Abstract
Osteonecrosis of the jaw is an adverse effect of bisphosphonates. While the etiopathogenesis of this condition has been investigated, the interactions and effects of bisphosphonates on oral mucosa cells remain unclear. It is hypothesized that cell culture models, such as co-culture or three-dimensional cell culture models, can provide valuable insight. Therefore, the aim of this study was to evaluate the effects of zoledronic acid (ZA) on epithelial cells and gingival fibroblasts in a co-culture model. Briefly, epithelial cells were seeded on transwell inserts and gingival fibroblasts were seeded in the lower well of 24-well plates. The latter were treated with ZA (5 μM) for 24 or 48 h. Cell viability and synthesis of the inflammatory chemokine, CCL2, were subsequently assessed. Data were subjected to statistical analysis with a 5% significance level. In the presence of ZA, the epithelial cells exhibited significant toxicity in both cell culture models and at both time points. However, greater cytotoxicity was observed in the co-culture model. Greater viability for the gingival fibroblasts was also associated with the co-culture model, and ZA-mediated toxicity was observed for the 48 h time point. ZA promoted a significant increase in CCL2 synthesis in both sets of cells, with greater CCL2 synthesis detected in the gingival fibroblasts. However, this effect was diminished in the co-culture model. Taken together, these results confirm the specific response patterns of the cells seeded in the co-culture model and also demonstrate the protective mechanism that is mediated by epithelial/mesenchymal cell interactions upon exposure to ZA.
Cell Culture Techniques; Epithelial Cells; Fibroblasts; Diphosphonates
Introduction
The etiopathogenesis of bisphosphonate-related oral osteonecrosis of the jaw (BRONJ) has not been completely elucidated; however, the toxic effects of this class of drugs on oral mucosal cells appears to play an important role.11. Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67(5 Suppl):61-70. doi:10.1016/j.joms.2009.01.007
https://doi.org/10.1016/j.joms.2009.01.0...
,22. Allen MR. Bisphosphonates and osteonecrosis of the jaw: moving from the bedside to the bench. Cells TissueS Organs. 2009;189(1-4):289-94. doi:10.1159/000151371
https://doi.org/10.1159/000151371...
In order to provide better care for patients receiving bisphosphonate treatment, the events related to BRONJ are important to elucidate.
Several studies have demonstrated that bisphosphonates are highly toxic to oral epithelial cells and gingival fibroblasts, as characterized by decreased cell viability and cell proliferation, and even cell death.33. Scheper MA, Badros A, Chaisuparat R, Cullen KJ, Meiller TF. Effect of zoledronic acid on oral fibroblasts and epithelial cells: a potential mechanism of bisphosphonate-associated osteonecrosis. Br J Haematol. 2009;144(5):667-76. doi:10.1111/j.1365-2141.2008.07504.x
https://doi.org/10.1111/j.1365-2141.2008...
,44. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
Decreased migration and reduced growth factor expression have also been observed, and these phenomena are directly related to cell proliferation and local neovascularization.55. Basso FG, Turrioni AP, Hebling J, Souza Costa CA. Zoledronic acid decreases gene expression of vascular endothelial growth factor and basic fibroblast growth factor by human epithelial cells. Br J Oral Maxillofac Surg. 2013;51(8):971-3. doi:10.1016/j.bjoms.2013.05.010
https://doi.org/10.1016/j.bjoms.2013.05....
It is possible that these effects may be related to the poor specificity of bisphosphonates for clastic cells, which could lead to nonspecific activity against oral mucosal cells and inhibition of the farnesil synthetase pathway. The latter is responsible for cell migration and proliferation control in several cell types, and has been shown to promote cell death when activated.66. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961-78. doi:10.1002/1097-0142 (20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L
https://doi.org/10.1002/1097-0142 (20000...
,77. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150-62. doi:10.1542/peds.2006-2023H
https://doi.org/10.1542/peds.2006-2023H...
,88. Ravosa MJ, Ning J, Liu Y, Stack MS. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch Oral Biol. 2011;56(5):491-8. doi:10.1016/j.archoralbio.2010.11.003
https://doi.org/10.1016/j.archoralbio.20...
,99. Saracino S, Canuto RA, Maggiora M, Oraldi M, Scoletta M, Ciuffreda L et al. Exposing human epithelial cells to zoledronic acid can mediate osteonecrosis of jaw: an in vitro model. Oral Pathol Med. 2012;41(10):788-92. doi:10.1111/j.1600-0714.2012.01173.x
https://doi.org/10.1111/j.1600-0714.2012...
While many studies have demonstrated the cytotoxic effects of bisphosphonates,44. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
,55. Basso FG, Turrioni AP, Hebling J, Souza Costa CA. Zoledronic acid decreases gene expression of vascular endothelial growth factor and basic fibroblast growth factor by human epithelial cells. Br J Oral Maxillofac Surg. 2013;51(8):971-3. doi:10.1016/j.bjoms.2013.05.010
https://doi.org/10.1016/j.bjoms.2013.05....
,66. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961-78. doi:10.1002/1097-0142 (20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L
https://doi.org/10.1002/1097-0142 (20000...
,1010. Basso FG, Pansani TN, Turrioni AP, Kurachi C, Bagnato VS, Hebling J et al. Biostimulatory effects of low level laser therapy on epithelial cells and gingival fibroblast treated with zoledronic acid. Laser Phys. 2013;23(5):055601-9. doi:10.1088/1054-660X/23/5/055601
https://doi.org/10.1088/1054-660X/23/5/0...
a great number of these studies were performed with monoculture cell models. However, oral mucosal homeostasis is directly related to interactions that occur between the oral epithelium and subjacent connective tissues, with both tissues able to be stimulated or regulated by the other.1111. Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24(1):127-52. doi:10.1034/j.1600-0757.2000.024001127.x
https://doi.org/10.1034/j.1600-0757.2000...
,1212. Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998-1008. doi:10.1038/sj.jid.5700786
https://doi.org/10.1038/sj.jid.5700786...
Moreover, during the healing of oral tissues, expression of growth factors by gingival fibroblasts has been found to promote the proliferation of oral epithelial cells.1111. Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24(1):127-52. doi:10.1034/j.1600-0757.2000.024001127.x
https://doi.org/10.1034/j.1600-0757.2000...
Additionally, epithelial wound closure has been found to be directly related to fibroblast proliferation and the expression of factors related to local vascularization.1212. Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998-1008. doi:10.1038/sj.jid.5700786
https://doi.org/10.1038/sj.jid.5700786...
Therefore, in vitro models that are able to represent the interactions between both cell lines in the same microenvironment are predicted to provide better insight into the behavior of these cells when they are exposed to various drugs, including bisphosphonates.1313. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112(12) 1843-53.,1414. Grøn B, Stoltze K, Andersson A, Dabelsteen E. Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS. 2002;110(12):892-8. doi:10.1034/j.1600-0463.2002.1101208.x
https://doi.org/10.1034/j.1600-0463.2002...
,1515. Berg E, Hsu YC, Lee JA. Consideration of the cellular microenvironment: physiologically relevant co-culture systems in drug discovery. Adv Drug Deliv Rev. 2014;69-70:190-204. doi:10.1016/j.addr.2014.01.013
https://doi.org/10.1016/j.addr.2014.01.0...
Thus, in the present study, the effects of ZA on epithelial cells and gingival fibroblasts in monolayer and co-culture models were evaluated and compared.
Methodology
Cell culture
Two human cell lineages, epithelial cells (HaCaT - CLS 300493) and gingival fibroblasts (HGF Ethics Committee of Piracicaba Dental School – UNICAMP # 64/99), were used. Both sets of cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, USA) containing 10% fetal bovine serum (FBS; Gibco). Two cell culture models were established: a monolayer cell culture model and a co-culture model. For the monolayer model, cells (epithelial cells or gingival fibroblasts) were seeded (1.5 x 1044. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
cells/cm22. Allen MR. Bisphosphonates and osteonecrosis of the jaw: moving from the bedside to the bench. Cells TissueS Organs. 2009;189(1-4):289-94. doi:10.1159/000151371
https://doi.org/10.1159/000151371...
/well) in 24-well plates. For the co-culture model, gingival fibroblasts were seeded as a monolayer (1.5 x 1044. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
cells/cm22. Allen MR. Bisphosphonates and osteonecrosis of the jaw: moving from the bedside to the bench. Cells TissueS Organs. 2009;189(1-4):289-94. doi:10.1159/000151371
https://doi.org/10.1159/000151371...
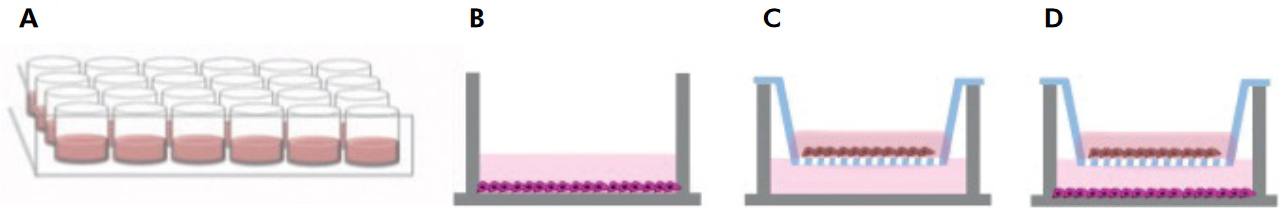
/well) in 24-well plates, and epithelial cells were cultured in transwell inserts (0.4 μm pores; Corning Inc., Lowell, USA) which were placed in the same wells containing the fibroblasts. Interactions between the two cell types involved paracrine signaling by macromolecules, thereby simulating the in vivo situation (Figure 1).1414. Grøn B, Stoltze K, Andersson A, Dabelsteen E. Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS. 2002;110(12):892-8. doi:10.1034/j.1600-0463.2002.1101208.x
https://doi.org/10.1034/j.1600-0463.2002...
The monolayer cell model was established as a control to represent the behavior of each cell line individually as opposed to in the co-culture system.
Schematic representation of the monolayer and co-culture models employed: (A) 24-well cell culture plate; (B) monolayer model for gingival fibroblasts; (C) monolayer model for epithelial cells; (D) co-culture model with epithelial cells plated on the insert and gingival cells in the lower well.
ZA treatment
After cells were grown for 48 h in DMEM/10% FBS, the cells were incubated for 24 h in serum-free DMEM. ZA (5-µM; Zometa 4 mg; Novartis Biociências S.A., São Paulo, Brazil) was then added to the serum-free DMEM and the cells were incubated for an additional 24 or 48 h. This drug concentration was selected based on a previous study by Scheper et al.,1616. Scheper MA, Badros A, Salama AR, Warburton G, Cullen KJ, Weikel DS, et al. A novel bioassay model to determine clinically significant bisphosphonate levels. Support Care Cancer. 2009;17(12):1553-7. doi:10.1007/s00520-009-0710-7
https://doi.org/10.1007/s00520-009-0710-...
where it was demonstrated that 5 µM ZA is present in the saliva and oral bone of patients under ZA treatment. This concentration has also been used in previous studies,44. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
,55. Basso FG, Turrioni AP, Hebling J, Souza Costa CA. Zoledronic acid decreases gene expression of vascular endothelial growth factor and basic fibroblast growth factor by human epithelial cells. Br J Oral Maxillofac Surg. 2013;51(8):971-3. doi:10.1016/j.bjoms.2013.05.010
https://doi.org/10.1016/j.bjoms.2013.05....
,1010. Basso FG, Pansani TN, Turrioni AP, Kurachi C, Bagnato VS, Hebling J et al. Biostimulatory effects of low level laser therapy on epithelial cells and gingival fibroblast treated with zoledronic acid. Laser Phys. 2013;23(5):055601-9. doi:10.1088/1054-660X/23/5/055601
https://doi.org/10.1088/1054-660X/23/5/0...
thereby facilitating a comparison of the results.
Cell viability
The effect of ZA treatment on the viability of epithelial cells and gingival fibroblasts seeded in either the monolayer or the co-culture model was determined by a MTT assay. This method determines the mitochondrial activity of viable cells based on cleavage of the MTT salt by a dehydrogenase succinic enzyme.44. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
For the monolayer cell cultures, the medium was removed and fresh culture medium supplemented with MTT salt (10%) was added. From each culture, 200 μL-aliquots were transferred to a 96-well plate. After 4 h, formazan crystals were dissolved in acid isopropilic ethanol and absorbance values at 570 nm were determined with a spectrophotometer (Synergy H1). For the co-culture model, the inserts containing seeded epithelial cells were transferred to a new cell culture plate and were subjected to the same steps as the monolayer culture.
Inflammatory chemokine synthesis
Expression of CCL2 was evaluated with an immunoenzymatic ELISA assay (R&D Systems, Inc., Minneapolis, USA). Standardized kits were used for this test, based on antigen-antibody detection, according to the manufacturer’s instructions.1717. Madureira DF, Taddei SA, Abreu MH, Pretti H, Lages EM, Silva TA. Kinetics of interleukin-6 and chemokine ligands 2 and 3 expression of periodontal tissues during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2012;142(4):494-500. doi:10.1016/j.ajodo.2012.05.012
https://doi.org/10.1016/j.ajodo.2012.05....
Briefly, 96-well ELISA plates were coated with a primary antibody overnight. The plates were then rinsed with wash solution and blocked with a 1% BSA solution for 1 h. After rinsing the wells, 100-μL aliquots of each sample were added to the plates and were incubated for 2 h at room temperature, then were incubated with the appropriate secondary antibodies (100 ng/mL) for an additional 2 h. Streptavidin solution was added to each sample and incubated for 20 min, followed by the addition of a reagent solution and a stop solution. The absorbance values at 455 nm for each sample were recorded by a spectrophotometer (Synergy). The concentration of CCL2 for each sample was determined according to a standard curve that was generated from known CCL2 concentrations.
Statistical analysis
The cell viability data exhibited normal distribution (Kolmogorov-Smirnov, p > 0.05) and homoscedasticity (Levene, p > 0.05). Therefore, a parametric two-way analysis of variance (ANOVA) was applied, complemented by Tukey’s test. In contrast, the CCL2 synthesis data did not exhibit normal distribution and the non-parametric Kruskal-Wallis test was applied, followed by the Mann-Whitney test. A P-value < 0.05 was considered statistically significant.
Results
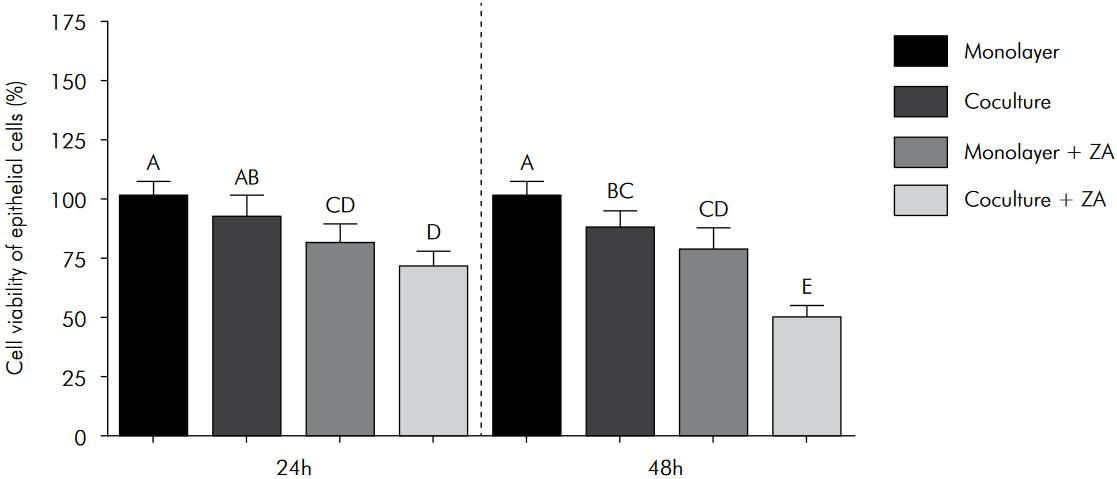
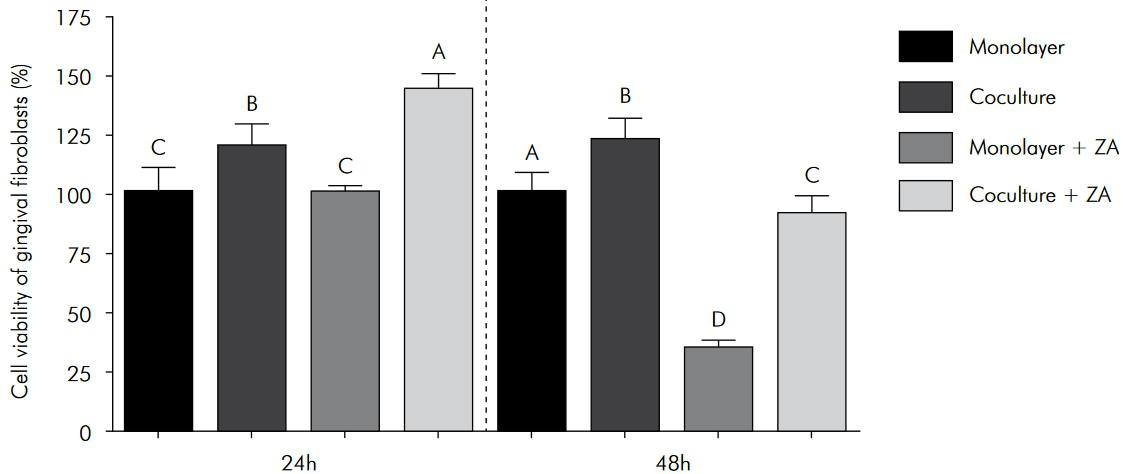
After 24 h, the viability of the epithelial cells in the monolayer and co-culture models was similar. However, after 48 h, greater viability was observed in the monolayer model. For the gingival fibroblasts, greater viability was detected in the co-culture model at both time points.
For both the monolayer and co-culture models, ZA treatment significantly decreased the viability of the epithelial cells at both the 24 h and 48 h time points. However, the toxic effect was greater in the co-culture model (Figure 2). Conversely, a significant increase in fibroblast viability was observed in both models following ZA treatment for 24 h, particularly in the co-culture model. However, after 48 h of ZA exposure, fibroblast viability significantly decreased, and this negative effect was greater in the monolayer model (Figure 3).
Viability of the epithelial cells that were exposed to ZA in the monolayer and co-culture models. The columns represent the mean values ± standard deviation. The different letter labels indicate statistically significant differences for each period (Tukey’s test, p < 0.05).
Viability of the gingival fibroblasts that were treated with ZA in both the monolayer and co-culture models. The columns represent the mean values ± standard deviation. The different letter labels indicate statistically significant differences for each period (Tukey’s test, p < 0.05).
Diverse patterns of CLL2 synthesis were observed for each cell line. For the gingival fibroblasts, greater synthesis of CLL2 was detected compared with the epithelial cells. However, when the gingival fibroblasts were included in the co-culture model, CCL2 synthesis decreased. ZA-treated cells also showed enhanced synthesis of CCL2, even in the co-culture model. However, decreased synthesis was observed for this model (Figure 4).
