Abstract
The relationship among the phenotypes resistance to infection, virus replication in the brain and isotype production was investigated in genetically modified High (H) or Low (L) antibody responder mouse lines. Although they express the same innate susceptibility to rabies infection, these lines differ as to different viral replication rates in the central nervous system and L mice showed a higher permissible state. After intramuscular infection with the Pasteur rabies strain (PV), the H-L interline differences on the earlier stage of virus replication were 1000 and 80 folds on days 5 and 6, respectively. The isotype profile in sera of the experimentally infected mice reflected an interline difference of 25 folds for IgG2a throughout the infection period, and for the IgE production the H-L difference was highly significant only at the beginning of the process. These results confirm the multi-specific effect of antibody immune responsiveness and the general isotype distribution of antibodies in these genetically selected mice. Contrary to the clear correlation between antibody responsiveness and the acquired resistance to rabies infection, the present study demonstrates that the constitutive genetic character of High and Low responder individuals does not intervene in the degree of resistance following infection. Altogether, this study contributes to the knowledge of the protective role of the general innate responsiveness on the pathological pattern to rabies virus infection.
rabies; infection; isotypes; nervous system
ORIGINAL PAPER
Lack of correlation between rabies virus replication in the brain and antibody isotype profile in genetically modified mice
Consales C. A.I; Pereira C. A.II; Passos E. C.I; Carrieri M. L.I; Galina N. M. F.II; Sant'Anna O. A.II
IPasteur Institute, São Paulo, Brazil
IIButantan Institute, São Paulo, Brazil
Correspondence to Correspondence to: Cleide Aschenbrenner Consales Instituto Pasteur Av. Paulista 393 01311-000, São Paulo, Brasil Phone: +55 11 3288 0088 ext. 116 Email: cleide135@yahoo.com.br
ABSTRACT
The relationship among the phenotypes resistance to infection, virus replication in the brain and isotype production was investigated in genetically modified High (H) or Low (L) antibody responder mouse lines. Although they express the same innate susceptibility to rabies infection, these lines differ as to different viral replication rates in the central nervous system and L mice showed a higher permissible state. After intramuscular infection with the Pasteur rabies strain (PV), the H-L interline differences on the earlier stage of virus replication were 1000 and 80 folds on days 5 and 6, respectively. The isotype profile in sera of the experimentally infected mice reflected an interline difference of 25 folds for IgG2a throughout the infection period, and for the IgE production the H-L difference was highly significant only at the beginning of the process. These results confirm the multi-specific effect of antibody immune responsiveness and the general isotype distribution of antibodies in these genetically selected mice. Contrary to the clear correlation between antibody responsiveness and the acquired resistance to rabies infection, the present study demonstrates that the constitutive genetic character of High and Low responder individuals does not intervene in the degree of resistance following infection. Altogether, this study contributes to the knowledge of the protective role of the general innate responsiveness on the pathological pattern to rabies virus infection.
Key words: rabies, infection, isotypes, nervous system.
INTRODUCTION
Rabies is an acute lethal disease known for ages. Humans and animals are susceptible to the RNA Lyssavirus of the Rhabdoviridae family and due to the wide geographic distribution, the variety of population isolates and the diversity of mammalian reservoirs, rabies is still a serious public health problem. Susceptibility varies among wild species and the intervention of 2-4 loci determining the acquired resistance to rabies infection has been experimentally proved. Moreover, it must be considered that vaccines exert eventual selective pressure on the evolution of pathogenicity and influence the generation of new virus strains.
The distinct strains of rabies virus are strictly neurotropic pathogens. Experimental infection reveals that the virus initially remains next to the inoculation site, where it replicates, and the accumulation of mononuclear and other leukocytes is a secondary process developed during this early stage. Unless neutralized at this stage, the virus particles enter the peripheral nerves and reach the central nervous system (CNS) where they can be found at high concentrations with limited neuropathological events during the early phase. These events include CNS inflammation associated with gliosis reaction and perivascular lymphocytes infiltration (7, 17, 22, 30). Several specific or non-specific immunobiological mechanisms of defense have been suggested to be involved in the restriction of virus replication at different sites of infection, which may result in individual resistance. Among these, both T-cell mediated immune responses and B-derived antibody responsiveness are active mechanisms for the final protective state, and the level of soluble factors such as INF-g correlates with the degree of resistance (1, 8, 11, 14, 20).
Results of research developed in the last thirty years include demonstration of the fundamental participation of genetic factors in the regulation of the immune response and over the innate or acquired resistance to infections. Two broad types of genetic control were established: a) specific monogenic regulation of immune response related to genes often linked with the major histocompatibility complex [MHC] (5, 19), and b) polygenic control of quantitative innate and acquired immune responsiveness (3). To study the natural or acquired resistance against rabies, distinct animal models have been established allowing for the analysis and characterization of effective protection mechanisms. It was demonstrated that resistance to rabies infection is genetically controlled by at least two loci in isogenic mice (16, 18). In these inbred lines responsiveness to rabies vaccination and resistance to virus challenge were showed to be monogenic (28). However, isogenic mice were not selected for immunobiological characters and in these mouse strains susceptibility is presumably due to defective recessive alleles fixed by chance during selective cross.
Investigation of the genes controlling the specific acquired or innate immune mechanisms acting on the resistance to infection demonstrated that the major immunobiological phenotypes such as inflammatory responses, quantitative antibody production, immunological tolerance and T-cell mediated reactivity, in spite of their functional integration, are, at least in part, under the additive control of independent segregating loci (3, 9, 13). The resulting polymorphic regulation of immunity is the essential characteristic ensuring the survival of a genetically heterogeneous natural population. As pointed by Boyartchuk and Dietrich (4), the plasticity and redundancy of the immune system complicates the experimental study of these distinct components. The goal of genetic selection is to create mouse lines with extreme and easily differentiated phenotypes. If the selected genes modify the immune function, they can intervene in the responsiveness to toxins and infectious agents. Thus, the opposite capacity of the independent genetically modified mice to establish protective mechanisms reflects a dynamic relation between the gene frequency and the nature of immunogens or pathogens. Results of the factors responsible for the complex pleiotropic network of the immune system may be indicative of the main protective mechanisms of survival. Thus, the advantage of the genetically selected H and L mouse lines model in relation to inbred mice is the availability of individuals genetically homogeneous at the relevant loci controlling general characters, allowing for the study of the real biological signification of innate and/or acquired immune traits and the establishment of eventual correlation between distinct immunobiological parameters (26, 29). In fact, studies on distinct H and L lines of mice clearly demonstrated the positive correlation between neutralizing antibody responsiveness to rabies vaccination and resistance to infection (21) and it was proved that these traits are under polygenic control (10, 23). It was also demonstrated that differences in resistance were directly correlated with the intrinsic activities of their macrophages (8). Altogether, these studies suggest that innate and acquired resistance against rabies involve distinct genetic traits other than antibody responsiveness or inflammatory aptness, making evident the complexity of factors acting during the infective process (25).
In the present study the chosen analyzed phenotypes were resistance to infection and virus replication in the brain. The antibody isotype profile after experimental infection with the PV strain was also titrated, providing further data for the comprehension of the general phenomenon of the rabies virus pathogenesis. The analysis of these phenotypes conjointly with those already reported ascertained the complex mechanisms acting on resistance/susceptibility to rabies virus infection.
MATERIALS AND METHODS
Animals
HIII and LIII mice were from Selection III according to the maximal or minimal antibody responsiveness to flagellar antigens of Salmonellae (27). Adult males and females were used when 3 months old; they were obtained and maintained at the animal facilities of the Immunogenetics Laboratory of the Butantan Institute. All experiments were performed following the guidelines for animal use approved by the Ethics Committee in Animal Experimentation.
Virus and infection
In the experiment we used PV strain adapted to Vero cells culture with a titer of 104.9 LD50/0.03ml, being one mouse intracerebral 50% lethal dose equal to a titer of 104.9, as calculated by the method described by Reed and Muench (24).
HIII and LIII antibody responder lines were intramuscularly infected with 0.1 ml of a PV suspension and observed daily for mortality and mean survival time determinations. At different times after infection, two sets of experiments were executed: 1) Some infected animals expressing paralysis symptoms were sacrificed and the brain collected for evaluation of virus titration. This was measured following intracerebral inoculation into 14-16g Swiss mice, as described by Koprowsky (15), and expressed as LD50/0.03ml; 2) Groups of mice were bled from the retro-orbital venous plexus on days 3, 6, 12, 22 and 30 post infection.
Assay for rabies virus-specific Ab IgG isotypes
The individual antibody isotypes of IgG2a and IgE were measured by the ELISA test. Briefly, microplates (Nunc, MaxiScorp) were covered with 50µl of purified anti-mouse IgG2a monoclonal antibody [mAb] R19-15V at 8µg/ml (Pharmingen, USA), and 50µl of anti-mouse mAb R35-72 at 2µg/ml (Pharmingen) was added for capture. Plates were blocked with PBS/1%BSA/0.05%Tween. Individual double serum dilutions in ELISA buffer (PBS/0.1%BSA) were carried out including serum from uninfected animals as controls. After 18h at 4°C biotin-conjugated anti-mouse IgG2a mAb R19-15 and biotin-conjugated anti-IgE R35-72 were added. Reactions were developed with p-nitrophenyl phosphate (Sigma Chemical) in diethanolamine buffer. Absorbance was measured at l405nm and antibody isotype titers expressed as log2 of the reciprocal of the highest serum dilution at which the absorbance was equivalent to three times the background values (12).
RESULTS
Susceptibility to rabies infection
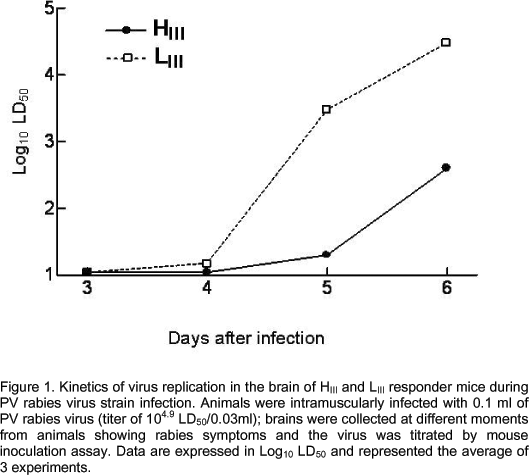
HIII and LIII mice were equally susceptible to the experimental rabies infection, showing 39 and 45 of mortality, respectively, and mean survival time of 12 days (Table 1). Nevertheless, as shown in Figure 1, LIII mice were more permissible to viral replication in the central nervous system than HIII. Titers as high as 103.5 and 104.3 LD50/0.03ml were found in brains of LIII mice on days 5 and 6 of infection, respectively, in comparison to 101.1 and 102.3 LD50/0.03ml found in brains of HIII mice on the same days. The HIII-LIII mice interline differences in virus replication were 1000 and 80 folds on days 5 and 6, respectively.
Isotype profiles
The isotype detected in sera of mice after different times of infection showed that HIII mice were capable of regularly producing a higher amount of IgG2a when compared to LIII mice (Figure 2). Interline differences of at least 25 folds were observed throughout the infection period. HIII mice were also capable of producing IgE in high amounts from the beginning to the end of the infection and LIII mice showed at the initial phase a quite low amount of IgE, which rose during a late period of the infection. Thus, interline differences for IgE syntheses were highly significant at the beginning of the infection only.
DISCUSSION
Rabies virus is highly neurotropic being extremely and selectively adapted to neurons. As clearly exposed by Baloul and Lafond (2), the degree of pathogenicity depends on the neuroinvasive capacity of the rabies virus strain. Thus, an attenuated virus may promote effective humoral and T-cell immune responses and would be eventually protector to subsequent challenge with a highly pathogenic strain. Several studies suggest that both innate and acquired resistance against rabies virus intrinsic neuroinvasive characteristics involve distinct genetic traits other than antibody responsiveness or inflammatory aptness, making evident the complexity of factors acting during the infective process (25). Recently, it was demonstrated that apoptosis is a relevant phenomenon related to the neuroinvasion and transmission of rabies virus within the CNS. Microglia are the tissue macrophages, the principal immune cells of the CNS. The activation of microglial cells is controlled by cytokines such as IFN-g, a particularly potent activator of microglia that can up-regulate MHC classe II expression, phagocytosis and production of different cytokines. Virus neuroinvasion might be controlled if microglia and activated T cells eliminate the infected neurons (2, 6).
The present results indicate lack of correlation between serum neutralizing antibody responses following infection and resistance to rabies in genetically modified mice selected according to the maximal [HIII line] or minimal [LIII line] antibody responsiveness. Previous and pioneer studies on distinct H and L lines of mice demonstrated the positive correlation between acquired resistance to infection and neutralizing antibody responsiveness to rabies virus (21) and it was proved that these traits are under polygenic control (10, 23). It was also demonstrated that differences in resistance were directly correlated with the intrinsic activities of macrophages and the antiviral effect mediated by IFN-g (8). Although HIII and LIII mice showed a quite different magnitude of virus replication in the brain as well as a distinct capacity to synthesize IgG2a or IgE, the resistance degree against experimental rabies infection was comparable. The antibody isotype pattern observed in infected mice may reflect a distinct capability of these animals to mount a cell immune response, which in turn could be more or less effective in neutralizing the virus in the brain. The possibility that the higher virus replication in the brain of LIII mice is compensated by a greater capability of these animals to mount a more efficient cell immune response can not be excluded, and may explain the final comparable resistance of both mouse lines to the rabies infection.
These results confirm that high and low responsiveness is not restricted to the selection immunogens but is inclusive for distinct antigen specificities and antibody isotypes. They also indicate that this multi-specific trait could be likewise extensive for the adaptive immune response during an infectious process. Finally, the analysis of the three described phenotypes conjointly with those previously reported (8, 10, 18, 28) contribute to the understanding of the complex mechanisms acting on the protective or immunopathological states following rabies virus infection or immunization.
ACKNOWLEDGEMENTS
The authors thank Maria das Graças Silva for her help with the manuscript. This work was supported in part by grants from the Pasteur Institute, São Paulo; FAPESP; and Butantan Foundation. Carlos Augusto Pereira and Osvaldo Augusto Sant'Anna are researchers of The National Council for Scientific and Technological Development (CNPq).
Received: November 25, 2005
Accepted: January 23, 2006
Abstract published online: January 31, 2006
Full paper published online: August 31, 2006
- 1 BAER GM., CLEARY WF., DIAZ AM., PERL DF. Characteristics of 11 rabies virus isolates in mice: titers and relative invasiveness of virus, incubation period of infection, and survival of mice with sequelae. J. Infect. Dis., 1977, 136, 336-45.
- 2 BALOUL L., LAFON M. Apoptosis and rabies virus neuroinvasion. Biochimie, 2003, 85, 777-88.
- 3 BIOZZI G., MOUTON D., SANT'ANNA OA., PASSOS HC., GENNARI M., REIS MH., FERREIRA VC., HEUMANN AM., BOUTHILLIER Y., IBAÑEZ OM., STIFFEL C., SIQUEIRA M. Genetics of immunoresponsiveness to natural antigens in the mouse. Curr. Top. Microbiol. Immunol, 1979, 85, 31-98.
- 4 BOYARTCHUK V., DIETRICH W. Genetic dissection of host immune response.Genes Immun, 2002, 3, 119-22.
- 5 BRILES DE., BENJAMIN Jr. WH., HUSTER WJ., POSEY B. Genetic approaches to the study of disease resistance: with special emphasis on the use of recombinant inbred mice. Curr. Top. Microbiol. Immunol., 1986, 124, 21-35.
- 6 CHAN A., MAGNUS T., GOLD R. Phagocytosis of apoptotic inflammatory cells by microglia and modulation by different cytokines: mechanism for removal of apoptotic cells in the inflamed nervous system. Glia, 2001, 33, 87-95.
- 7 CHARLTON KM. The pathogenesis of rabies. In: CAMPBELL JB., CHARLTON KM. Eds. Rabies Boston: Kluwer, 1988: 102-50.
- 8 CONSALES CA., MENDONÇA RZ., LUCCHIARI MA., VASSÃO RC., PEREIRA CA. Macrophage activity in rabies virus infection of genetically selected high and low antibody responder lines of mice. Res. Virol., 1990, 141, 57-67.
- 9 DA SILVA AC., DE SOUZA KW., MACHADO RC., DA SILVA MF., SANT'ANNA OA. Genetics of immunological tolerance: I. Bidirectional selective breeding of mice for oral tolerance. Res. Immunol., 1998, 149, 151-61.
- 10 DE FRANCO M., MASSA S., VASSÃO RC., SIQUEIRA M., SANT'ANNA OA. Polygenic control of antibody production and correlation with vaccine induced resistance to rabies virus in high and low antibody responder mice. Arch. Virol., 1996, 141, 1397-406.
- 11 FU ZF. Rabies and rabies research: past, present and future. Vaccine, 1997, 15, suppl. S20-4.
- 12 GUESDON JL., TERNYNCK T., AVRAMEAS S. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cytochem, 1979, 27, 1131-9.
- 13 IBAÑEZ OM., STIFFEL C., RIBEIRO OG., CABRERA WK., MASSA S., DE FRANCO M., SANT'ANNA OA., DECREUSEFOND C., MOUTON D., SIQUEIRA M., BIOZZI G. Genetics of nonspecific immunity: I. Bidirectional selective breeding of lines of mice endowed with maximal or minimal inflammatory responsiveness. Eur. J. Immunol, 1992, 22, 2555-63.
- 14 KAWANO H., MIFUNE K., OHUCHI M., MANNEN K., CHO S., HIRAMATSU K., SHICHIJO A. Protection against rabies in mice by a cytotoxic T cell clone recognizing the glycoprotein of rabies virus. J. Gen. Virol, 1990, 71, 281-7.
- 15 KOPROWSKI H. The mouse inoculation test. In: MESLIN FX., KAPLAN MM., KOPROWSKI H. Eds. Laboratory techniques in rabies 4.ed. Geneva: World Health Organization, 1996: 80-6.
- 16 LODMELL DL., CHESEBRO B. Murine resistance to street rabies virus: genetic analysis by testing second-backcross progeny and verification of allelic resistance genes in SJL/J and CBA/J mice. J. Virol., 1984, 50, 359-62.
- 17 LODMELL DL., ESPOSITO JJ., EWALT LC. Rabies virus antinucleoprotein antibody protects against rabies virus challenge in vivo and inhibits rabies virus replication in vitro. J. Virol., 1993, 67, 6080-6.
- 18 LODMELL DL., EWALT LC. Pathogenesis of street rabies virus infections in resistant and susceptible strain of mice. J. Virol, 1985, 55, 788-95.
- 19 MCDEVITT H. The discovery of linkage between the MHC and genetic control of the immune response. Immunol. Rev., 2002, 185, 78-85.
- 20 MENDONÇA RZ., PEREIRA CA. Relationship of interferon synthesis and the resistance of mice infected with street rabies virus. Braz. J. Med. Biol. Res., 1994, 27, 691-5.
- 21 NILSSON MR., SANT'ANNA OA., SIQUEIRA M., NILSSON TT., GENNARI M. Rabies virus immunity in genetically selected high and low responder lines of mice. Infect. Immun., 1979, 25, 23-6.
- 22 PLOTKIN SA. Rabies. Clin. Infect. Dis., 2000, 30, 4-12.
- 23 QUEIROZ-DA-SILVA LH., DE-FRANCO M., SANT'ANNA OA. Rabies infection and specific effect of vaccination in mice selected for high and low immunobiological parameters. Braz. J. Med. Biol. Res., 1997, 30, 1309-13.
- 24 REED LJ., MÜENCH H. A simple method of estimating fifty percent end points. Am. J. Hyg., 1938, 27, 493-7.
- 25 SANT'ANNA OA., MOUTON D., BIOZZI G. Multigenic control of specific and non-specific immunity in mice. A review. Livest. Prod. Sci., 1988, 20, 277-86.
- 26 SANT'ANNA OA., MOUTON D., IBAÑEZ OM., BOUTHILLIER Y., MEVEL JC., REIS MH., BIOZZI G. Basal immunoglobulin serum concentration and isotype distribution in relation to the polygenic control of antibody responsiveness in mice. Immunogenetics, 1985, 22, 131-9.
- 27 SIQUEIRA M., BANDIERI A., REIS MS., SANT'ANNA OA., BIOZZI G. Selective breeding of mice for antibody responsiveness to flagellar and somatic antigens of Salmonellae. Eur. J. Immunol, 1976, 6, 241-9.
- 28 TEMPLETON JW., HOLMBERG C., GARBER T., SHARP RM. Genetic control of serum neutralizing-antibody response to rabies vaccination and survival after a rabies challenge infection in mice. J. Virol., 1986, 59, 98-102.
- 29 TREZENA AG., SOUZA CM., BORREGO A., MASSA S., SIQUEIRA M., DE FRANCO M., SANT'ANNA OA. Co-localization of quantitative trait loci regulating resistance to Salmonella typhimurium infection and specific antibody production phenotypes. Microbes Infect., 2002, 4, 1409-15.
- 30 TSIANG H. Pathophysiology of rabies virus infection of the nervous system. Adv. Virus Res, 1993, 42, 375-412.
Correspondence to:
Publication Dates
-
Publication in this collection
19 Sept 2006 -
Date of issue
2006
History
-
Received
25 Nov 2005 -
Accepted
23 Jan 2006