(1), 1186-1192 (2011).). For this study, the air flow rate and inlet air relative humidity were kept constant during all cultivations, at 20 mL min-1 and 80%, respectively.
Microorganism Screening
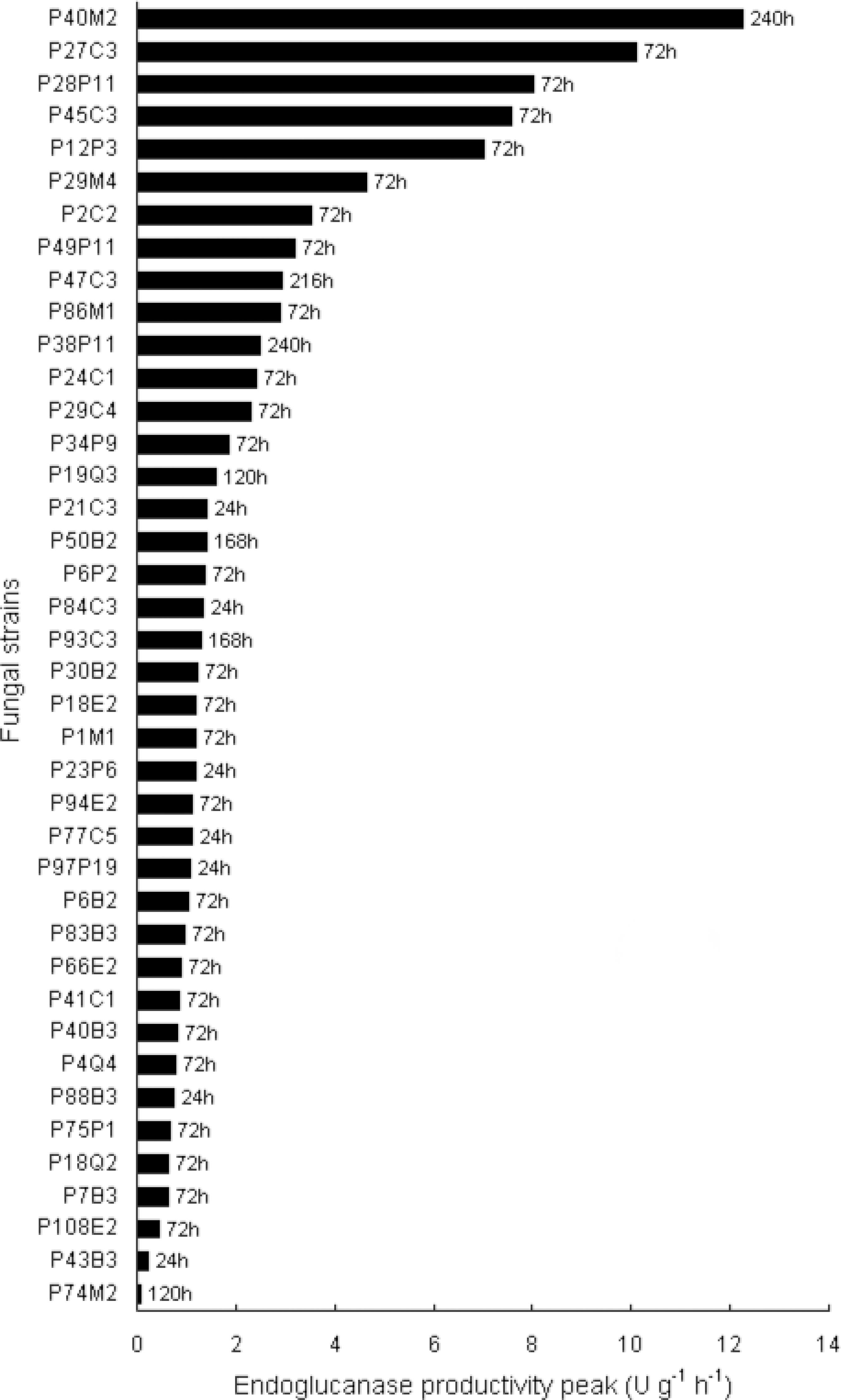
An initial screening of 40 fungal strains isolated from the Amazon Forest (Delabona et al., 2012Delabona, P., Pirota, R., Codima, C., Tremacoldi, C., Rodrigues, A., Farinas, C., Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass and Bio energy, 37, 243-250 (2012).) was carried out in terms of endoglucanase production under SSF. In this preliminary step, a set of SSF cultivations was carried out in 250 mL Erlenmeyer flasks for 240 hours, using a solid substrate composed of wheat bran moistened at 60% with a nutrient medium (Mandels and Sternberg, 1976Mandels, M., Sternberg, D., Recent advances in cel lulase technology. Journal of Fermentation Tech nology, 54(4), 267-286 (1976).). Enzymes were extracted at 24 hour intervals and analyzed as described below. The selection criterion for this study was based on endoglucanase productivity val ues (IU g-1h-1) of the different strains, calculated in terms of activity units per mass of initial dry solid substrate (IU g-1), as a function of time (in hours).
Identification of the Selected Strain
The microorganism selected for further use in this study was a strain of Aspergillus oryzae (P27C3) deposited in the Embrapa Food Technology microorganism collection (Rio de Janeiro, Brazil). The culture was maintained on PDA slants at 32 °C for 5 days before inoculation for SSF cultivation studies. The identification of the selected strain was per formed by extraction of the genomic DNA and amplification of the Internal Transcribed Spacer (ITS) regions, using the same procedure described in a previous work (Pirota et al., 2013Pirota, R., Tonelotto, M., Delabona, P. D., Fonseca, R. F., Paixao, D. A. A., Baleeiro, F. C. F., Neto, V. B., Farinas, C. S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under con trolled operation conditions. Industrial Crops and Products, 45, 465-471 (2013).). The sequence of the ITS region corresponding to rDNA was submitted for registration at the National Center for Biotechnology Information (NCBI) and received the access number JX163919.