Synthesis of CCL2 by the gingival fibroblasts that were treated with ZA in both the monolayer and co-culture models. The boxplots represent the median (25th to 75th percentiles) values. The different letter labels indicate statistically significant differences for each period (Mann-Whitney, p < 0.05). *statistically significant difference between the groups at 24 h and 48 h.
Discussion
In previous in vivo studies and clinical trials, ZA treatment has inhibited the healing of oral mucosa and delayed wound closure.1818. Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66(5):839-47. doi:10.1016/j.joms.2008.01.026
https://doi.org/10.1016/j.joms.2008.01.0...
,1919. Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010;28(2):165-75. doi:10.1007/s00774-009-0128-9
https://doi.org/10.1007/s00774-009-0128-...
In vitro studies have further demonstrated that ZA induces marked toxicity against oral mucosa cells, including gingival fibroblasts and epithelial cells.44. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
,55. Basso FG, Turrioni AP, Hebling J, Souza Costa CA. Zoledronic acid decreases gene expression of vascular endothelial growth factor and basic fibroblast growth factor by human epithelial cells. Br J Oral Maxillofac Surg. 2013;51(8):971-3. doi:10.1016/j.bjoms.2013.05.010
https://doi.org/10.1016/j.bjoms.2013.05....
,66. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961-78. doi:10.1002/1097-0142 (20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L
https://doi.org/10.1002/1097-0142 (20000...
,77. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150-62. doi:10.1542/peds.2006-2023H
https://doi.org/10.1542/peds.2006-2023H...
,2020. Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318-20. doi:10.1016/j.bone.2007.04.196
https://doi.org/10.1016/j.bone.2007.04.1...
In addition a study by Werner et al.1212. Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998-1008. doi:10.1038/sj.jid.5700786
https://doi.org/10.1038/sj.jid.5700786...
showed that interactions between epithelial cells and connective tissues are directly related to successful healing. Therefore, in the present study, both co-culture and monolayer cell culture models were exposed to ZA, and the data were compared.
After 24 h and 48 h of ZA treatment, decreased cell viability was observed for both cell lines in the monolayer culture. These negative results were expected, since previous studies have demonstrated a similar cell response after ZA treatment.44. Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
https://doi.org/10.1590/0103-64402013022...
,55. Basso FG, Turrioni AP, Hebling J, Souza Costa CA. Zoledronic acid decreases gene expression of vascular endothelial growth factor and basic fibroblast growth factor by human epithelial cells. Br J Oral Maxillofac Surg. 2013;51(8):971-3. doi:10.1016/j.bjoms.2013.05.010
https://doi.org/10.1016/j.bjoms.2013.05....
,66. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961-78. doi:10.1002/1097-0142 (20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L
https://doi.org/10.1002/1097-0142 (20000...
,77. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150-62. doi:10.1542/peds.2006-2023H
https://doi.org/10.1542/peds.2006-2023H...
In the co-culture model, treatment with ZA also negatively affected the viability of the epithelial cells, although this affect appeared to protect the gingival fibroblasts since their viability was largely unaffected.
Interesting behavioral data were also observed for the co-cultured cells that were not exposed to ZA. For example, the gingival fibroblasts exhibited the highest levels of viability in the untreated co-culture model. These results, in combination with the results of previous studies,1313. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112(12) 1843-53.,1414. Grøn B, Stoltze K, Andersson A, Dabelsteen E. Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS. 2002;110(12):892-8. doi:10.1034/j.1600-0463.2002.1101208.x
https://doi.org/10.1034/j.1600-0463.2002...
,2121. Florin L, Maas-Szabowski N, Werner S, Szabowski A, Angel P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci. 2005;118(Pt 9):1981-9. doi:10.1242/jcs.02303
https://doi.org/10.1242/jcs.02303...
support the hypothesis that growth factors play an important role. It has also been observed that the co-culture model facilitates interactions between epithelial cells and mesenchymal cells, thereby providing a better environment for the expression of cell phenotypes.1313. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112(12) 1843-53. Despite the fact that the co-culture model used in this study did not allow for direct cell-cell interactions, paracrine communication via the secretion of soluble molecules such as growth factors and inflammatory chemokines/cytokines was possible.1313. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112(12) 1843-53.
