Abstract
The present study offers a broad comparative analysis of the dorsolateral head musculature in the Gymnotiformes, with detailed descriptions and illustrations of the dorsolateral head muscles of 83 species representing combined all valid genera. Results permit a detailed assessment of primary homologies and taxonomically-relevant variation across the order. This provides the basis for a myological synonymy, which organizes 33 previously proposed names for 15 recognized muscles. Morphological variation derived from dorsolateral head musculature was coded into 56 characters. When analyzed in isolation, that set of characters results in Gymnotidae as the sister group of remaining gymnotiforms, and all other currently recognized families as monophyletic groups. In a second analysis, myological characters were concatenated with other previously proposed characters into a phenotypic matrix. Results of that analysis reveal new myological synapomorphies for nearly all taxonomic categories within Gymnotiformes. A Partitioned Bremer Support (PBS) was used to asses the significance of comparative myology in elucidating phylogenetic relationships. PBS values show strongly non-uniform distributions on the tree, with positive scores skewed towards more inclusive taxa, and negative PBS values concentrated on less inclusive clades. Our results provide background for future studies on biomechanical constraints evolved in the early stages of gymnotiform evolution.
Keywords:
Anatomy; Electric fishes; Myology; Phylogeny; Partitioned Bremer Support
Resumo
O presente estudo fornece uma ampla análise comparativa da musculatura dorsolateral da cabeça dos Gymnotiformes, com descrições detalhadas e ilustrações dos músculos dorsolaterais da cabeça de 83 espécies representando quase todos os gêneros válidos. Resultados permitem uma avaliação das homologias primárias e da variação taxonomicamente relevante na ordem. Isto fornece a base para uma sinonímia da nomenclatura miológica que organiza 33 nomes previamente propostos para os 15 músculos reconhecidos. As variações morfológicas da musculatura dorsolateral da cabeça foram codificadas em 56 caracteres. Este conjunto de dados foi inicialmente analisado isoladamente, resultando em Gymnotidae como grupo-irmão dos demais Gymnotiformes; e todas as famílias como grupos monofiléticos. Numa segunda análise, os caracteres musculares foram concatenados com uma matriz fenotípica previamente proposta compondo uma ampla matriz morfológica combinada. Os resultados desta análise revelaram novas sinapomorfias miológicas para todas as categorias taxonômicas em Gymnotiformes. O Suporte de Bremer Particionado (SBP) foi implementado para acessar a influência da miologia em elucidar os relacionamentos filogenéticos. Os valores de SBP exibem uma distribuição não uniforme na árvore, com indicadores positivos para agrupamentos mais inclusivos e valores negativos de SBP em clados menos inclusivos. Nossos resultados fornecem subsídios para investigações futuras sobre as restrições biomecânicas envolvidas nos estágios inicias da evolução dos Gymnotiformes.
Palavras-chave:
Anatomia; Peixes elétricos; Miologia; Filogenia; Suporte de Bremer particionado
INTRODUCTION
Popularly known as “tuvíras”, “sarapós”, “knifefishes” or “neotropical electric eels”, the fishes of the order Gymnotiformes have a broad distribution in neotropical freshwater environments, occurring from southern Mexico to northern Argentina (Ferraris et al., 2017Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
https://doi.org/10.1590/1982-0224-201600...
), with particularly rich diversity in the Amazonas-Orinoco-Guiana system (Albert, Crampton, 2005aAlbert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005a. p.360–409. https://doi.org/10.1007/0-387-28275-0_13
https://doi.org/10.1007/0-387-28275-0_13...
; Dagosta, de Pinna, 2019Dagosta FC, de Pinna MCC. The fishes of the Amazon: Distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1–163. Available from: http://digitallibrary.amnh.org/handle/2246/6940
http://digitallibrary.amnh.org/handle/22...
). Those fishes are important components mostly in the nocturnal ichthyofauna (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), but also represent relevant diurnal elements, and occupy a wide range of habitats, from small streams to large rivers, including waterfalls, flooded forests and caves (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Albert, Crampton, 2005Albert JS, Crampton WGR. Electroreception and electrogenesis. In: Evans DH, Claiborne JB, editors. The Physiology of fishes. Boca Raton: CRC Press; 2005b. p.431–72.b). Gymnotiformes comprises about 260 valid species allocated in 34 genera (Ferraris et al., 2017Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
https://doi.org/10.1590/1982-0224-201600...
) and five families: Apteronotidae, Gymnotidae, Hypopomidae, Rhamphichthyidae and Sternopygidae (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.).
The order is easily distinguished from other Neotropical fish lineages by their extremely elongated, cylindrical or laterally compressed body, with the anal fin extending for much of the ventral margin and by the absence of dorsal, adipose and pelvic fins. The caudal fin is present only in Apteronotidae and in Electrophorus Gill, 1864 (Gymnotidae) (Mago-Leccia, 1994Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.; de Santana et al., 2013de Santana CD, Vari RP, Wosiacki WB. The untold story of the caudal skeleton in the electric eel (Ostariophysi: Gymnotiformes: Electrophorus). PLoS ONE. 2013; 8(7):e68719. https://doi.org/10.1371/journal.pone.0068719
https://doi.org/10.1371/journal.pone.006...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). Such body pattern is related to the most conspicuous biological characteristics of gymnotiforms: electroreception and electrogenesis (Moller, 1995Moller P. Electric fishes: history and behavior. London: Chapman & Hall; 1995.; Crampton, Albert, 2006Crampton WGR, Albert JS. Evolution of electric signal diversity in gymnotiform fishes. In: Ladich F, Collin SP, Moller P, Kapoor BG, editors. Communication in fishes. Enfield: Science Publishers; 2006. p.647–731.). These fishes move by rippling of the anal-fin rays, allowing for body stability during swimming and thus uniformity of the electric field generated around the fish. The electric field is used in fish orientation and communication, or in prey detection (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.). Discharges from the electrical organs can be of the “pulse type”, characterized by short-duration sequential discharges generated at rates from 1 to 120Hz, with a long pause period or “electrical silence” (Gymnotidae, Hypopomidae and Rhamphichthyidae); or “wave type”, at rates from 20 to 2200Hz, without intervals (Sternopygidae and Apteronotidae) (Albert, Crampton, 2005Albert JS, Crampton WGR. Electroreception and electrogenesis. In: Evans DH, Claiborne JB, editors. The Physiology of fishes. Boca Raton: CRC Press; 2005b. p.431–72.b).
Anatomical studies on Gymnotiformes follow the historical trend in other groups of Teleostei and focused on relatively detailed descriptions of osteological complexes (e.g., Chardon, de la Hoz, 1974Chardon M, de la Hoz E. Towards an improved classification of the gymnotiform fishes by the use of the splanchnocranium characters. Acta Biol Jugoslav, Beograd. 1974; 6:15–25., 1977Chardon M, de la Hoz E. Remarques anatomiques et fonctionnelles à propos du suspensorium et de la série operculaire chez Sternopygus macrurus (Bloch & Schneider) et Egenmannia virescens (Val) (Teleostei, Gymnotoidei). Ann Soc R Zool Belg. 1977; 106(2–4):177–91.; Mago-Leccia, 1978Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51.; Hilton et al., 2007Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
; Carvalho, Albert, 2011Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
). These are complemented by surveys of neuroanatomic structures (e.g., Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Crampton et al., 2013Crampton WGR, Rodríguez-Cattáneo A, Lovejoy NR, Caputi AA. Proximate and ultimate causes of signal diversity in the electric fish Gymnotus. J Exp Biol. 2013; 216(13):2523–41. https://doi.org/10.1242/jeb.083261
https://doi.org/10.1242/jeb.083261...
), and components associated with electrogenesis and electroreception (e.g., Carr et al., 1982Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory nerves in gymnotiform fish. J Comp Neurol. 1982; 211(2):139–53. https://doi.org/10.1002/cne.902110204
https://doi.org/10.1002/cne.902110204...
; Lannoo et al., 1989Lannoo MJ, Maler L, Tinner B. Ganglion cell arrangement and axonal trajectories in the anterior lateral line nerve of the weakly electric fish Apteronotus leptorhynchus (Gymnotiformes). J Comp Neurol. 1989; 280(3):331–42. https://doi.org/10.1002/cne.902800302
https://doi.org/10.1002/cne.902800302...
; Vischer et al., 1989Vischer HA, Lannoo MJ, Heiligenberg W. Development of the electrosensory nervous system in Eigenmannia (Gymnotiformes): I. The peripheral nervous system. J Comp Neurol. 1989; 290:16–40. https://doi.org/10.1002/cne.902900103
https://doi.org/10.1002/cne.902900103...
; Hopkins, 1999Hopkins CD. Design features for electric communication. J Exp Biol. 1999; 202(10):1217–28. https://doi.org/10.1242/jeb.202.10.1217
https://doi.org/10.1242/jeb.202.10.1217...
; Crampton, 1998Crampton WGR. Electric signal design and habitat preferences in a species rich assemblage of gymnotiform fishes from the upper Amazon basin. An Acad Bras Cienc. 1998; 70:805–47., 2019Crampton WGR. Electroreception, electrogenesis and signal evolution. J Fish Biol. 2019; 95(1):92–134. https://doi.org/10.1111/jfb.13922
https://doi.org/10.1111/jfb.13922...
). Other studies have focused on structures recently discovered in Gymnotiformes, such as the caudal skeleton in Electrophorus (de Santana et al., 2013de Santana CD, Vari RP, Wosiacki WB. The untold story of the caudal skeleton in the electric eel (Ostariophysi: Gymnotiformes: Electrophorus). PLoS ONE. 2013; 8(7):e68719. https://doi.org/10.1371/journal.pone.0068719
https://doi.org/10.1371/journal.pone.006...
) and the pseudotympanum in several subgroups of the order (Dutra et al., 2015Dutra GM, Jerep FC, Vari RP, de Santana CD. The pseudotympanum in the Gymnotiformes (Teleostei, Ostariophysi, Otophysi): homology and evolution of a previously unexplored system in Neotropical electric fishes. Zool J Linn Soc. 2015; 174(1):114–29. https://doi.org/10.1111/zoj.12221
https://doi.org/10.1111/zoj.12221...
). Finally, secondary sexual dimorphism in Gymnotiformes has been discussed in a phylogenetic paradigm (Cox Fernandes et al., 2002Cox Fernandes C, Lundberg JG, Riginos C. Largest of all electric-fish snouts: hypermorphic facial growth in male Apteronotus hasemani and the identity of Apteronotus anas (Gymnotiformes: Apteronotidae). Copeia. 2002; 2002(1):52–61. https://doi.org/10.1643/0045-8511(2002)002[0052:LOAEFS]2.0.CO;2
https://doi.org/10.1643/0045-8511(2002)0...
; Rapp Py-Daniel, Cox Fernandes, 2005Rapp Py-Daniel LH, Cox Fernandes C. Dimorfismo sexual em Siluriformes e Gymnotiformes (Ostariophysi) da Amazônia. Acta Amaz. 2005; 35(1):97–110. https://doi.org/10.1590/S0044-59672005000100015
https://doi.org/10.1590/S0044-5967200500...
; Hilton, Cox Fernandes, 2006Hilton EJ, Cox Fernandes C. Sexual dimorphism in Apteronotus bonapartii (Gymnotiformes: Apteronotidae). Copeia. 2006; 2006(4):826–33. http://dx.doi.org/10.1643/0045-8511(2006)6[826:SDIABG]2.0.CO;2
http://dx.doi.org/10.1643/0045-8511(2006...
; Albert, Crampton, 2009Albert JS, Crampton WGR. A new species of electric knifefish, genus Compsaraia (Gymnotiformes: Apteronotidae) from the Amazon River, with extreme sexual dimorphism in snout and jaw length. Syst Biodivers. 2009; 7(1):81–92. https://doi.org/10.1017/S1477200008002934
https://doi.org/10.1017/S147720000800293...
; Evans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
, 2019aEvans KM, Bernt MJ, Kolmann MA, Ford KL, Albert JS. Why the long face? Static allometry in the sexually dimorphic phenotypes of Neotropical electric fishes. Zool J Linn Soc. 2019a; 186(3):633–49. https://doi.org/10.1093/zoolinnean/zly076
https://doi.org/10.1093/zoolinnean/zly07...
,bEvans KM, Vidal-García M, Tagliacollo VA, Taylor SJ, Fenolio DB. Bony patchwork: mosaic patterns of evolution in the skull of electric fishes (Apteronotidae: Gymnotiformes). Integr Comp Biol. 2019b; 59(2):420–31. https://doi.org/10.1093/icb/icz026
https://doi.org/10.1093/icb/icz026...
; Keeffe et al., 2019Keeffe R, Hilton EJ, De Souza MJFT, Cox Fernandes C. Cranial morphology and osteology of the sexually dimorphic electric fish, Compsaraia samueli Albert & Crampton (Apteronotidae, Gymnotiformes), with comparisons to C. compsa (Mago-Leccia). Zootaxa. 2019; 4555(1):101–12. https://doi.org/10.11646/zootaxa.4555.1.8
https://doi.org/10.11646/zootaxa.4555.1....
). In general, studies of comparative anatomy in Gymnotiformes have been restricted to traditional sources of information (e.g., osteology and external anatomy), with complexes from soft anatomy being largely neglected. As a result, several biologically interesting and potentially relevant complexes remain almost entirely uncharted in the group.
Despite being one of the main anatomical complexes of vertebrates, the skeletal musculature of fishes is seldom studied (Datovo, Bockmann, 2010Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol. 2010; 8(2):193–246. http://dx.doi.org/10.1590/S1679-62252010000200001
http://dx.doi.org/10.1590/S1679-62252010...
). In Gymnotiformes, our current knowledge is limited to observations of the dorsolateral head muscles of a few species, or brief descriptions of specific myological components. Chardon, de la Hoz, (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10. were pioneers in myological studies of gymnotiforms, with descriptions and illustrations of the dorsolateral head muscles of Sternopygus macrurus (Bloch & Schneider, 1801) (Sternopygidae), and comparisons with some other Ostariophysi species. Subsequently, Howes, (1983)Howes JG. Cranial muscles of loricarioid catfishes, their homologies and value as taxonomic characters (Teleostei: Siluroidei). Bull Br Mus Nat Hist Zool. 1983; 45:309–45. https://doi.org/10.5962/bhl.part.28003
https://doi.org/10.5962/bhl.part.28003...
presented data on ligament components of some gymnotiform species, along with brief descriptions of the insertion of subsections of the adductor mandibulae in Sternopygus, Eigenmannia Jordan & Evermann, 1896 (Sternopygidae) and Rhamphichthys Müller & Troschel, 1846 (Rhamphichthyidae). The first contribution focusing specifically on the striated musculature in Gymnotiformes was de la Hoz, Chardon, (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53., who offered a detailed description of S. macrurus, including descriptions and illustrations of osteology, myology and ligaments.
Although such studies comprise crucial background information on the musculature of Gymnotiformes, Aguilera, (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23. was the first contribution to tackle myology in gymnotiforms in a relatively broad comparative context. The author presented detailed descriptions of the dorsolateral muscles of thirteen species of the order, including representatives of all families, with emphasis on Apteronotidae. Later, Aguilera, Machado-Allison, (1993)Aguilera O, Machado-Allison A. La musculatura em los peces Gymnotiformes (Teleostei-Ostariophysi): Arcos Branquiales. Acta Biol Venez. 1993; 14:21–32. described and illustrated details of the gill arch muscles of Gymnotiformes, also offering a discussion on their phylogenetic implications.
Subsequent to these contributions, the study of gymnotiform myology underwent a long hiatus, dotted by specific descriptive contributions (e.g., Diogo, Chardon, 2000Diogo R, Chardon M. Homologies among different adductor mandibulae sections of teleostean fishes, with special regard to catfishes (Teleostei: Siluriformes). J Morphol. 2000; 243(2):193–208. https://doi.org/10.1002/(SICI)1097-4687(200002)243:2<193::AID-JMOR8>3.0.CO;2-2
https://doi.org/10.1002/(SICI)1097-4687(...
) and comparative surveys of a broad scope (Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). The later paper offered detailed descriptions of the adductor mandibulae of Gymnotus carapo Linnaeus, 1758 (Gymnotidae) and Brachyhypopomus pinnicaudatus (Hopkins, Comfort, Bastian & Bass, 1990) (Hypopomidae), along with a synonymic list for this complex in Gymnotiformes.
Studies on the phylogenetic relationships in Gymnotiformes have expectedly emphasized osteology and external-anatomical characters. Myological characters were either under-represented (e.g., Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
) or entirely absent (e.g., Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130., 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Bernt et al., 2018Bernt MJ, Crampton WGR, Orfinger AB, Albert JS.Melanosternarchus amaru, a new genus and species of electric ghost knifefish (Gymnotiformes: Apteronotidae) from the Amazon Basin. Zootaxa. 2018; 4378(2):451–79. https://doi.org/10.11646/zootaxa.4378.4.1
https://doi.org/10.11646/zootaxa.4378.4....
, 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
). In a compehensive study on the phylogenetic relationships in Gymnotiformes, Albert, Campos-da-Paz, (1998)Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60. was the first to use myology as potential source of phylogenetic signal, and listed four such characters in a data matrix with 250 characters (the same characters were later analyzed in Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.). Further, Albert et al., (2005)Albert JS, Crampton WGR, Thorsen DH, Lovejoy NR. Phylogenetic systematics and historical biogeography of the Neotropical electric fish Gymnotus (Teleostei: Gymnotidae). Syst Biodivers. 2005; 2(4):375–417. https://doi.org/10.1017/S1477200004001574
https://doi.org/10.1017/S147720000400157...
listed two characters from the adductor mandibulae from a total of 113 in a study focusing on the phylogenetic relationships in Gymnotus. Similarly, de Santana, Vari, (2010)de Santana CD, Vari RP. Electric fishes of the genus Sternarchorhynchus (Teleostei, Ostariophysi, Gymnotiformes); phylogenetic and revisionary studies. Zool J Linn Soc. 2010; 159(1):223–371. https://doi.org/10.1111/j.1096-3642.2009.00588.x
https://doi.org/10.1111/j.1096-3642.2009...
, in a matrix with 88 characters, utilized a single myological character. Recently, Tagliacollo et al. (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
proposed the first phylogenetic hypothesis grounded in a total-evidence model in Gymnotiformes, with a morphological database with 223 characters, only four of which were from myology. As a result, characters from myology currently represent less than 0.2% of the entire universe of morphological characters so far explored in cladistic studies of Gymnotiformes.
The present paper aims to fill out a large gap in the anatomical knowledge of this important group of freshwater fishes and to assist in the understanding of their diversity and evolution. We offer a detailed description of the dorsolateral musculature of the head in representatives of all major subgroups of the Gymnotiformes. This information forms the basis for primary homology assessments and a new standard of the myological nomenclature in the order, which is synthesized as a synonymic list. The variation detected is evaluated in a phylogenetic context by isolated and concatenated analyses combining our data with those from previous studies. Our results, set within a context of an integrated phenotypic matrix, reveal several new synapomorphies for major groups of Gymnotiformes, and provides additional data for resolving phylogenetic relationships within the order.
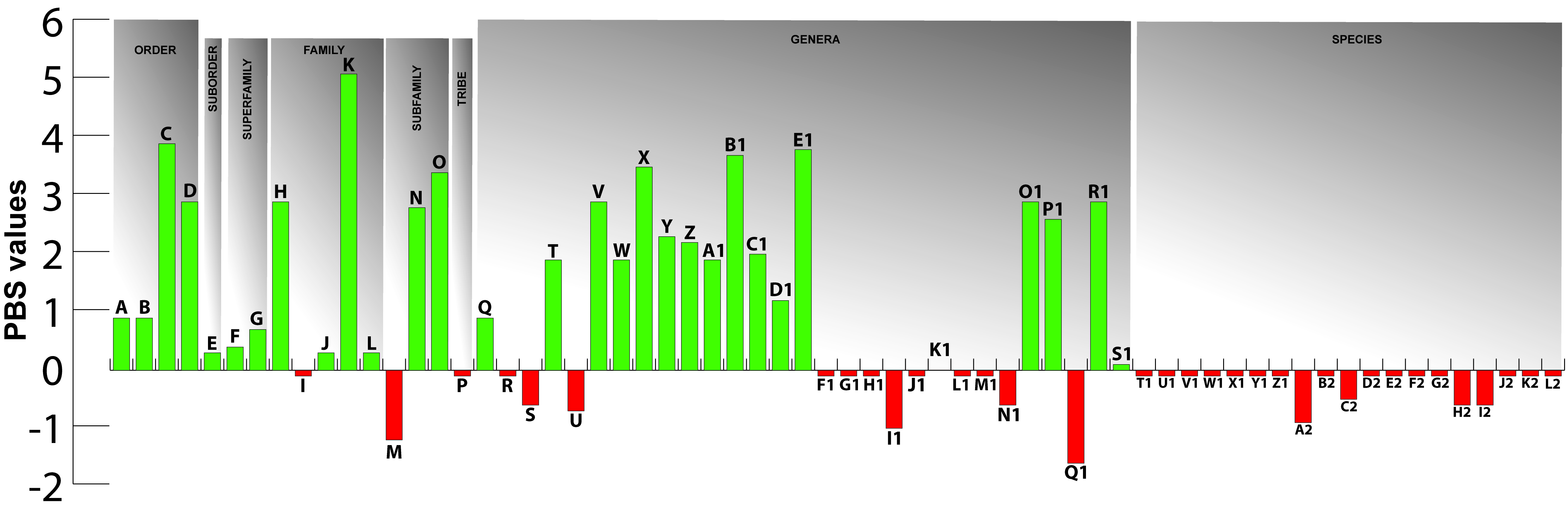
Finally, Partitioned Bremer Support (PBS), a technique for describing the distribution of character support and conflict among different datasets in a concatenated analysis, was used to assess the influence of myological characters in elucidating evolutionary relationships, allowing an evaluation of the contribution of dorsolateral head muscles in global analyses of the Gymnotiformes.
MATERIAL AND METHODS
Taxonomic and terminological nomenclature. Taxonomic nomenclature follows Albert, (2001)Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127., with the modifications of Tagliacollo et al. (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
, except for “Sinusoidea”, which is not based on an available generic name and therefore invalid (Ferraris et al., 2017Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
https://doi.org/10.1590/1982-0224-201600...
; Betancur-R et al., 2017Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol. 2017; 17(162):1–40. https://doi.org/10.1186/s12862-017-0958-3
https://doi.org/10.1186/s12862-017-0958-...
). Sternopygoidea is used as the correct name for that taxon. For the same reason, “Navajini” (sensu Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.) is also invalid and not used in this work. The taxonomic status of all analyzed taxa follows Ferraris et al., (2017)Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
https://doi.org/10.1590/1982-0224-201600...
and Fricke et al. (2020)Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2020. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
. In phylogenetic context, the terms “basal” and “apical” refer to the phylogenetic position of a taxon in relation to the root in a tree topology.
Anatomical nomenclature. Myological nomenclature follows Winterbottom, (1974a)Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
, except for the adductor mandibulae and associated structures, which follows Datovo, Vari (2013, 2014). Conservatively, in this study the name adductor hyomandibulae is used for the myological component located posterior to the adductor arcus palatini and anterior to the adductor operculi in gymnotiforms (Huysentruyt et al., 2009Huysentruyt F, Moerkerke B, Devaere S, Adriaens D. Early development and allometric growth in the armoured catfish Corydoras aeneus (Gill, 1858). Hydrobiologia. 2009; 627:45–54. https://doi.org/10.1007/s10750-009-9714-z
https://doi.org/10.1007/s10750-009-9714-...
; but see Datovo, Rizzato, 2018Datovo A, Rizzato PP. Evolution of the facial musculature in basal ray-finned fishes. Front Zool. 2018; 15(40):1–29. https://doi.org/10.1186/s12983-018-0285-6
https://doi.org/10.1186/s12983-018-0285-...
). Osteological terminology follows Albert, Fink, (1996)Albert JS, Fink WL. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
https://doi.org/10.2307/1446944...
, Albert, (2001)Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127., Hilton et al., (2007)Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
and Peixoto et al. (2015), with elements not covered therein following Weitzman, (1962)Weitzman SH. The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichth Bull. 1962; 8:1–77.. Lateral-line nomenclature follows Pastana et al., (2020)Pastana MNL, Bockmann FA, Datovo A. The cephalic lateral-line system of Characiformes (Teleostei: Ostariophysi): anatomy and phylogenetic implications. Zool J Linn Soc. 2020; 189(1):1–46. https://doi.org/10.1093/zoolinnean/zlz105
https://doi.org/10.1093/zoolinnean/zlz10...
. The terms “origin” and “insertion” are used to the stationary connection site of the muscle (more stable) and the connection point that moves from muscle contraction (relatively more mobile), respectively (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
).
In some gymnotiforms, two or more sections of the adductor mandibulae may be partly or entirely undifferentiated from each other, with extensive continuity among their fibers, resulting in composite sections. In such cases, sections are named according to their conformity to the homology of the adductor mandibulae subcomponents (e.g., Adductor mandibulae, pars ricto-malaris and stego-malaris), according to Datovo, Vari, (2013)Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
. Although it is possible to infer correspondence between sets of fibers of undifferentiated bundles and separate sections in taxa with complete differentiation, we maintain a composite nomenclature because in many cases it is possible to observe a subtle differentiation in regions of origin and insertion.
The anteroventral portion of the lateral line nerve is called “recurrent ramus of anteroventral part of anterior lateral line nerve” (R-Avn) according to Carr et al., (1982)Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory nerves in gymnotiform fish. J Comp Neurol. 1982; 211(2):139–53. https://doi.org/10.1002/cne.902110204
https://doi.org/10.1002/cne.902110204...
and Vischer et al., (1989)Vischer HA, Lannoo MJ, Heiligenberg W. Development of the electrosensory nervous system in Eigenmannia (Gymnotiformes): I. The peripheral nervous system. J Comp Neurol. 1989; 290:16–40. https://doi.org/10.1002/cne.902900103
https://doi.org/10.1002/cne.902900103...
. That branch originates from the electro-sensorial lobe of the lateral line and innervates electro-receptors of the trunk, being arranged differently in relation to the opercular muscles in Gymnotiformes. Recently, this nerve has been named as a “lateral line nerve” (Dutra et al., 2015Dutra GM, Jerep FC, Vari RP, de Santana CD. The pseudotympanum in the Gymnotiformes (Teleostei, Ostariophysi, Otophysi): homology and evolution of a previously unexplored system in Neotropical electric fishes. Zool J Linn Soc. 2015; 174(1):114–29. https://doi.org/10.1111/zoj.12221
https://doi.org/10.1111/zoj.12221...
), a nomenclature not adopted here because it does not adequately reflect the positional homology of the ramus. Terminology for other cranial nerves follows Freihofer, (1978)Freihofer WC. Cranial nerves of a percoid fish, Polycentrus schomburgkii (family Nandidae), a contribution to the morphology and classification of the order Perciformes. Occas Pap Calif Acad Sci. 1978; 128:1–178. .
Synonymy. The synonymic list of names of the dorsolateral head musculature aims to include all names previously employed for that complex in Gymnotiformes. Species mentioned in previous studies were either directly examined or, if not available, represented by a close relative. In a few cases, some of the components described or illustrated in previous contributions could not be definitely identified and in those instances, they are indicated as “?”, followed by comments in brackets.
Anatomical descriptions. In order to avoid excessive redundancy in anatomical descriptions, we adopt a method of hierarchical descriptions that minimizes the need for repetition. Via this style, descriptions of more inclusive groups precede those of less inclusive groups, so that general traits for each taxonomic category are described only once. For example, descriptions under the heading “Gymnotiformes” include characteristics common to all members in the order. Within “Gymnotidae”, in turn, only those traits common to all members of the family yet different from the general previously-provided gymnotiform pattern are described. Finally, within Gymnotus, only the states exclusive to that genus are included. In all cases, there are allowances for relevant exceptions and intra-taxon variation. Due to the great morphological variability of the adductor mandibulae and levator arcus palatini among the genera of each family, these muscles are presented separately in detailed descriptions. The dilatator operculi and levator operculi are presented separately only in Gymnotidae, due to the compositional variation of muscles in the family. Descriptions of the dorsolateral head musculature follow an anteroposterior and lateromedial arrangement of the muscles in their natural position in the head .
Illustrations. Photographs were made with a Zeiss Discovery V20 stereomicroscope coupled with the Axiocam 506 color digital camera, using a self-assembling procedure, with multifocal images combined with Combine ZP program (Hadley, 2009Hadley A. CombineZP: GNU public license software [Internet]; 2009. Available from: https://combinezp.software.informer.com/
https://combinezp.software.informer.com/...
) and later edited in Adobe Photoshop CS4 and Adobe Illustrator CS5. Anatomical abbreviations are presented in Tab. 1.
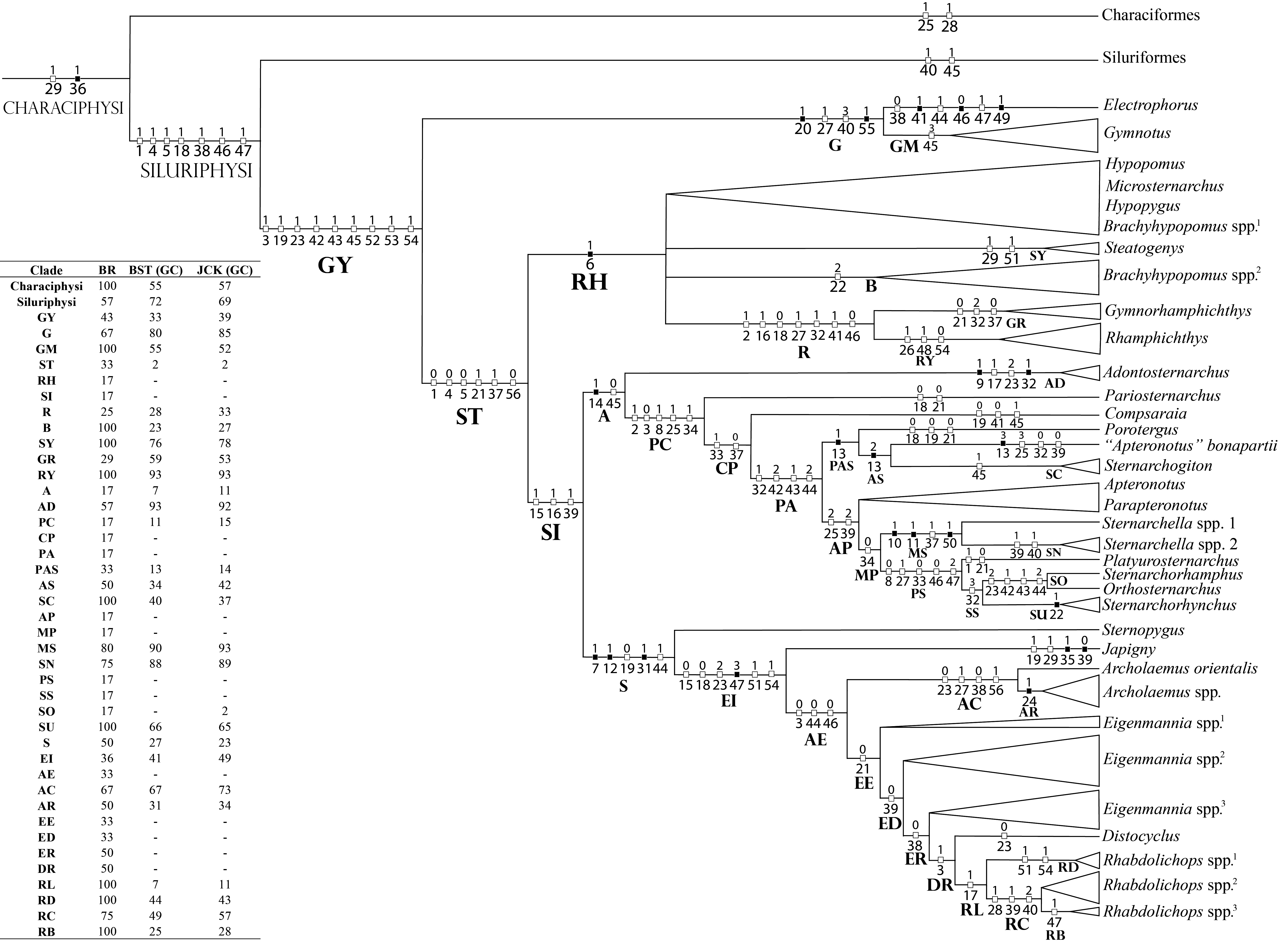
Phylogenetic inference. Two analyses were performed. “Analysis 1” includes solely the dorsolateral head musculature characters. Its main objective is to infer cladistic congruence among myological characters when analyzed in isolation and to draw comparisons with previous studies. “Analysis 2” is the myological matrix concatenated into an integrated phenotypic matrix. It aims to infer new synapomorphies and the influence of the dorsolateral head musculature within a large phenotypic dataset. Results of each analysis are synthesized in the “Discussion: ANALYSIS 1 – Dorsolateral head musculature and phylogenetic inference in Gymnotiformes: comparisons with previous studies” and “Discussion: ANALYSIS 2 – Influence of myological characters on the relationships of Gymnotiformes relationships”, respectively.
ANALYSIS 1 - Dorsolateral head musculature and phylogenetic methodology. Characters from dorsolateral head musculature were compiled in a matrix of 87 terminal taxa and 56 characters from dorsolateral head musculature (Tab. S1) built in Notepad ++ 7.5.1 (Ho, 2019Ho D. Notepad++. 7.5.3 [Internet]; 2019. Available from: http://notepad-plus-plus.org
http://notepad-plus-plus.org...
). The matrix was treated with parsimony analysis with the TNT program (“Tree Analysis using New Technology” – Goloboff, Catalano, 2016Goloboff PA, Catalano SA. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016; 32(3):221–38. https://doi.org/10.1111/cla.12160
https://doi.org/10.1111/cla.12160...
). The tree was rooted at Chanos chanos (Fabricius, 1775) (Gonorynchiformes), widely recognized as the sister group to remaining Ostariophysi included in the analysis (e.g., Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
, 1996Fink S, Fink WL. Interrelationships of Ostariophysan fishes (Teleostei). In: Stiassny ML, Parenti LR, Johnson D, editors. Interrelationships of fishes. San Diego: Academic Press; 1996. p.209–49.; Saitoh et al., 2003Saitoh K, Miya M, Inoué JG, Ishiguro NB, Nishida M. Mitochondrial genomics of ostariophysan fishes: perspectives on phylogeny and biogeography. J Mol Evol. 2003; 56:64–472. https://doi.org/10.1007/s00239-002-2417-y
https://doi.org/10.1007/s00239-002-2417-...
; Ortí, Meyer, 1996Ortí G, Meyer A. Molecular evolution of ependymin and the phylogenetic resolution of early divergences among euteleost fishes. Mol Biol Evol. 1996; 13(4):556–73. https://doi.org/10.1093/oxfordjournals.molbev.a025616
https://doi.org/10.1093/oxfordjournals.m...
, 1997Ortí G, Meyer A. The radiation of characiform fishes and the limits of resolution of mitochondrial ribosomal DNA sequences. Syst Biol. 1997; 46(1):75–100. https://doi.org/10.1093/sysbio/46.1.75
https://doi.org/10.1093/sysbio/46.1.75...
; Lavoué et al., 2011Lavoué S, Miya M, Inoue JG, Saitoh K, Ishiguro NB, Nishida M. Molecular systematics of the gonorynchiform fishes (Teleostei) based on whole mitogenome sequences: implications for higher-level relationships within the Otocephala. Mol Phylogenet Evol. 2011; 37(1):165–77. https://doi.org/10.1016/j.ympev.2005.03.024
https://doi.org/10.1016/j.ympev.2005.03....
; Nakatani et al., 2011Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011; 11(177):1–25. https://doi.org/10.1186/1471-2148-11-177
https://doi.org/10.1186/1471-2148-11-177...
; Chen et al., 2013Chen WJ, Lavoué S, Mayden RL. Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution. 2013; 67(8):2218–39. https://doi.org/10.1111/evo.12104
https://doi.org/10.1111/evo.12104...
). With the exception of character 13 (see section on that character), multi-state characters were treated as unordered.
Most parsimonious trees (MPT’s) were found by traditional heuristic search analysis with 1000 replications of RAS + TBR (“tree-bisection reconnection”), saving 90 trees by replication and hitting the best score at least 50 times. This strategy best suits our data set, and is recommended for the location of all the global optima in medium-sized datasets (Giribet, 2007Giribet G. Efficient tree searches with available algorithms. Evol Bioinform Online. 2007; 3:341–56. https://doi.org/10.1177/117693430700300014
https://doi.org/10.1177/1176934307003000...
; Goloboff et al., 2008Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008; 24(5):774–86. https://doi.org/10.1111/j.1096-0031.2008.00217.x
https://doi.org/10.1111/j.1096-0031.2008...
). Ambiguous character-state distributions were optimized by ACCTRAN (Accelerated Transformation Optimization) optimization (de Pinna, 1991de Pinna MCC. Concepts and tests of homology in the cladistic paradigm. Cladistics. 1991; 7(4):367–94. https://doi.org/10.1111/j.1096-0031.1991.tb00045.x
https://doi.org/10.1111/j.1096-0031.1991...
). A strict consensus tree was computed in TNT (ne*) and only synapomorphies common to all trees are presented and discussed. Consistency (CI) and retention (RI) indices were used as measures-of-fit between characters and trees (Farris, 1969Farris J. A successive approximations approach to character weighting. Syst Zool. 1969; 18(4):374–85. https://doi.org/10.2307/2412182
https://doi.org/10.2307/2412182...
, 1989Farris J. The retention index and the rescaled consistency index. Cladistics. 1989; 5(4):417–19. https://doi.org/10.1111/j.1096-0031.1989.tb00573.x
https://doi.org/10.1111/j.1096-0031.1989...
) and were calculated with a TNT script “wstats.run”. CI and RI are presented as ranges for characters with different performances among recovered MPT’s. RI for characters that have a state in a single terminal and another state in all other terminals are mathematically indeterminate and indicated as “AUT”.
Relative Bremer support (Goloboff, Farris, 2001Goloboff PA, Farris JS. Methods for quick consensus estimation. Cladistics. 2001; 17(1):26–34. https://doi.org/10.1111/j.1096-0031.2001.tb00102.x
https://doi.org/10.1111/j.1096-0031.2001...
) was calculated using 10 additional calculation runs. The relative measure corrects the distortion of the absolute value of support (Bremer support; Bremer, 1994Bremer K. Branch support and tree stability. Cladistics. 1994; 10(3):295–304. https://doi.org/10.1111/j.1096-0031.1994.tb00179.x
https://doi.org/10.1111/j.1096-0031.1994...
), since it is expressed as a proportion of evidence in favor and against a given clade (Goloboff, Farris, 2001Goloboff PA, Farris JS. Methods for quick consensus estimation. Cladistics. 2001; 17(1):26–34. https://doi.org/10.1111/j.1096-0031.2001.tb00102.x
https://doi.org/10.1111/j.1096-0031.2001...
). In addition, Bootstrap (Felsenstein, 1985Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985; 39(4):783–91.) and Jackknife values were calculated and expressed in GC (“group present / contradicted”; Goloboff et al., 2003Goloboff PA, Farris JS, Källersjö M, Oxelman B, Ramírez MJ, Szumik CA. Improvements to resampling measures of group support. Cladistics. 2003; 19:324–32. https://doi.org/10.1111/j.1096-0031.2003.tb00376.x
https://doi.org/10.1111/j.1096-0031.2003...
). Zero-length branches were collapsed (“rule 3”).
ANALYSIS 2 - Myological data concatenated with an integrated phenotypic matrix, and the influence of myological characters in phylogenies using PBS. Characters from dorsolateral head musculature mentioned above were concatenated with the morphological character matrix originally presented in Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
and subsequently modified by Peixoto et al. (2019) (Tab. S2). Searches were made on TNT under equal weights using new technologies (20 iterations of fuse, drift, ratchet and sectorial search), reaching the best score 50 times (hit = 50), and with all the fundamental trees submitted to additional TBR analyses. Following Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
, the tree was rooted at Carassius auratus (Linnaeus, 1758). The influence of characters from dorsolateral head musculature in concatenated analyses was estimated by Partitioned Bremer support (PBS; Baker, DeSalle, 1997Baker RH, DeSalle R. Multiple sources of character information and the phylogeny of Hawaiian drosophilids. Syst Biol. 1997; 46(4):654–73. https://doi.org/10.1093/sysbio/46.4.654
https://doi.org/10.1093/sysbio/46.4.654...
; Lambkin et al., 2002Lambkin CL, Lee MSY, Winterton SL, Yeates DK. Partitioned Bremer support and multiple trees. Cladistics. 2002; 18(4):436–44. https://doi.org/10.1111/j.1096-0031.2002.tb00159.x
https://doi.org/10.1111/j.1096-0031.2002...
; Lambkin, 2004Lambkin CL. Partitioned Bremer support localises significant conflict in bee flies (Diptera: Bombyliidae: Anthracinae). Invertebr Syst. 2004; 18:351–60. https://doi.org/10.1071/IS04004
https://doi.org/10.1071/IS04004...
), using the “pbsup.run” script available for TNT (Peña et al., 2006Peña C, Wahlberg N, Weingartner E, Kodandaramaiah U, Nylin S, Freitas AVL, Brower AVZ. Higher level phylogeny of Satyrinae butterflies (Lepidoptera: Nymphalidae) based on DNA sequence data. Mol Phylogenet Evol. 2006; 40(1):29–49. https://doi.org/10.1016/j.ympev.2006.02.007
https://doi.org/10.1016/j.ympev.2006.02....
).
Material examined. Material examined is listed below. Museum acronyms follow Sabaj, (2020)Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
https://doi.org/10.1643/ASIHCODONS2020...
. Size of specimens is expressed in Standard Length (SL, measured from the tip of the snout to the insertion of the median rays of the caudal fin), Total Length (TL, measured from the tip of the snout to the posterior margin of the longest caudal-fin ray or caudal filament) or Length at End of the Anal Fin (LEA, measured from the tip of the snout until the insertion of the last ray of the caudal fin). Length ranges refer to specimens examined, not necessarily to all specimens in lot. Museum specimens were stained according to Datovo, Bockmann, (2010)Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol. 2010; 8(2):193–246. http://dx.doi.org/10.1590/S1679-62252010000200001
http://dx.doi.org/10.1590/S1679-62252010...
. All specimens listed were prepared as myological dissections, except those indicated by an asterisk. Cleared and stained specimens are indicated by “c&s” and dry skeletons by “skl”.
Clupeiformes:Denticeps clupeoides: Benin: MZUSP 84776, 2, 31.7-40.1 mm SL. Gonorynchiformes:Chanos chanos: Australia: USNM 173572, 1, 167.3 mm SL. Cypriniformes:Carassius auratus*: Germany: MZUSP 91472, 3, 74.1-129.4 mm SL. Labeo chrysophekadion: Thailand: USNM 271352, 1, 81.2 mm SL. Characiformes: Brycon falcatus: Brazil: MZUSP 18089, 1, 102.83 mm SL. Cyphocharax festivus*: Brazil: MZUSP 103174, 5, 38.2-50.1 mm SL. Cyphocharax leucostictus: Brazil: MZUSP 21156, 1 c&s, not measured. Erythrinus erythrinus*: Brazil: MZUSP 34352, 13, 66.1-134.6 mm SL; MZUSP 34350, 2, c&s, 67.1-72.1 mm SL. Serrasalmus rhombeus*: Brazil: MZUSP 94907, 9, 84.8-89.7 mm SL; MZUSP 95862, skl, not measured; MZUSP 94082, skl, not measured. Dianema longibarbis*: Peru: MZUSP 26413, 6, 51.5-84.9 mm SL. Siluriformes: Dianema sp.: Brazil: MZUSP 30862, 2, c&s, not measured. Diplomystes mesembrinus: Argentina: MZUSP 62595, 2, 81.2-105.8 mm SL. Pterygoplichthys sp.*: Brazil: MZUSP, 92363, 2, not measured; MZUSP 117325, skl, not measured. Pseudostegophilus nemurus*: Brazil: MZUSP 57717, 5, 62.6-78.5 mm SL. Gymnotiformes: Apteronotidae: Adontosternarchus balaenops: Brazil: MZUSP 83219, 2, 165.2-175.3 mm LEA. Adontosternarchus clarkae: Brazil: MZUSP 30072, 1, 79.3 mm LEA. Adontosternarchus sachsi: Brazil: MPEG 2435, 1, 116.5 mm LEA. Apteronotus albifrons: Brazil: MZUSP 89044, 1, 75.8 mm LEA; MZUSP 22251, 1, 150.1 mm LEA. Apteronotus bonapartii: Brazil: MPEG 3038, 2, 204.6-217.5 mm LEA. Apteronotus camposdapazi: Brazil: MZUSP 114249, 1, 120. 7 mm TL [regenerated]. Apteronotus rostrarus: Colombia: USNM 317229, 1, 142.3 mm LEA. Compsaraia compsa: Brazil: MZUSP 56206, 1, 95.4-123.4 mm LEA. Orthosternarchus tamandua: Brazil: MZUSP 55955, 1, 286.3 mm LEA; MZUSP 56541*, 112.1 mm LEA. Parapteronotus hasemani: Brazil: MPEG 1161, 1, 191.5 mm LEA. Platyurosternarchus macrostomus: Brazil: MZUSP 105584, 194.2 mm LEA; MZUSP 57686, 1, 189.5 mm LEA. Pariosternarchus amazonensis: Brazil: MZUSP 58258, 109.4 mm LEA; MZUSP 57061*, 129.1 mm LEA. Porotergus gimbeli: Brazil: MZUSP 83300, 1, 148.8 mm LEA. MZUSP 57426, 2, 127.7-154.3 mm LEA. Tenebrosternarchus preto: Brazil: MPEG 22758, 2, 248.2-268.5 mm LEA. Sternarchogiton porcinum: Brazil: MZUSP 56319, 1, 202.2 mm LEA. Sternarchella duccis: Brazil: MZUSP 57370, 1, 146.9 mm LEA. Sternarchella raptor: Brazil: USNM 374014, 1, 71.9 mm LEA. Sternarchella schotti: Brazil: MZUSP 58187, 1, 141. 9 mm LEA. Sternarchella schotti: Brazil: MPEG 3481, 2, 154.05-155.3 mm TL [regenerated]; MPEG 7989, 1, 185.6 mm LEA. Sternarchorhynchus goeldii: Brazil: MPEG 1193, 1, 148.3 mm LEA. Sternarchorhynchus oxyrhynchus: Brazil: MZUSP 55851, 1, 227.0 mm LEA. Sternarchorhamphus mulleri: Brazil: MPEG 3712, 2, 335.1-335.4* mm LEA; USNM 373030, 1, 222.2 mm LEA. Gymnotidae: Electrophorus cf. electricus: Brazil: MZUSP 103699, 1, 530.12 mm LEA; MZUSP 85509, 1, 488.2 mm LEA. Gymnotus coatesi: Brazil: MPEG 27120, 1, 115.7 mm LEA. Gymnotus coropinae: Brazil: MPEG 21510, 1, 112.5 mm LEA; MZUSP 80142, 1, 137.0 mm LEA. Gymnotus gr. carapo: Brazil: MPEG 3012, 1, 232.2 mm LEA; MZUSP 90618, 1, 177.8 mm LEA. Gymnotus maculosus: Guatemala: USNM 114539, 1, 189.4 mm LEA. Gymnotus gr. pantherinus: Brazil: MZUSP 113616, 1, 151.3 mm LEA. Gymnotus cylindricus: Guatemala: USNM 134701, 1, 178.5 mm LEA. Hypopomidae: Brachyhypopomus sp.: Brazil: MPEG 12067, 1, 70.5 mm LEA. Brachyhypopomus bombilla: Brazil: MZUSP 59441, 1, 66.2 mm LEA. Brachyhypopomus beebei: Brazil: MZUSP 103275, 1, 74.6 mm LEA. Brachyhypopomus brevirostris: Brazil: MPEG 2397, 2, 65.9-71.2 mm LEA; MPEG 7295, 2, 50.0-61.3 mm LEA. MZUSP 30047, 1, 144.2 mm LEA. Brachyhypopomus draco: Brazil: UFRS 8887, 1, 140.4 mm LEA. Brachyhypopomus gaudeiro: Brazil: MZUSP 25165, 1, 79.1 mm LEA. Brachyhypopomus hendersoni: Brazil: MZUSP 113218, 1, 77.8 mm LEA; MZUSP 30050, 1, 67.5 mm LEA. Brachyhypopomus janeiroensis: Brazil: MZUSP 22702, 1, 80.9 mm LEA. Brachyhypopomus pinnicaudatus: Brazil: MZUSP 23216, 1, 87.8 mm LEA. Brachyhypopomus regani: Brazil: MZUSP 110609, 1, 107. 3 mm LEA. Brachyhypopomus sullivani: Brazil: MZUSP 105803, 1, 72.7 mm LEA. Hypopomus artedi: Suriname: USNM 408442, 1, 202. 7 mm LEA. Microsternarchus aff. bilineatus: Brazil: MPEG 12757, 1, 69.5 mm LEA; MZUSP 102314, 1, 71.19 mm LEA. Microsternarchus cf. bilineatus: Venezuela: MBUCV-V 7298, 1, 59.2 mm LEA. Hypopygus lepturus: Brazil: MPEG 10169, 1, 61.0 mm LEA. MZUSP 102317, 1, 45.8 mm LEA. Peru: MZUSP 91426, 3, 55.4 mm LEA. Steatogenys duidae: Brazil: MPEG 14670, 1, not measured. Steatogenys elegans: Brazil: MZUSP 83331, 1, 120.5 mm LEA. Rhamphichthyidae: Gymnorhamphichthys rosemariae: Brazil: MZUSP 56317, 1, 116.3 mm LEA. Gymnorhamphichthys rondoni: Brazil: MPEG 14681, 1, 107.1 mm LEA. MZUSP 85130, 1, 159.8 mm LEA. Rhamphichthys depranium: Brazil: MZUSP 36144, 1, 282.3 mm TL [regenerated]. Rhamphichthys hahni: Brazil: MZUSP 24736, 1, 479.5 mm TL [regenerated]. MZUSP 52514*, 280 mm LEA. Rhamphichthys lineatus*: Brazil: MZUSP 44823, 1, 417.2 LEA. Rhamphichthys marmoratus: Brazil: MPEG 8833, 1, 65.8 mm HL [head only]; MZUSP 44574*, 1, 258 mm LEA; MZUSP 36016*, 1, 290 mm LEA. Rhamphichthys rostratus*: Brazil: MZUSP 32233, 1, 643.6 mm LEA. Sternopygidae: Archolaemus cf. blax: Brazil: MZUSP 89304, 1, 101.5 mm LEA. Archolaemus ferreirai: Brazil: INPA-ICT 6496, 1, 128.5 mm LEA. Archolaemus janeae: Brazil: MZUSP 97383, 1, 171.0 mm LEA. Archolaemus luciae: Brazil: MPEG 23607, 1, 220.5 mm LEA. Archolaemus orientalis: Brazil: MPEG 21509, 1, paratype, 110 mm LEA. Archolaemus santosi: Brazil: LIRP 13010, 1, 171.5 mm LEA. Distocyclus conirostris: Brazil: MZUSP 23316, 1, 242.2 mm LEA; MPEG 20022, 1, 152.3 mm LEA; MCP 26287, 1, 133.0 mm LEA. Eigenmannia oradens: Venezuela: ANSP 190768, 1, paratype, 101.4 mm LEA. Eigenmannia antonioi: Brazil: MPEG 29487, 1, 80.0 mm LEA. Eigenmannia besouro: Brazil: MZUSP 98748, 1, paratype, 89.2 mm LEA. Eigenmannia desantanai: Brazil: MZUSP 38169, 1, 133.5 mm LEA. Eigenmannia guairaca: Brazil: LBP 9911, 1, 107.4 mm TL [regenerated]. Eigenmannia humboldtii: Colombia: FMNH 56812, 1, 186.2 mm LEA. Eigenmannia limbata: Brazil: MZUSP 75569, 1, 160.0 mm LEA. Eigenmannia macrops: Guyana: USNM 405266, 1, 103.2 mm LEA. Eigenmannia cf. macrops: Brazil: MZUSP 102072, 1, 269.4 mm LEA. Eigenmannia matintaperera: Brazil: MZUSP 29979, 113.0 mm LEA. Eigenmannia meeki: Panamá: MZUSP 119018, 1, paratype, 160.2 mm LEA. Eigenmannia microstoma: Brazil: MCP 45216, 1, 80.0 mm LEA. Eigenmannia muirapinima: Brazil: MZUSP 97577, 1, 117.0 mm LEA. Eigenmannia nigra: Brazil: MPEG 2430, 1, 154.1 mm LEA. MPEG 27121, 2, 170.6-180.1 mm LEA. Eigenmannia pavulagem: Brazil: MPEG 7308, 1, 90.9 mm LEA. Eigenmannia sayona: Venezuela: MPEG 33926, 1, paratype, 103.7 mm LEA. Eigenmannia trilineata: Argentina: MZUSP 111146, 305.0 mm LEA. Eigenmannia vicentespelaea: Brazil: MZUSP 83467, 1, 115.9 mm LEA. Eigenmannia virescens: Argentina: MZUSP 6319, 1, 155.4 mm LEA. Eigenmannia waiwai: Brazil: MZUSP 15882, 99.1 mm LEA. Japigny kirschbaum: Guyana: FMNH 50185, 1, 137.2 mm LEA. Rhabdolichops caviceps: Brazil: INPA 20157, 1, 103.9 mm LEA. Rhabdolichops eastwardi: Brazil: MZUSP 81178, 1, 188.3 mm LEA; MPEG 8148, 1, 113.7 mm LEA. