Abstract
A rapid decrease in parasitaemia remains the major goal for new antimalarial drugs and thus, in vivo models must provide precise results concerning parasitaemia modulation. Hydroxyethylamine comprise an important group of alkanolamine compounds that exhibit pharmacological properties as proteases inhibitors that has already been proposed as a new class of antimalarial drugs. Herein, it was tested the antimalarial property of new nine different hydroxyethylamine derivatives using the green fluorescent protein (GFP)-expressing Plasmodium berghei strain. By comparing flow cytometry and microscopic analysis to evaluate parasitaemia recrudescence, it was observed that flow cytometry was a more sensitive methodology. The nine hydroxyethylamine derivatives were obtained by inserting one of the following radical in the para position: H, 4Cl, 4-Br, 4-F, 4-CH3, 4-OCH3, 4-NO2, 4-NH2 and 3-Br. The antimalarial test showed that the compound that received the methyl group (4-CH3) inhibited 70% of parasite growth. Our results suggest that GFP-transfected P. berghei is a useful tool to study the recrudescence of novel antimalarial drugs through parasitaemia examination by flow cytometry. Furthermore, it was demonstrated that the insertion of a methyl group at the para position of the sulfonamide ring appears to be critical for the antimalarial activity of this class of compounds.
experimental malaria; novel antimalarial drugs; hydroxyethylamine
Malaria is the most relevant parasitic disease and, despite the many efforts made to eradicate malaria, the disease still accounts for 0.5 million deaths per year globally (WHO 2015WHO - World Health Organization 2015. World malaria report 2014. Available from: who.int/malaria/publications/world_malaria_report_2014/en/.
who.int/malaria/publications/world_malar...
). In Brazil, despite the number of cases has been decreasing, it still accounts for 177,767 cases in 2013 (de Pina-Costa et al. 2014de Pina-Costa A, Brasil P, Di Santi SM, de Araujo MP, Suárez-Mutis MC, Santelli ACFS, Oliveira-Ferreira J, Lourenço-de-Oliveira R, Daniel-Ribeiro CT 2014. Malaria in Brazil: what happens outside the Amazonian endemic region. Mem Inst Oswaldo Cruz 109: 618-633., WHO 2015WHO - World Health Organization 2015. World malaria report 2014. Available from: who.int/malaria/publications/world_malaria_report_2014/en/.
who.int/malaria/publications/world_malar...
). The current antimalarial treatment recommended by World Health Organization (WHO) is artemisinin-based combination therapy because of artemisinin’s efficacy and ability to lower the rate at which resistance emerges (WHO 2010WHO - World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed., WHO, Geneva, 194 pp.). However, several cases of resistance to artemisinin derivatives have been observed, first at the Cambodia-Thailand border (Dondorp et al. 2010Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L 2010. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8: 272-280.) and now spread across Southeast Asia (Ashley et al. 2014Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371: 411-423.). Such a scenario compels the discovery of novel antimalarial drugs. Several approaches have been used in antimalarial drug discovery, including the use of drugs that prevent transmission or new infection, stop relapse or can be used in cases of uncomplicated and severe malaria (Aguiar et al. 2012aAguiar ACC, da Rocha EMM, de Souza NB, França TCC, Krettli AU 2012a. New approaches in antimalarial drug discovery and development - A Review. Mem Inst Oswaldo Cruz 107: 831-845., Anthony et al. 2012Anthony MP, Burrows JN, Duparc S, Moehrle J, Wells TN 2012. The global pipeline of new medicines for the control and elimination of malaria. Malar J 11: 316-341.). However, a rapid decrease in parasitaemia remains the major goal for new drugs (Burrows et al. 2013Burrows JN, van Huijsduijnen RH, Möhrle JJ, Oeuvray C, Wells TN 2013. Designing the next generation of medicines for malaria control and eradication. Malar J 12: 187-207.).
The biological activities of hydroxyethylamine core have been extensively studied. Hydroxyethylamines have been described as human immunodeficiency virus (HIV) protease inhibitors (Ghosh et al. 2014Ghosh AK, Schiltz GE, Rusere LN, Osswald HL, Walters DE, Amano M, Mitsuya H 2014. Design and synthesis of potent macrocyclic HIV-1 protease inhibitors involving P1-P2 ligands. Org Biomol Chem 12: 6842-6854.) and, over the last several years, this class have been studied for their antimalarial activity (de Souza et al. 2012de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272.). The antimalarial mechanism of action of hydroxyethylamines comprises the selective inhibition of plasmodium proteases such as falcipain and plasmepsin without interfering with human proteases (Muthas et al. 2005Muthas D, Noteberg D, Sabnis YA, Hamelink E, Vrang L, Samuelsson B, Karlén A, Hallberg A 2005. Synthesis, biological evaluation and modeling studies of inhibitors aimed at the malarial proteases plasmepsins I and II. Bioorg Med Chem 13: 5371-5390., Rathi et al. 2013Rathi B, Singh AK, Kishan R, Singh N, Latha N, Srinivasan S, Pandey KC, Tiwari HK, Singh BK 2013. Functionalized hydroxyethylamine based peptide nanostructures as potential inhibitors of falcipain-3, an essential proteases of Plasmodium falciparum. Bioorg Med Chem 21: 5503-5509.). Indeed, the study of hydroxyethylamine derivatives as a new class of antimalarial drugs could represent a safe antimalarial drug. Recently, it was demonstrated that the insertion of a ciclohexyl group in hydroxyethylamine core synthesised from alkylamines increase the antimalarial of such molecule (de Souza et al. 2012de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272.).
Herein, it was tested newly synthesised nine different hydroxyethylamine derived from ring-opening of the (2S,3S)-Boc-phenylalanine epoxide with benzylamine in refluxing isopropanol, according its antimalarial activity using the mouse in vivo model of infection with green fluorescent protein-expressing Plasmodium berghei (PbGFP).
MATERIALS AND METHODS
Ethics statement - This work was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on Ethical Use of Laboratory Animals of the Oswaldo Cruz Foundation (Fiocruz) (Rio de Janeiro, Brazil) (permit LW52/12).
Mice and the model of infection - C57BL/6 mice (4-5 weeks old) were provided by the Fiocruz breeding unit and caged with free access to food and fresh water in a room at the Farmanguinhos experimental facility, with a temperature ranging from 22-24ºC and a 12 h light/dark cycle, until use.
For the nontransfected and PbGFP ANKA infection [GFPcon 259cl2 was kindly provided by Dr L Carvalho (Fiocruz) and is a donations from the Malaria Research and Reference Reagent Resource Center - MR4, deposited by CJ Janse and AP Waters (MRA-865)], the mice were intraperitoneally (i.p.) inoculated with 5 x 106 P. berghei-parasitised red blood cells withdrawn from a previously infected mouse. Artesunate, chloroquine or primaquine was orally administered to mice on the third day of infection (100 mg/kg, diluted in 10% ethanol and 90% propylene glycol; Farmanguinhos). For the evaluation of survival rate, lethality was registered every day until day 14 post-infection. Mice were euthanised by an i.p. injection with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) prior to pentobarbital (150 mg/kg).
Parasitaemia evaluation - At the indicated time points after infection, a thin blood smear was performed for parasitaemia determination by Diff-Quick staining. The determination of parasitaemia by microscopy was performed by counting five fields of approximately 200 erythrocytes per field. To evaluate parasitaemia by flow cytometry, 4 µL of blood was resuspended in 500 µL of phosphate buffered saline/0.1% azide and the cell suspension was immediately submitted to flow cytometry (FACSCalibur, BD Biosciences), as described (Franke-Fayard et al. 2004Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ 2004. A Plasmodium bergheireference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol 137: 23-33.). Forward scatter and side scatter were set to gate the total erythrocytes and the percentage of PbGFP-infected erythrocytes was determined by fluorescence intensity. At least 10,000 events were acquired in the gate. The data analyses were performed using CellQuest software (BD Immunocytometry Systems, USA).
Antimalarial activity of hydroxyethylamine derivatives - The target compounds 5a-i were obtained as previously described (Facchinetti et al. 2014Facchinetti V, Gomes CRB, de Souza MVN, Vasconcelos TRA, Wardell S, Wardell JL 2014. Solvates of two ethyl 6-(2-(aryl)-4-oxothiazolidin-3-yl)-1-ethyl-4-oxo-1,4-dihydroquinoline-3- carboxylates. J Chem Crystallogr 44: 471-479., Moreth et al. 2014Moreth M, Gomes CR, Lourenço MC, Soares RP, Rocha MN, Kaiser CR, de Souza MV, Wardell SM, Wardell JL 2014. Syntheses and antimycobacterial activities of (2S,3R)-2-(amino)-4-(arenesulfonamido)-3-hydroxy-1-phenylbutane derivatives. Med Chem 10: 189-200.). To evaluate the in vivo antimalarial efficacy of hydroxyethylamine derivatives, the PbGFP four-day suppressive test was used (Fidock et al. 2004Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3: 509-520.). Two hours after infection with PbGFP, mice were randomly assigned to 11 groups: nontreated (vehicle, 200 μL i.p.), artesunate treated [10 mg/kg/day diluted in 5% dimethyl sulfoxide (DMSO)] and a group for each hydroxyethylamine derivatives (5a-i; 10 mg/kg/day diluted in 5% DMSO). Mice were treated daily up to day 4 after infection when parasitaemia determination was performed by flow cytometry. The results are expressed as drug activity as described previously (Fidock et al. 2004Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3: 509-520.). The difference between the mean value of the control group (taken as 100%) and that of the experimental groups was calculated and expressed as percent reduction (= activity) using the following equation: activity = 100 - [(mean parasitaemia treated/mean parasitaemia control) x 100].