Other studies have evaluated the expression of inflammatory cytokines by cells seeded in monolayer or co-culture models.1313. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112(12) 1843-53.,1414. Grøn B, Stoltze K, Andersson A, Dabelsteen E. Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS. 2002;110(12):892-8. doi:10.1034/j.1600-0463.2002.1101208.x
https://doi.org/10.1034/j.1600-0463.2002...
In these co-culture models, expression levels of inflammatory cytokines were up-regulated. In the present study, the co-culture model appeared to provide a protective interaction among the epithelial/mesenchymal cells, since decreased synthesis of CCL2 was detected. The aim of this study was to evaluate the effects of ZA on cells that play an important role in oral mucosal wound healing. Therefore, up-regulation of chemokine synthesis after ZA treatment may represent, at least in part, delayed wound healing as a result of this treatment.2222. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x
https://doi.org/10.1111/j.1524-475X.2008...
Correspondingly, the capacity for CCL2 to regulate alveolar bone resorption, which may enhance the solubilization of ZA from bone tissue, has previously been found to increase the exposure of oral mucosal cells to ZA.2323. Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86(4):306-19. doi:10.1177/154405910708600403
https://doi.org/10.1177/1544059107086004...
In the present study, higher expression of this chemokine was observed in the co-culture model compared with the epithelial cells that were seeded in the monolayer. This is probably due to the presence of gingival fibroblasts, which exhibited an increase in CCL2 expression in the monolayer model. In the co-culture model, ZA treatment enhanced the expression of CCL2, although the expression levels did not increase over time, and a positive effect was observed.
Conclusion
The results of this study confirm the different response patterns of cells seeded in the co-culture model and the protective mechanism provided by epithelial/mesenchymal cell interactions when these cells were exposed to ZA.
Acknowledgements
The authors acknowledge the São Paulo Research Foundation (FAPESP, grants: 2013/05879-0 and PD: 2012/17947-8) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant: 301029/2010-1) for their financial support.
References
-
1Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67(5 Suppl):61-70. doi:10.1016/j.joms.2009.01.007
» https://doi.org/10.1016/j.joms.2009.01.007 -
2Allen MR. Bisphosphonates and osteonecrosis of the jaw: moving from the bedside to the bench. Cells TissueS Organs. 2009;189(1-4):289-94. doi:10.1159/000151371
» https://doi.org/10.1159/000151371 -
3Scheper MA, Badros A, Chaisuparat R, Cullen KJ, Meiller TF. Effect of zoledronic acid on oral fibroblasts and epithelial cells: a potential mechanism of bisphosphonate-associated osteonecrosis. Br J Haematol. 2009;144(5):667-76. doi:10.1111/j.1365-2141.2008.07504.x
» https://doi.org/10.1111/j.1365-2141.2008.07504.x -
4Basso FG, Pansani TN, Oliveira CF, Turrioni AP, Soares DG, Hebling J et al. Cytotoxic effects of zoledronic acid on human epithelial cells and gingival fibroblasts. Braz Dent J. 2013;24 (6):551-8. doi:10.1590/0103-6440201302229
» https://doi.org/10.1590/0103-6440201302229 -
5Basso FG, Turrioni AP, Hebling J, Souza Costa CA. Zoledronic acid decreases gene expression of vascular endothelial growth factor and basic fibroblast growth factor by human epithelial cells. Br J Oral Maxillofac Surg. 2013;51(8):971-3. doi:10.1016/j.bjoms.2013.05.010
» https://doi.org/10.1016/j.bjoms.2013.05.010 -
6Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12 Suppl):2961-78. doi:10.1002/1097-0142 (20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L
-
7Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150-62. doi:10.1542/peds.2006-2023H
» https://doi.org/10.1542/peds.2006-2023H -
8Ravosa MJ, Ning J, Liu Y, Stack MS. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch Oral Biol. 2011;56(5):491-8. doi:10.1016/j.archoralbio.2010.11.003
» https://doi.org/10.1016/j.archoralbio.2010.11.003 -
9Saracino S, Canuto RA, Maggiora M, Oraldi M, Scoletta M, Ciuffreda L et al. Exposing human epithelial cells to zoledronic acid can mediate osteonecrosis of jaw: an in vitro model. Oral Pathol Med. 2012;41(10):788-92. doi:10.1111/j.1600-0714.2012.01173.x
» https://doi.org/10.1111/j.1600-0714.2012.01173.x -
10Basso FG, Pansani TN, Turrioni AP, Kurachi C, Bagnato VS, Hebling J et al. Biostimulatory effects of low level laser therapy on epithelial cells and gingival fibroblast treated with zoledronic acid. Laser Phys. 2013;23(5):055601-9. doi:10.1088/1054-660X/23/5/055601
» https://doi.org/10.1088/1054-660X/23/5/055601 -
11Häkkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24(1):127-52. doi:10.1034/j.1600-0757.2000.024001127.x
» https://doi.org/10.1034/j.1600-0757.2000.024001127.x -
12Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998-1008. doi:10.1038/sj.jid.5700786
» https://doi.org/10.1038/sj.jid.5700786 -
13Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112(12) 1843-53.
-
14Grøn B, Stoltze K, Andersson A, Dabelsteen E. Oral fibroblasts produce more HGF and KGF than skin fibroblasts in response to co-culture with keratinocytes. APMIS. 2002;110(12):892-8. doi:10.1034/j.1600-0463.2002.1101208.x
» https://doi.org/10.1034/j.1600-0463.2002.1101208.x -
15Berg E, Hsu YC, Lee JA. Consideration of the cellular microenvironment: physiologically relevant co-culture systems in drug discovery. Adv Drug Deliv Rev. 2014;69-70:190-204. doi:10.1016/j.addr.2014.01.013
» https://doi.org/10.1016/j.addr.2014.01.013 -
16Scheper MA, Badros A, Salama AR, Warburton G, Cullen KJ, Weikel DS, et al. A novel bioassay model to determine clinically significant bisphosphonate levels. Support Care Cancer. 2009;17(12):1553-7. doi:10.1007/s00520-009-0710-7
» https://doi.org/10.1007/s00520-009-0710-7 -
17Madureira DF, Taddei SA, Abreu MH, Pretti H, Lages EM, Silva TA. Kinetics of interleukin-6 and chemokine ligands 2 and 3 expression of periodontal tissues during orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2012;142(4):494-500. doi:10.1016/j.ajodo.2012.05.012
» https://doi.org/10.1016/j.ajodo.2012.05.012 -
18Landesberg R, Cozin M, Cremers S, Woo V, Kousteni S, Sinha S et al. Inhibition of oral mucosal cell wound healing by bisphosphonates. J Oral Maxillofac Surg. 2008;66(5):839-47. doi:10.1016/j.joms.2008.01.026
» https://doi.org/10.1016/j.joms.2008.01.026 -
19Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K et al. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010;28(2):165-75. doi:10.1007/s00774-009-0128-9
» https://doi.org/10.1007/s00774-009-0128-9 -
20Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318-20. doi:10.1016/j.bone.2007.04.196
» https://doi.org/10.1016/j.bone.2007.04.196 -
21Florin L, Maas-Szabowski N, Werner S, Szabowski A, Angel P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci. 2005;118(Pt 9):1981-9. doi:10.1242/jcs.02303
» https://doi.org/10.1242/jcs.02303 -
22Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x
» https://doi.org/10.1111/j.1524-475X.2008.00410.x -
23Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86(4):306-19. doi:10.1177/154405910708600403
» https://doi.org/10.1177/154405910708600403
Publication Dates
-
Publication in this collection
2016
History
-
Received
16 Dec 2015 -
Accepted
16 Aug 2016 -
Reviewed
8 Sept 2016