Selection of Operational Conditions for SSF Cul tivations
Bioreactor cultivations were carried out for 72 hours using wheat bran as solid substrate. The solid medium was sterilized by autoclaving at 121 °C for 20 minutes before inoculation. A spore suspension volume corresponding to 107 conidia per g of dry solid medium was inoculated into the solid medium by gently stirring with a glass rod until a uniform mixture was obtained. The strategy adopted for se lection of SSF operational conditions was to evaluate each variable individually and then select the best value for incorporation in the next variable selection step. Initially, cultivations were carried out at 35 °C with different moisture levels of 50, 60, 70, and 80%. The moisture content was adjusted with a nutrient medium solution (Mandels and Sternberg, 1976Mandels, M., Sternberg, D., Recent advances in cel lulase technology. Journal of Fermentation Tech nology, 54(4), 267-286 (1976).). After selection of the most favorable moisture content, different temperatures (28, 30, 32, 35, and 37 °C) were evaluated using an initial moisture content of 70%. All cultivations were carried out in 250 mL Erlenmeyer flasks under similar conditions to enable comparison of the results obtained using forced aeration (bioreactor) with those achieved using the static aeration system. After the cultivation period of 72 hours, the enzymes were extracted and analyzed as described below. Means for each condition were subjected to a statistical analysis using the Origin (version 8.0) software.
Cellulase Production Kinetic Profiles
The kinetic profiles of cellulase production were evaluated during a 120 hour cultivation period using the selected SSF operational conditions (28 °C, air flow rate of 20 mL min-1, inlet air relative humidity of 80%, and initial substrate moisture content of 70%). Cultivations were carried out in 250 mL Erlenmeyer flasks under similar conditions in order to compare forced aeration (bioreactor) with the static aeration system. Samples were withdrawn at 24 hour inter vals, and the enzymes were extracted and analyzed as described below.
Enzyme Extraction
After the cultivation period, the solid medium was transferred to Erlenmeyer flasks (in the case of column cultivations) and the enzymes were extracted by adding a sufficient volume of 0.05 mol L-1 citrate/ acetate buffer, at pH 4.8, to achieve a solid/liquid ratio of 1:10. The suspension was stirred at 120 rpm for 30 minutes at room temperature, and the enzymatic solution was recovered by filtration. The enzyme extracts were stored at -18 °C prior to the analyses.
Respirometric Analyses
Respirometric analyses were carried out by meas uring CO2 in the outlet air stream from the columns of the bioreactor system, using a GMM 220 instrument (Vaisala, Finland). The cumulative amount of CO2 produced was calculated from the area under the CO2 vs. cultivation time curve. The data obtained for CO2 production up to the end of the exponential phase (approximately 15 hours after the end of the lag phase) were fitted to two different functions of cell growth behavior for comparison. These were the logistic and the deceleration equations, and the fit ting was performed by means of non-linear regression using numerical software (Origin, version 8.0). The logistic equation (Equation (1)) was chosen for estimation of the specific growth velocity (µ) for each experimental condition varying the initial substrate moisture content and the temperature, as well as the cultivation carried out for estimation of the kinetic profile under the selected operational conditions.
In Equation (1), C is the biomass content (in terms of mol CO2), Cm is the maximum biomass content (in terms of mol CO2), C0 is the initial biomass content (in terms of mol CO2), µ is the specific growth rate (h-1), and t is the time. Here, the CO2 produced was used to describe cell growth. This assumption is reasonable, since in the exponential period the CO2 de rived from maintenance is much lower than the CO2 produced from growth, which makes it possible to fit the CO2 production during this period with a logistic curve, as for a biomass profile. The amount of initial CO2 (C0) was assumed to be the same (1.99 × 10-4 mol of CO2) in each experiment, since the inoculum concentration was fixed for these studies. The maximum CO2 (Cm) was estimated as the total amount of CO2 produced up to the end of the exponential phase (which corresponded to approximately 15 hours after the end of the lag phase).
Enzyme Activity Assays
The activities of FPase and endoglucanase were measured according to the methodology described by Ghose (1987)Ghose, T., Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257-268 (1987).. One unit of activity corresponds to 1 mmol of glucose released per minute per mL, under the reaction conditions. The quantification of the reducing groups was performed using the dinitrosalicylic acid (DNS) method (Miller, 1959Miller, G., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chem istry, 31(3), 426-428 (1959).). The β-glucosidase activity was determined using cellobiose (Sigma, St. Louis, USA) as substrate and quantifying the sugars released by means of an enzymatic kit for glucose measurement (Laborlab, São Paulo, Brazil). The results were expressed as activity units per mass of initial dry solid substrate (IU g-1).
RESULTS AND DISCUSSION
Screening of Cellulase-Producing Fungal Strains
The efficiency of cellulase production in terms of endoglucanase productivity is presented in Figure 1 for 40 fungal strains isolated from the Amazon Forest and cultivated under SSF on wheat bran.
The two strains that presented the highest en doglucanase productivity values were P40M2 (12.3 IU g-1h-1), followed by P27C3 (10.1 IU g-1h-1). The P40M2 strain has been previously identified as Aspergillus fumigatus (Delabona et al., 2012Delabona, P., Pirota, R., Codima, C., Tremacoldi, C., Rodrigues, A., Farinas, C., Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass and Bio energy, 37, 243-250 (2012).). Since some strains of A. fumigatus can exhibit a degree of pathogenesis (Meyer et al., 2011Meyer, V., Wu, B., Ram, A. F. J., Aspergillus as a multi-purpose cell factory: Current status and per spectives. Biotechnology Letters, 33(3), 469-476 (2011).), the P27C3 strain was selected for identification. The ITS region of the fungal rRNA was amplified and sequenced. The ITS rDNA sequence of isolate P27C3 was submitted to alignment using the Basic Local Alignment Search Tool (BLASTN) program, and showed satisfactory homology of 100% with the Aspergillus oryzae spe cies. Since there have been very few bioprocess de velopment studies of cellulase production by A. ory zae, the P27C3 strain was selected for further charac terization of its potential as a new source of industrial enzymes.
Effect of Initial Moisture Content on Cellulase Pro duction by A. oryzae
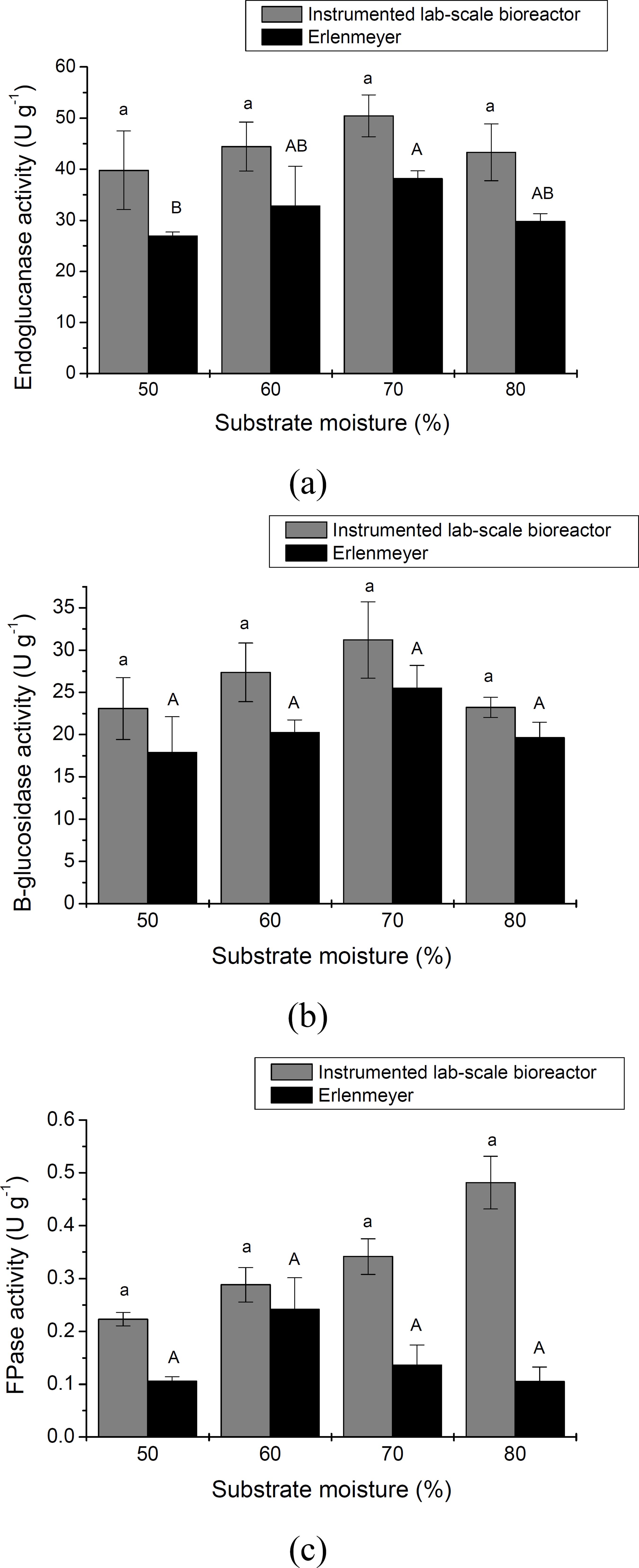
Bioprocess development for cellulase production by A. oryzae P27C3 cultivated under controlled SSF operational conditions was carried out using the in strumented bioreactor. In order to compare the effec tiveness of this system, a similar set of cultivations was conducted under static aeration conditions. The effect of initial moisture content on cellulase pro duction was investigated by cultivating the fungus for 72 hours at 35 °C using different initial substrate moisture contents (50, 60, 70, and 80%). Compari sons of the influence of moisture content on endoglu canase, β-glucosidase, and FPase production under forced and static aeration conditions are presented in Figures 2a, 2b, and 2c, respectively. As can be ob served, there was a marked positive effect of cultivation under controlled conditions of forced aeration, where cellulase production was significantly higher than in cultivations carried out under static conditions. Statistical analysis indicated that the population means of activities obtained using the instru mented lab-scale bioreactor and Erlenmeyer flasks were significantly different after a Tukey test com parison (p < 0.05). In these experiments, endoglu canase, β-glucosidase, and FPase activities were up to 48, 29, and 336% higher, respectively, using forced rather than static aeration. Lower enzyme production under static conditions is probably related to oxygen limitation during fungal growth, since O2 is transferred mainly by diffusion. Besides oxygen transfer, heat transfer and CO2 dissipation are also favored under dynamic conditions of forced aeration (Pirota et al., 2013Pirota, R., Tonelotto, M., Delabona, P. D., Fonseca, R. F., Paixao, D. A. A., Baleeiro, F. C. F., Neto, V. B., Farinas, C. S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under con trolled operation conditions. Industrial Crops and Products, 45, 465-471 (2013).).
An initial substrate moisture content of 70% was most favorable for endoglucanase production (Figure 2a). Nevertheless, endoglucanase production was not significantly affected by the initial moisture content used, for either of the aeration systems, as indicated by the statistical analysis. Endoglucanase activity values varied from 39.8 to 50.4 IU g-1 when using forced aeration and from 26.9 to 38.2 IU g-1 when using static aeration.
Effect of initial moisture content on (a) endoglucanase, (b) β-glucosidase, and (c) FPase production by A. oryzae cultivated under SSF using forced and static aeration. Lower case and capital letters compare results for activities obtained using either the instrumented lab-scale bioreactor or Erlen meyer flasks, respectively. Means with different let ters are significantly different after a Tukey test com parison (p < 0.05). The population means of activi ties obtained using the instrumented lab-scale biore actor and Erlenmeyer flasks were significantly dif ferent after a Tukey test comparison (p < 0.05).
A more pronounced effect was therefore observed for static aeration, since modulating the initial moisture content from 50 to 70% resulted in a 42% increase in endoglucanase activity, compared to a 27% increase when using forced aeration. This can be explained by the ability of the bioreactor system to maintain the moisture content of the medium by humidification of the air passing through the columns. This is a very positive result in terms of bioprocess development; since it implies that endoglucanase biosynthesis by A. oryzae P27C3 was not very sensitive to variations in substrate initial moisture content when using a bioreactor system with control of aeration and inlet air relative humidity.
A similar trend was observed for β-glucosidase, since an initial substrate moisture content of 70% also favored production of this enzyme (Figure 2b). β-Glucosidase activity values varied from 23.1 to 31.2 IU g-1 when using forced aeration and from 17.9 to 25.4 IU g-1 when using static aeration. As ob served for endoglucanase, there was a more pro nounced effect of moisture content on β-glucosidase activity using static aeration, with a 42% increase in activity achieved by modulating the initial moisture content from 50 to 70%, as compared to a 35% in crease when using forced aeration.
In terms of FPase activity, higher values were achieved using an initial substrate moisture content of 80%. FPase activity values varied from 0.22 to 0.48 IU g-1 when using forced aeration and from 0.11 to 0.14 IU g-1 when using static aeration. A more pronounced effect of moisture content was observed for forced aeration, with a 118% increase in FPase activity achieved by modulating the initial moisture content from 50 to 80%, as compared to a 27% increase when using static aeration. This finding can be explained by restricted production of the cellulase enzymes that account for FPase activity due to oxy gen limitation during fungal growth, together with reduced heat transfer, when using the static aeration system. In addition, since total cellulase activity as says are measured using insoluble substrates, prob lems can result from the heterogeneity of insoluble cellulose and the complexity of the cellulase system, especially regarding reproducibility when the values are of the magnitude encountered here.
Moisture content is one of the most important factors that affect SSF process efficiency. The effect of the initial substrate moisture content on the pro duction of cellulase by other Aspergillus strains cultivated using SSF has been described previously. Mamma et al. (2008)Mamma, D., Kourtoglou, E., Christakopoulos, P., Fungal multienzyme production on industrial by-products of the citrus-processing industry. Biore source Technology, 99(7), 2373-2383 (2008). evaluated enzyme production under SSF, using the fungus A. niger with orange peel as substrate, and were able to significantly in crease enzyme activities after optimizing the initial moisture content of the solid medium. Gao et al. (2008)Gao, J., Weng, H., Zhu, D., Yuan, M., Guan, F., Xi, Y., Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal As pergillus terreus M11 under solid-state cultivation of corn stover. Bioresource Technology, 99(16), 7623-7629 (2008). found that an increase in the initial moisture content enhanced enzyme production by the ther moacidophilic fungus Aspergillus terreus M11, culti vated under SSF using corn stover as substrate. Our results demonstrated that the initial moisture content also played an important role in cellulase production by A. oryzae P27C3, especially for cultivations car ried out using static aeration. Nevertheless, A. oryzae P27C3 was not very sensitive to variations in sub strate moisture content when using a bioreactor sys tem with controlled forced aeration. Based on these results, an initial substrate moisture content of 70% was selected infurther studies to evaluate the effect of temperature on cellulase production.
Effect of Temperature on Cellulase Production by A. oryzae
Comparisons of the effect of temperature on en doglucanase, β-glucosidase, and FPase production, using forced and static aeration, are presented in Figures 3a, 3b, and 3c, respectively. Cultivations using temperatures of 28, 30, 32, 35, and 37 °C were carried out using both forced and static aeration sys tems, with a fixed initial substrate moisture content of 70%. In this set of cultivations, there was also a large positive effect of controlled forced aeration, with cellulase production being significantly higher than under static conditions. Statistical analysis indi cated that the population means of activities obtained using the instrumented lab-scale bioreactor and Er lenmeyer flasks were significantly different after a Tukey test comparison (p < 0.05). Endoglucanase, β-glucosidase, and FPase activities were up to 87, 281, and 210% higher, respectively, using forced aeration. This can be explained by the more favorable envi ronmental conditions encountered in the bioreactor system, such as efficient heat dissipation and avoid ance of temperature gradients caused by the heat generated during microbial activity (Pirota et al., 2013Pirota, R., Tonelotto, M., Delabona, P. D., Fonseca, R. F., Paixao, D. A. A., Baleeiro, F. C. F., Neto, V. B., Farinas, C. S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under con trolled operation conditions. Industrial Crops and Products, 45, 465-471 (2013).).
The most favorable temperature for endoglucanase production by A. oryzae was 28 °C (Figure 3a). Pro duction of the enzyme was significantly influenced by temperature, in both cultivation systems. Endoglu canase activity values varied from 36.7 to 79.4 IU g-1 using forced aeration and from 30.2 to 70.3 IU g-1 using static aeration. There was therefore a more pronounced effect of temperature for static aeration, since a 133% increase in endoglucanase activity was achieved by modulating the temperature, as com pared to a 116% increase when using forced aeration. In terms of bioprocess development, it is important to highlight that endoglucanase biosynthesis by A. oryzae P27C3 was significantly sensitive to variations in temperature.
Effect of temperature on (a) endoglu canase, (b) β-glucosidase, and (c) FPase production by A. oryzae cultivated under SSF using forced and static aeration. Lower case and capital letters com pare results for activities obtained using either the instrumented lab-scale bioreactor or Erlenmeyer flasks, respectively. Means with different letters are significantly different after a Tukey test comparison (p < 0.05). The population means of activities ob tained using the instrumented lab-scale bioreactor and Erlenmeyer flasks were significantly different after a Tukey test comparison (p < 0.05).
A different trend was observed for β-glucosidase, with higher temperatures (35 and 37 °C) favoring production of the enzyme (Figure 3b). β-glucosidase activity values varied from 9.7 to 29.5 IU g-1 using forced aeration and from 2.9 to 26.5 IU g-1 using static aeration. Such different behavior could be pos sibly related to a different mechanism for b-gluco sidase production, given the different fungal accessi bility to the soluble sugars which act as inducers for such enzymes. Nevertheless, as observed for endo glucanase, there was a more pronounced effect of temperature on β-glucosidase activity when static aeration was employed, with temperature modulation resulting in an increase of 813% in enzyme activity, compared to a 204% increase when forced aeration was used.
Using forced aeration, cellulase production in terms of FPase activity was highest at 37 °C, fol lowed by 28 °C (Figure 3c). FPase activity values varied from 0.19 to 0.31 IU g-1 using forced aeration and from 0.10 to 0.12 IU g-1 using static aeration. A more pronounced effect of temperature was observed for forced aeration, with a 63% increase in FPase activity achieved by modulating the temperature, as compared to a 20% increase when static aeration was used.
Temperature is an important variable that affects microbial growth under SSF, thereby influencing product formation, while the time at which a maxi mum enzyme activity level is reached will also vary according to the cultivation temperature. The influ ence of temperature on cellulase production has been reported for other Aspergillus strains cultivated un der SSF. Jecu (2000)Jecu, L., Solid state fermentation of agricultural wastes for endoglucanase production. Industrial Crops and Products, 11(1), 1-5 (2000). studied the effect of tempera tures between 25 and 37 °C on endoglucanase pro duction by A. niger and found that the optimum for endoglucanase production was in the range 28 to 34 °C. Jabasingh and Nachiyar (2011)Jabasingh, S., Nachiyar, C., Utilization of pretreated bagasse for the sustainable bioproduction of cel lulase by Aspergillus nidulans MTCC344 using response surface methodology. Industrial Crops and Products, 34(3), 1564-1571 (2011). studied the in fluence of different variables on cellulase production by A. nidulans MTCC344 using response surface methodology. The effect of temperature was evalu ated in the range 20 to 60 °C, and the optimum value for cellulase production was found to be 37.5 °C. As with the effect of moisture content, the characterization of each particular microorganism in terms of the influence of temperature on the kinetics of growth and product formation is essential for SSF biopro cess development. Based on the results, an initial moisture content of 70% and a temperature of 28 °C were selected for evaluation of the kinetic profiles of cellulase production over a cultivation period of 120 hours.
Kinetic Profiles of Cellulase Production Under the Selected Conditions
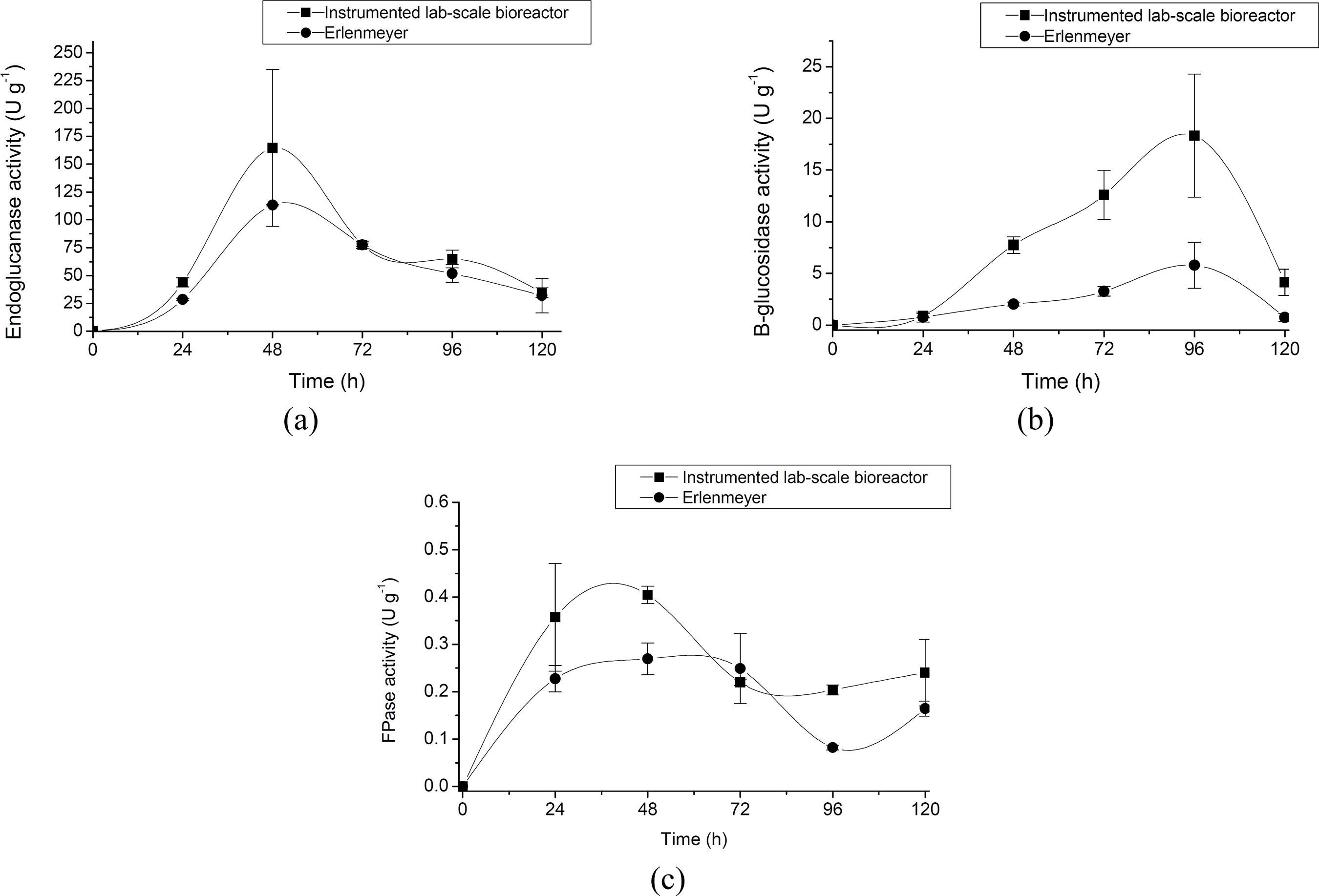
The kinetic profiles of cellulase production over a period of 120 hours, using the selected operational conditions (temperature of 28 °C, initial substrate mois ture content of 70%, inlet air humidity of 80%, and
flow rate of 20 mL min-1), are illustrated in Figures 4a, 4b, and 4c for endoglucanase, β-glucosidase, and FPase, respectively. In order to compare the effec tiveness of the system used, a similar set of cultivations was conducted under static aeration conditions. Both endoglucanase and FPase activities reached maximum values (123.64and 0.40 IU g-1, respec tively) after 48 hours of cultivation, whereas the maximum value for β-glucosidase (18.32 IU g-1) was only achieved after around 96 hours of cultivation, for both cultivation systems. Nevertheless, the ad vantages of performing SSF under forced aeration conditions can be observed by comparing the results with those obtained under static conditions (Figures 4a, 4b, and 4c). The dynamic system used here was therefore suitable for controlling SSF operational conditions to achieve higher enzyme production efficiency. Zhang et al. (2003)Zhang, X. Y., Mo, H., Zhang, J., Li, Z. H., A solid-state bioreactor coupled with forced aeration and pressure oscillation. Biotechnology Letters, 25(5), 417-420 (2003). and Mo et al. (2004)Mo, H. T., Zhang, X. Y., Li, Z. H., Control of gas phase for enhanced cellulase production by Peni cillium decumbens in solid-state culture. Process Biochemistry, 39(10), 1293-1297 (2004). also found that forced aeration had a positive effect on cellulase production by Penicillium decumbens under SSF.
In previous work, endoglucanase production of up to 56.1 IU g-1 was achieved using wheat bran as solid substrate and a selected strain of Aspergillus niger (Farinas et al., 2011Farinas, C., Vitcosque, G., Fonseca, R., Neto, V., Couri, S., Modeling the effects of solid state fer mentation operating conditions on endoglucanase production using an instrumented bioreactor. Industrial Crops and Products, 34(1), 1186-1192 (2011).). The values for endoglu canase production achieved here were more than two times higher, demonstrating the importance of the characterization of new fungal strains in terms of their ability to produce biomass-degrading enzymes.
Respirometric Analyses
The evolution of CO2 during the SSF process was monitored using a sensor connected to the gas stream exiting the columns of the bioreactor system. CO2 data can provide an important means of under standing the relationship between fungal growth and enzyme production, since it is difficult to measure biomass in SSF due to the problem of separating the biomass from the substrate (Raimbault, 1998Raimbault, R., General and microbiological aspects of solid substrate fermentation. In: Eletronic Jour nal of Biotechnology, 3-45 (1998).). Figures 5a, 5b, and 5c show CO2 evolution during the cultivations, investigating the initial moisture content, temperature, and kinetic profiles, respec tively, under the selected operational conditions.
The CO2 evolution curves shown in Figure 5a are very similar, which could imply that fungal growth was not very much affected by the different substrate moisture contents used. This is in agreement with the previous observation that endoglucanase production was not significantly affected by the initial moisture content when using the bioreactor system with con trolled conditions of aeration and inlet air relative humidity (Figure 2a).
Cellulase profile in terms of (a) endoglucanase, (b) β-glucosidase, and (c) FPasefor A.oryzae cultivated under SSF over a period of 120 hours, using a temperature of 28 °C, an initial substrate moisture content of 70%, an inlet air humidity of 80%, and a flow rate of 20 mL min-1.
CO2 evolution during cellulase production by A. oryzae cultivated using (a) different initial substrate moisture contents, (b) different temperatures, and (c) the selected operational conditions (temperature of 28 °C, initial substrate moisture content of 70%, inlet air humidity of 80%, and flow rate of 20 mL min-1).
In order to further evaluate the significance of the CO2 evolution curves obtained using different initial moisture contents, the specific growth rate (µ) was obtained by regression analysis of Equation (1). The growth profile was fitted very well by the logistic equation, as reflected by the good correlation coeffi cients achieved, which were all above 0.99 (Table 1). The deceleration equation was also evaluated and resulted in a similar good correlation (data not shown). However, the logistic equation was chosen due to the familiarity of its parameters and its wider use in the literature concerning SSF. The calculated specific growth rates were similar for all conditions of initial moisture content investigated (Table 1). Neverthe less, the highest value of µ (0.532 h-1) was obtained using an initial moisture content of 70%. This was in agreement with the moisture condition selected based on cellulase production. There was no signifi cant effect of the initial substrate moisture content on the duration of the lag phase.
Effect of initial substrate moisture content on fermentation parameters obtained using the logistic equation.
The CO2 evolution curves revealed a distinct in fluence of temperature on fungal growth (Figure 5b), which was favored at 28 °C, in agreement with the condition identified for higher endoglucanase pro duction (Figure 3a). Similarly, there were distinct tem perature-related differences in total cumulative CO2 evolution, as well as the calculated specific growth rates (Table 2). The highest value of µ (0.430h-1) was achieved using a temperature of 28 °C, in agreement with the temperature selected based on cellulase production. Figure 5c illustrates the evolution of CO2 over a period of 120 hours during the cultivation carried out to obtain the kinetic profiles of cellulase production under the selected operational conditions (temperature of 28 °C, initial substrate moisture con tent of 70%, inlet air humidity of 80%, and flow rate of 20 mL min-1). The total CO2 produced during the exponential phase (9.09 × 10-3 ± 1.0× 10-4 mol), as well as the value of µ(0.40 ± 4.0 × 10-4 h-1), are in agreement with the results described previously (Table 2). Good correlation with enzyme production was shown by both the total amount of CO2 produced and the calculated specific growth rates, indicating the potential of these parameters for use in bioprocess development for cellulase production.
CONCLUSIONS
The performance of a new Amazon Forest strain of Aspergillus oryzae P27C3 in cellulase production using solid-state fermentation under controlled oper ational conditions was characterized using a lab-scale instrumented bioreactor. The results obtained enabled selection of the variables that could be ad justed in order to improve cellulase production. High est production of FPase (0.40 IU g-1), endoglucanase (123.64 IU g-1), and β-glucosidase (18.32 IU g-1) was achieved at 28 °C, using an initial substrate moisture content of 70%, an inlet air humidity of 80%, and an air flow rate of 20 mL min-1. The results of respirometric analyses were in good agreement with en zyme production values, showing that this information could contribute to bioprocess development for cellulase production.
-
*
To whom correspondence should be addressed
ACKNOWLEDGMENTS
The authors would like to thank Embrapa and the Brazilian agencies CNPq and Capes for financial support.
REFERENCES
- Barrios-Gonzalez, J., Solid-state fermentation: Physi ology of solid medium, its molecular basis and applications. Process Biochemistry, 47(2), 175-185 (2012).
- Begum, M. F., Alimon, A. R., Bioconversion and saccharification of some lignocellulosic wastes by Aspergillus oryzae ITCC-4857.01 for fermentable sugar production. Electronic Journal of Biotech nology, 14(5), 3-3 (2011)
- Bhat, M. K., Cellulases and related enzymes in biotechnology. Biotechnology Advances, 18(5), 355-383 (2000).
- Bogar, B., Szakacs, G., Tengerdy, R., Linden, J., Pandey, A., Production of alpha-amylase with As pergillus oryzae on spent brewing grain by solid substrate fermentation. Applied Biochemistry and Biotechnology, 102, 453-461 (2002).
- Chutmanop, J., Chuichulcherm, S., Chisti, Y., Siri nophakun, P., Protease production by Aspergillus oryzae in solid-state fermentation using agroin dustrial substrates. Journal of Chemical Technol ogy and Biotechnology, 83(7), 1012-1018 (2008).
- Delabona, P., Pirota, R., Codima, C., Tremacoldi, C., Rodrigues, A., Farinas, C., Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biomass and Bio energy, 37, 243-250 (2012).
- Farid, M., Shata, H., Amylase production from As pergillus oryzae LS1 by solid-state fermentation and its use for the hydrolysis of wheat flour. Iranian Journal of Biotechnology, 9(4), 267-274 (2011).
- Farinas, C., Vitcosque, G., Fonseca, R., Neto, V., Couri, S., Modeling the effects of solid state fer mentation operating conditions on endoglucanase production using an instrumented bioreactor. Industrial Crops and Products, 34(1), 1186-1192 (2011).
- Gao, J., Weng, H., Zhu, D., Yuan, M., Guan, F., Xi, Y., Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal As pergillus terreus M11 under solid-state cultivation of corn stover. Bioresource Technology, 99(16), 7623-7629 (2008).
- Ghose, T., Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257-268 (1987).
- Gusakov, A. V., Alternatives to Trichoderma reesei in biofuel production. Trends in Biotechnology, 29(9), 419-425 (2011).
- Holker, U., Lenz, J., Solid-state fermentation - are there any biotechnological advantages? Current Opinion in Microbiology, 8(3), 301-306 (2005).
- Hu, H., van den Brink, J., Gruben, B., Wosten, H., Gu, J., de Vries, R., Improved enzyme production by co-cultivation of Aspergillus niger and As pergillus oryzae and with other fungi. International Biodeterioration and Biodegradation, 65(1), 248-252 (2011).
- Jabasingh, S., Nachiyar, C., Utilization of pretreated bagasse for the sustainable bioproduction of cel lulase by Aspergillus nidulans MTCC344 using response surface methodology. Industrial Crops and Products, 34(3), 1564-1571 (2011).
- Jecu, L., Solid state fermentation of agricultural wastes for endoglucanase production. Industrial Crops and Products, 11(1), 1-5 (2000).
- Kareem, S., Akpan, I., Oduntan, S., Cowpea waste: A novel substrate for solid state production of am ylase by Aspergillus oryzae African Journal of Microbiology Research, 3(12), 974-977 (2009).
- Klein-Marcuschamer, D., Oleskowicz-Popiel, P., Simmons, B. A., Blanch, H. W., The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnology and Bioengineering, 109(4), 1083-1087 (2012).
- Kobayashi, T., Abe, K., Asai, K., Gomi, K., Juvvadi, P., Kato, M., Kitamoto, K., Takeuchi, M., Machida, M., Genomics of Aspergillus oryzae Bioscience Biotechnology and Biochemistry, 71(3), 646-670 (2007).
- Lio, J. Y., Wang, T., Solid-state fermentation of soy bean and corn processing coproducts for potential feed improvement. Journal of Agricultural and Food Chemistry, 60(31), 7702-7709 (2012).
- Lynd, L., Weimer, P., van Zyl, W., Pretorius, I., Microbial cellulose utilization: Fundamentals and biotechnology. Microbiology and Molecular Biol ogy Reviews, 66(3), 506 (2002).
- Mamma, D., Kourtoglou, E., Christakopoulos, P., Fungal multienzyme production on industrial by-products of the citrus-processing industry. Biore source Technology, 99(7), 2373-2383 (2008).
- Mandels, M., Sternberg, D., Recent advances in cel lulase technology. Journal of Fermentation Tech nology, 54(4), 267-286 (1976).
- Meyer, V., Wu, B., Ram, A. F. J., Aspergillus as a multi-purpose cell factory: Current status and per spectives. Biotechnology Letters, 33(3), 469-476 (2011).
- Miller, G., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chem istry, 31(3), 426-428 (1959).
- Mo, H. T., Zhang, X. Y., Li, Z. H., Control of gas phase for enhanced cellulase production by Peni cillium decumbens in solid-state culture. Process Biochemistry, 39(10), 1293-1297 (2004).
- Pengthamkeerati, P., Numsomboon, S., Satapanajaru, T., Chairattanamanokorn, P., Production of alpha-amylase by Aspergillus oryzae from cassava ba gasse and wastewater sludge under solid-state fer mentation. Environmental Progress and Sustaina ble Energy, 31(1), 122-129 (2012).
- Pirota, R., Tonelotto, M., Delabona, P. D., Fonseca, R. F., Paixao, D. A. A., Baleeiro, F. C. F., Neto, V. B., Farinas, C. S., Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under con trolled operation conditions. Industrial Crops and Products, 45, 465-471 (2013).
- Raimbault, R., General and microbiological aspects of solid substrate fermentation. In: Eletronic Jour nal of Biotechnology, 3-45 (1998).
- Sandhu, S. K., Oberoi, H. S., Dhaliwal, S. S., Babbar, N., Kaur, U., Nanda, D., Kumar, D., Ethanol pro duction from Kinnow mandarin (Citrus reticu lata) peels via simultaneous saccharification and fermentation using crude enzyme produced by Aspergillus oryzae and the thermotolerant Pichia kudriavzevii strain. Annals of Microbiology, 6(2)2, 655-666 (2012).
- Sivaramakrishnan, S., Gangadharan, D., Narnpoothiri, K., Soccol, C., Pandey, A., Alpha amylase pro duction by Aspergillus oryzae employing solid-state fermentation. Journal of Scientific and In dustrial Research, 66(8), 621-626 (2007).
- Szendefy, J., Szakacs, G., Christopher, L., Potential of solid-state fermentation enzymes of Aspergil lus oryzae in biobleaching of paper pulp. Enzyme and Microbial Technology, 39(6), 1354-1360 (2006).
- Thomas, L., Larroche, C., Pandey, A., Current de velopments in solid-state fermentation. Biochemi cal Engineering Journal, 81, 146-161 (2013).
- Vitcosque, G. L., Fonseca, R. F., Rodríguez-Zuniga, U. F., Bertucci Neto, V., Couri, S., Farinas, C. S., Production of Biomass-degrading multienzyme complexes under solid-state fermentation of soy bean meal using a bioreactor. Enzyme Research, 9 (2012).
- Xu, H., Sun, L., Zhao, D., Zhang, B., Shi, Y., Wu, Y., Production of alpha-amylase by Aspergillus oryzae As 3951 in solid state fermentation using spent brewing grains as substrate. Journal of the Sci ence of Food and Agriculture, 88(3), 529-535 (2008).
- Zhang, X. Y., Mo, H., Zhang, J., Li, Z. H., A solid-state bioreactor coupled with forced aeration and pressure oscillation. Biotechnology Letters, 25(5), 417-420 (2003).
Publication Dates
-
Publication in this collection
Jan-Mar 2016
History
-
Received
25 May 2014 -
Reviewed
08 Dec 2014 -
Accepted
08 Feb 2015