Statistical analysis - A log-rank (Mantel-Cox) test was used to compare the percentages of survival and the significance level was set at p < 0.05. The correlation coefficient and Bland-Altman limit were calculated. Additional statistical significance was assessed using ANOVA followed by the Newman-Keuls t test. The results are expressed as the mean ± standard error of the means and the significance level in all cases was set at p < 0.05.
RESULTS
Comparison of recrudescence test using Pb and PbGFP-infected mice - In view of the importance to observe the rapid decrease of parasitaemia after antimalarial treatment, it was first compared two methodologies used to the test of new antimalarial drugs (Aguiar et al. 2012b, de Souza et al. 2012de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272.). It was observed that Pb and PbGFP-infected mice exhibited similar survival curves (p = 1.00) (Fig. 1A). In addition, the parasitaemia in the Pb and PbGFP groups, as counted by microscopy, was not statistically different and increased up to day 6 post-infection (Fig. 1B). PbGFP-infected erythrocytes were further counted by flow cytometry and it was observed increased levels of parasitaemia up to day 6 post-infection (Fig. 1C). A positive correlation was observed between the parasitaemia counted by microscopy from Pb and PbGFP-infected mice (Fig. 2A). In addition, the evaluation of parasitaemia from PbGFP-infected mice analysed by microscopy or by flow cytometry revealed a significant positive linear correlation (p = 0.006) (Fig. 2B). To confirm that two different methodologies would infer the same result, it was performed a Bland-Altman analysis that also indicated that the evaluation of parasitaemia by cytometry and by microscopy are equivalent (bias = 0.1%; 95% limit of agreement = 4.2%) (Fig. 2C).
: survival rates for C57BL/6 mice infected with Plasmodium berghei (Pb) (solid line) or green fluorescent protein-expressing Pb (PbGFP) (dashed line). The log-rank test revealed no differences in the survival curves when the Pb-infected (n = 10) and PbGFP-infected C57BL/6 mice (n = 10) were compared. Evolution of parasitaemia in Pb (black symbols) or PbGFP-infected (white symbols) mice measured by microscopy (B) or cytometry (C). The results are expressed as the mean ± standard deviation from at least six animals per group in two different experiments. Gating strategy used to isolate total red blood cells (RBCs) based on forward scatter (FSC) and side scatter (SSC), and representative dot-plots demonstrate the increase in fluorescence, as indicated by an increase in GFP expression in the RBCs is shown in C.
: correlation analyses of parasitaemia estimated by microscopy and cytometry. A: correlation between parasitaemia in mice infected with Plasmodium berghei (Pb) or green fluorescent protein-expressing Pb (PbGFP) evaluated by microscopy; B: correlation between parasitaemia in PbGFP-infected mice evaluated by microscopy and cytometry; C: Bland-Altman plot representing the bias (0.1%) and 95% limit of agreement (4.2%) for the parasitaemia evaluation.
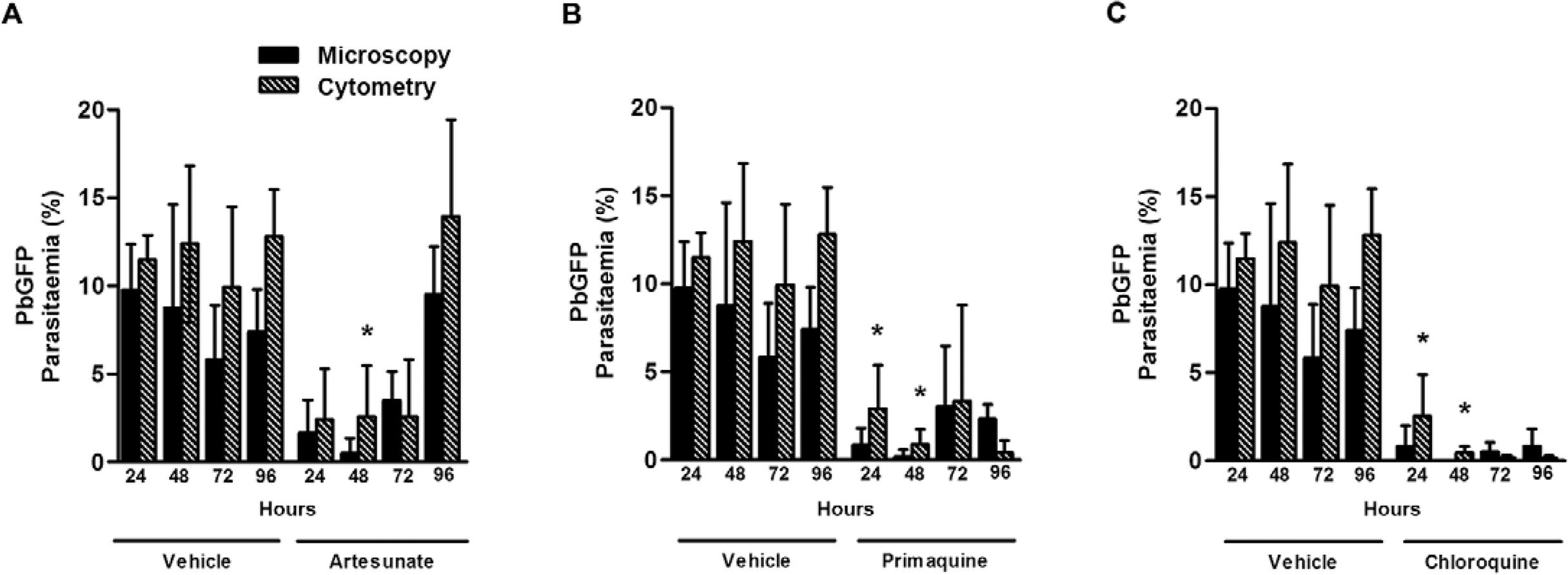
Concerning recrudescence studies, up to 48 h after treatment with chloroquine, no infected erythrocytes were found in the blood smears obtained from treated mice (Fig. 3A-C, respectively). However, using flow cytometry, an increase in the parasitaemia of treated mice was observed, especially in mice treated with primaquine or chloroquine. At 72 h and 96 h after treatment, infected erythrocytes were observed in the blood smears at the same extent observed by flow cytometry.
: evaluation of recrudescence after treatment with antimalarial drugs. Mice were infected with green fluorescent protein-expressing Plasmodium berghei(PbGFP) and treated with artesunate (A), chloroquine (B) or primaquine (C) at day 3 post-infection and parasitaemia was evaluated up to 96 h after treatment. Parasitaemia was evaluated by microscopy (black bars) and cytometry (hatched bars). The results are expressed as the mean ± standard deviaton from at least six animals per group in two different experiments. Statistically significant differences compared to the group evaluated by microscopy (p < 0.05) are indicated by an asterisk.
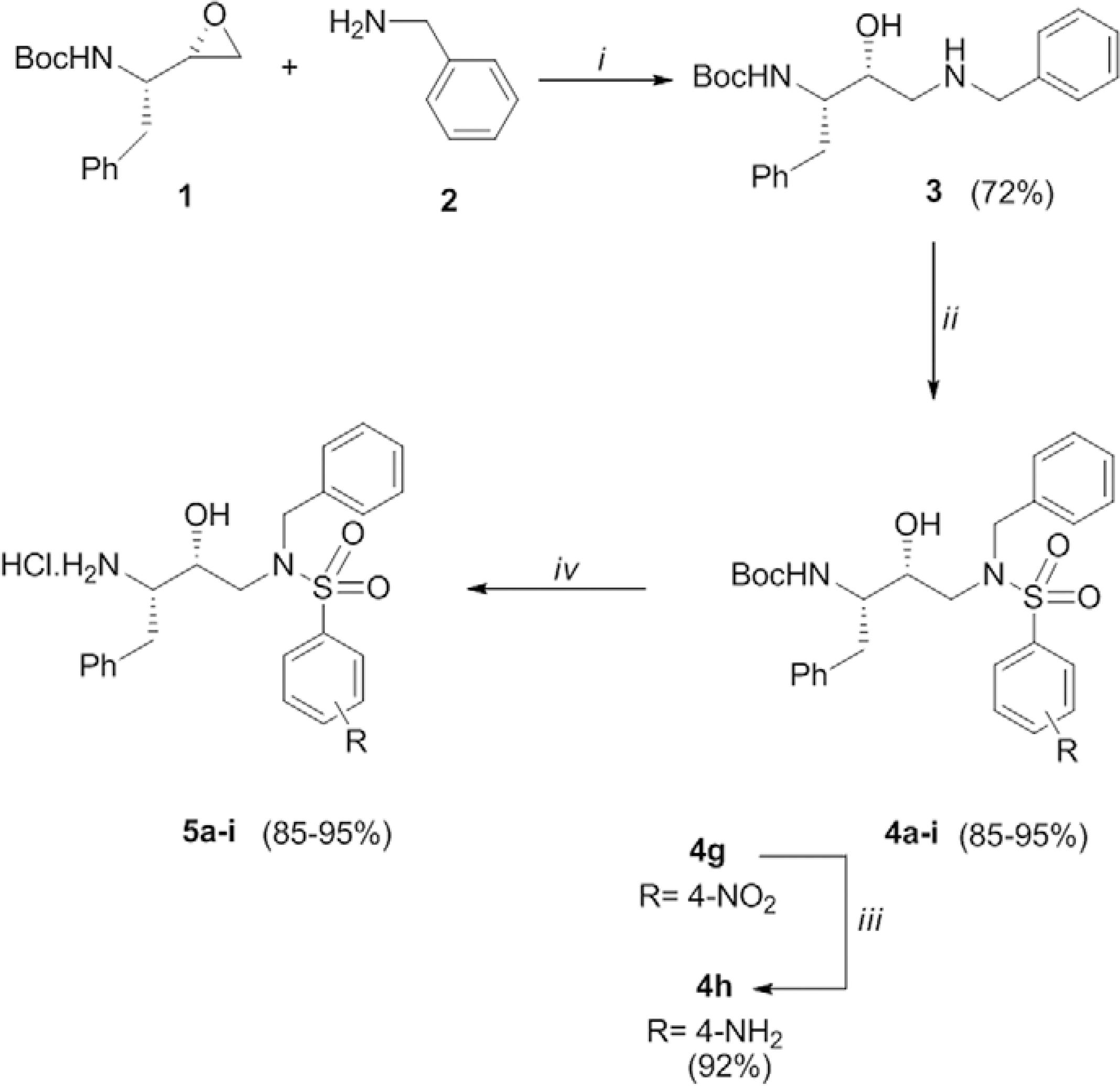
Antimalarial activity of hydroxyethylamine derivatives - Because PbGFP is an effective model to study antimalarial drugs, PbGFP-infected mice were treated with nine hydroxyethylamine derivatives ((2S,3R)-2-(amino)-[4-(N-benzylarenesulfonamido)-3-hydroxy-1-phenylbutane). The preparation of the