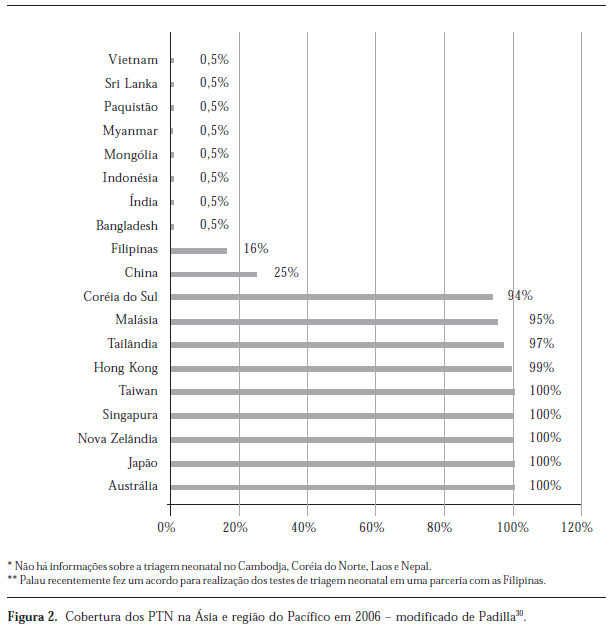
Newborn screening programs (NSP) aim to detect carriers of several congenital diseases among asymptomatic infants in order to warrant effective intervention. Specimen collection is the first step of a process that should be done in an universal and timely manner. A review of coverage and time of collection was done in NSP of several countries. The search was made in various sources, from 1998 to 2008, with "neonatal screening" and "coverage" as key words. The lack of a typical study design did not allow to the rigor required for a systematic review. Data were grouped in macro-regions. Canada had coverage of 71% in 2006 while the European coverage was of 69% in 2004, with data of 38 countries. In Asia and Pacific region, there were data of 19 countries. In Middle East and North Africa, there were data of 4 countries. In Latin America, the coverage was 49% in 2005, with data of 14 countries. In Brazil, coverage was 80%. Twelve reports had information about timeliness. The conclusion is that epidemiological transition has contributed to NSP success. Developed regions had more universal and timelier collection. In Brazil, government initiative increased access to the NSP, but late collections lead to the need of educational actions and participation of professional organizations in developing specific guidelines definition.
Neonatal screening; Coverage; Opportunity; Public Health Program