target compounds 5a-i (Fig. 4) has been previously described (Facchinetti et al. 2014Facchinetti V, Gomes CRB, de Souza MVN, Vasconcelos TRA, Wardell S, Wardell JL 2014. Solvates of two ethyl 6-(2-(aryl)-4-oxothiazolidin-3-yl)-1-ethyl-4-oxo-1,4-dihydroquinoline-3- carboxylates. J Chem Crystallogr 44: 471-479., Moreth et al. 2014Moreth M, Gomes CR, Lourenço MC, Soares RP, Rocha MN, Kaiser CR, de Souza MV, Wardell SM, Wardell JL 2014. Syntheses and antimycobacterial activities of (2S,3R)-2-(amino)-4-(arenesulfonamido)-3-hydroxy-1-phenylbutane derivatives. Med Chem 10: 189-200.).
: reaction and conditions (i: isopropanol, reflux, 16 h; ii: Et3N, DMF, RC6H4SO2Cl, CH2Cl2, r.t., 4 h; iii: H2, Pd/C 10%, EtOH, r.t., 16 h; iv: HCl gas, EtOH, r.t., 4 h).
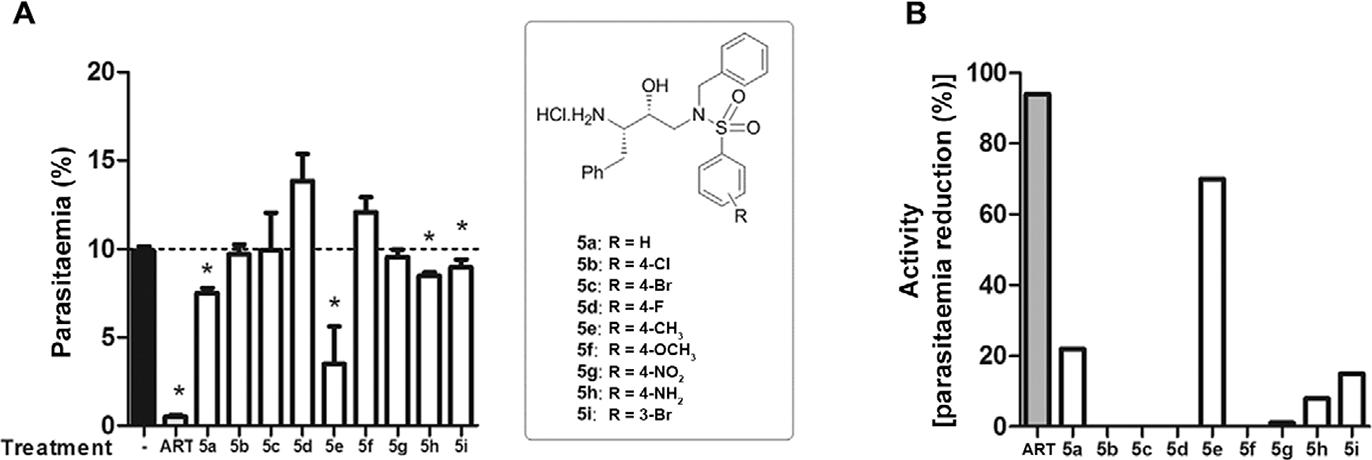
Of the nine tested compounds, 5a and 5e showed antimalarial activity. Compound 5e was able to reduce 70% of the parasitaemia and was the most active substance of this series (Fig. 5).
: antimalarial activity of the hydroxyethylamine derivatives. The mice were treated daily with artesunate (ART) or derivatives (10 mg/kg/day; intraperitoneally). Drug activity was evaluated at day 4 after infection and is expressed as (A) parasitaemia levels or as (B) activity, according the following equation: activity = 100 - [(mean parasitaemia treated/mean parasitaemia control) x 100]. It was used at least six animals per group in two different experiments.
DISCUSSION
Herein, it was proposed the study of newly synthesised hydroxyethylamine derivatives as antimalarial compounds using PbGFP. Furthermore, it was observed that parasitaemia evaluation by flow cytometry reveal low parasitaemia levels, which is not observed by microscopic analysis.
As described before, according the Medicine for Malaria Venture, the “ideal” candidate profile of an antimalarial drug is whom account for fast parasite clearance over 48 h after treatment (Burrows et al. 2013Burrows JN, van Huijsduijnen RH, Möhrle JJ, Oeuvray C, Wells TN 2013. Designing the next generation of medicines for malaria control and eradication. Malar J 12: 187-207.). In such way, it is important to use techniques for parasite evaluation that lead to accurate results. The construction of PbGFP was performed by Franke-Fayard et al. (2004)Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ 2004. A Plasmodium bergheireference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol 137: 23-33. and has been used in a wide range of studies (Sultan et al. 1999Sultan AA, Thathy V, Nussenzweig V, Menard R 1999. Green fluorescent protein as a marker in Plasmodium berghei transformation. Infect Immun 67: 2602-2606., Sanchez et al. 2004Sanchez BA, Mota MM, Sultan AA, Carvalho LH 2004. Plasmodium berghei parasite transformed with green fluorescent protein for screening blood schizontocidal agents. Int J Parasitol 34: 485-490., Tewari et al. 2010Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, Pain A, Billker O 2010. The systematic functional analysis of Plasmodium protein kinases identifies. Cell Host Microbe 8: 377-387., de Souza et al. 2012de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272.), including the screening of novel antimalarial drugs (de Souza et al. 2012de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272., Lam et al. 2013Lam CFC, Pearce AN, Tan SH, Kaiser M, Copp BR 2013. Discovery and evaluation of thiazinoquinones as anti-protozoal agents. Mar Drugs 11: 3472-3499., Wang et al. 2014Wang J, Kaiser M, Copp BR 2014. Investigation of indolglyoxamide and indolacetamide analogues of polyamines as antimalarial and antitrypanosomal agents. Mar Drugs 12: 3138-3160.). It is interesting to note the presence of infected erythrocytes up to 48 h after treatment with chloroquine by flow cytometry that was not detected by microscopic analysis. Flow cytometry allows faster and accurate parasitaemia examination because this technique can identify small amounts of parasites in the blood (Malleret et al. 2011Malleret B, Claser C, Ong AS, Suwanarusk R, Sriprawat K, Howland SW, Russell B, Nosten F, Rénia L 2011. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci Rep 1: 118-128.). Although flow cytometry is a costly and complex technology to examine parasitaemia for routine diagnosis purposes, the required instrumentation and materials are widely available in research and development institutions for research purpose (Shapiro et al. 2013Shapiro HM, Apte SH, Chojnowski GM, Hanscheid T, Rebelo M, Grimberg BT 2013. Cytometry in malaria - a practical replacement for microscopy? Curr Protoc Cytom 11: Unit 11.20.). Parasitaemia evaluation by microscopic examination, despite widely used as main test for diagnosis purposes (WHO 2015WHO - World Health Organization 2015. World malaria report 2014. Available from: who.int/malaria/publications/world_malaria_report_2014/en/.
who.int/malaria/publications/world_malar...
), is labour and time-consuming, as well as dependent on microscopist training and ability (Payne 1988Payne D 1988. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull World Health Organ 66: 621-626.). Limitations for PbGFP usage as a tool for the discovery of pyrimethamine-based drugs should be addressed, since the construction of PbGFP required the insertion of pyrimethamine-resistant gene at the same vector where GFP gene is insert aiming to select the successfully transfected parasites.
Hydroxyethylamine derivative has been used in different biological approaches, as HIV-1 protease inhibitor (Ghosh et al. 2014Ghosh AK, Schiltz GE, Rusere LN, Osswald HL, Walters DE, Amano M, Mitsuya H 2014. Design and synthesis of potent macrocyclic HIV-1 protease inhibitors involving P1-P2 ligands. Org Biomol Chem 12: 6842-6854.) and inhibitor of β-secretase 1, an enzyme associated with neurodegeneration (Nordeman et al. 2014Nordeman P, Estrada S, Odell LR, Larhed M, Antoni G 2014. (11)C-labeling of a potent hydroxyethylamine BACE-1 inhibitor and evaluation in vitro and in vivo. Nucl Med Biol 41: 536-543.). As well, the hydroxyethylamine-based compounds has been tested as antimalarial drugs since this compounds are able to inhibit the activity of plasmepsin (Muthas et al. 2005Muthas D, Noteberg D, Sabnis YA, Hamelink E, Vrang L, Samuelsson B, Karlén A, Hallberg A 2005. Synthesis, biological evaluation and modeling studies of inhibitors aimed at the malarial proteases plasmepsins I and II. Bioorg Med Chem 13: 5371-5390.) and falcipain (Rathi et al. 2013Rathi B, Singh AK, Kishan R, Singh N, Latha N, Srinivasan S, Pandey KC, Tiwari HK, Singh BK 2013. Functionalized hydroxyethylamine based peptide nanostructures as potential inhibitors of falcipain-3, an essential proteases of Plasmodium falciparum. Bioorg Med Chem 21: 5503-5509.), main enzymes involved in parasite development (Blackman 2008Blackman MJ 2008. Malarial proteases and host cell egress: an “emerging” cascade. Cell Microbiol 10: 1925-1934.). It was previously showed that hydroxyethylamine derivatives (ciclohexyl group inserted in hydroxyethylamine core) synthesised from alkylamines presented antimalarial activity (de Souza et al. 2012de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272.). Herein, it was tested the in vivo activity of new nine different ((2S,3R)-2-(amino)-[4-(N-benzylarenesulfonamido)-3-hydroxy-1-phenylbutane derivatives and observed that the insertion of a methyl group at the paraposition of the sulfonamide ring appears to be critical for the antimalarial activity of this class of compounds (Fig. 6). Interestingly, hydroxyethylamine exhibits no toxic effect on erythrocytes and does not inhibit human proteases (Muthas et al. 2005Muthas D, Noteberg D, Sabnis YA, Hamelink E, Vrang L, Samuelsson B, Karlén A, Hallberg A 2005. Synthesis, biological evaluation and modeling studies of inhibitors aimed at the malarial proteases plasmepsins I and II. Bioorg Med Chem 13: 5371-5390.), suggesting that hydroxyethylamine derivatives would be safe and effective novel antimalarial drugs. In fact, its biological activity may be attributed to a secondary alcohol structural element, which mimics the tetrahedral intermediate during metabolite cleavage by proteases (Cunico et al. 2009Cunico W, Gomes CR, Moreth M, Manhanini DP, Figueiredo IH, Penido C, Henriques MG, Varotti FP, Krettli AU 2009. Synthesis and antimalarial activity of hydroxyethylpiperazine derivatives. Eur J Med Chem 44: 1363-1368.). In addition, Jaudzems et al. (2014)Jaudzems K, Tars K, Maurops G, Ivdra N, Otikovs M, Leitans J, Kanepe-Lapsa I, Domraceva I, Mutule I, Trapencieris P, Blackman MJ, Jirgensons A 2014. Plasmepsin inhibitory activity and structure-guided optimization of a potent hydroxyethylamine-based antimalarial hit. ACS Med Chem Lett 5: 373-377. showed that the insertion of two methyl group in hydroxyethylamine-based compounds increased compound activity on Plasmodium faciparum enzymes when compared to nonmethylated compound.
Together, our results suggest that PbGFP is a useful tool to study the recrudescence of novel antimalarial drugs through parasitaemia examination by flow cytometry. Furthermore, it was demonstrated that the insertion of a methyl group at the para position of the sulfonamide ring appears to be critical for the antimalarial activity of this class of compounds.
REFERENCES
- Aguiar ACC, da Rocha EMM, de Souza NB, França TCC, Krettli AU 2012a. New approaches in antimalarial drug discovery and development - A Review. Mem Inst Oswaldo Cruz 107: 831-845.
- Aguiar ACC, Santos RM, Figueiredo FJB, Cortopassi WA, Pimentel AS, França TCC, Meneghetti WR, Krettli AU 2012b. Antimalarial activity and mechanisms of action of two novel 4-aminoquinolines against chloroquine-resistant parasites. PLoS ONE 7: e37259.
- Anthony MP, Burrows JN, Duparc S, Moehrle J, Wells TN 2012. The global pipeline of new medicines for the control and elimination of malaria. Malar J 11: 316-341.
- Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371: 411-423.
- Blackman MJ 2008. Malarial proteases and host cell egress: an “emerging” cascade. Cell Microbiol 10: 1925-1934.
- Burrows JN, van Huijsduijnen RH, Möhrle JJ, Oeuvray C, Wells TN 2013. Designing the next generation of medicines for malaria control and eradication. Malar J 12: 187-207.
- Cunico W, Gomes CR, Moreth M, Manhanini DP, Figueiredo IH, Penido C, Henriques MG, Varotti FP, Krettli AU 2009. Synthesis and antimalarial activity of hydroxyethylpiperazine derivatives. Eur J Med Chem 44: 1363-1368.
- de Pina-Costa A, Brasil P, Di Santi SM, de Araujo MP, Suárez-Mutis MC, Santelli ACFS, Oliveira-Ferreira J, Lourenço-de-Oliveira R, Daniel-Ribeiro CT 2014. Malaria in Brazil: what happens outside the Amazonian endemic region. Mem Inst Oswaldo Cruz 109: 618-633.
- de Souza MC, Gonçalves-Silva T, Moreth M, Gomes CR, Kaiser CR, Henriques MO, de Souza MV 2012. Synthesis and in vivo antimalarial evaluation of novel hydroxyethylamine derivatives. Med Chem 8: 266-272.
- Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L 2010. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8: 272-280.
- Facchinetti V, Gomes CRB, de Souza MVN, Vasconcelos TRA, Wardell S, Wardell JL 2014. Solvates of two ethyl 6-(2-(aryl)-4-oxothiazolidin-3-yl)-1-ethyl-4-oxo-1,4-dihydroquinoline-3- carboxylates. J Chem Crystallogr 44: 471-479.
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3: 509-520.
- Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ 2004. A Plasmodium bergheireference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol 137: 23-33.
- Ghosh AK, Schiltz GE, Rusere LN, Osswald HL, Walters DE, Amano M, Mitsuya H 2014. Design and synthesis of potent macrocyclic HIV-1 protease inhibitors involving P1-P2 ligands. Org Biomol Chem 12: 6842-6854.
- Jaudzems K, Tars K, Maurops G, Ivdra N, Otikovs M, Leitans J, Kanepe-Lapsa I, Domraceva I, Mutule I, Trapencieris P, Blackman MJ, Jirgensons A 2014. Plasmepsin inhibitory activity and structure-guided optimization of a potent hydroxyethylamine-based antimalarial hit. ACS Med Chem Lett 5: 373-377.
- Lam CFC, Pearce AN, Tan SH, Kaiser M, Copp BR 2013. Discovery and evaluation of thiazinoquinones as anti-protozoal agents. Mar Drugs 11: 3472-3499.
- Malleret B, Claser C, Ong AS, Suwanarusk R, Sriprawat K, Howland SW, Russell B, Nosten F, Rénia L 2011. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci Rep 1: 118-128.
- Moreth M, Gomes CR, Lourenço MC, Soares RP, Rocha MN, Kaiser CR, de Souza MV, Wardell SM, Wardell JL 2014. Syntheses and antimycobacterial activities of (2S,3R)-2-(amino)-4-(arenesulfonamido)-3-hydroxy-1-phenylbutane derivatives. Med Chem 10: 189-200.
- Muthas D, Noteberg D, Sabnis YA, Hamelink E, Vrang L, Samuelsson B, Karlén A, Hallberg A 2005. Synthesis, biological evaluation and modeling studies of inhibitors aimed at the malarial proteases plasmepsins I and II. Bioorg Med Chem 13: 5371-5390.
- Nordeman P, Estrada S, Odell LR, Larhed M, Antoni G 2014. (11)C-labeling of a potent hydroxyethylamine BACE-1 inhibitor and evaluation in vitro and in vivo. Nucl Med Biol 41: 536-543.
- Payne D 1988. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull World Health Organ 66: 621-626.
- Rathi B, Singh AK, Kishan R, Singh N, Latha N, Srinivasan S, Pandey KC, Tiwari HK, Singh BK 2013. Functionalized hydroxyethylamine based peptide nanostructures as potential inhibitors of falcipain-3, an essential proteases of Plasmodium falciparum Bioorg Med Chem 21: 5503-5509.
- Sanchez BA, Mota MM, Sultan AA, Carvalho LH 2004. Plasmodium berghei parasite transformed with green fluorescent protein for screening blood schizontocidal agents. Int J Parasitol 34: 485-490.
- Shapiro HM, Apte SH, Chojnowski GM, Hanscheid T, Rebelo M, Grimberg BT 2013. Cytometry in malaria - a practical replacement for microscopy? Curr Protoc Cytom 11: Unit 11.20.
- Sultan AA, Thathy V, Nussenzweig V, Menard R 1999. Green fluorescent protein as a marker in Plasmodium berghei transformation. Infect Immun 67: 2602-2606.
- Tewari R, Straschil U, Bateman A, Bohme U, Cherevach I, Gong P, Pain A, Billker O 2010. The systematic functional analysis of Plasmodium protein kinases identifies. Cell Host Microbe 8: 377-387.
- Wang J, Kaiser M, Copp BR 2014. Investigation of indolglyoxamide and indolacetamide analogues of polyamines as antimalarial and antitrypanosomal agents. Mar Drugs 12: 3138-3160.
- WHO - World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed., WHO, Geneva, 194 pp.
- WHO - World Health Organization 2015. World malaria report 2014. Available from: who.int/malaria/publications/world_malaria_report_2014/en/.
» who.int/malaria/publications/world_malaria_report_2014/en/
-
Financial support: CNPq, FAPERJ, CAPES, FIOCRUZ
Publication Dates
-
Publication in this collection
26 May 2015 -
Date of issue
June 2015
History
-
Received
9 Dec 2014 -
Accepted
24 Apr 2015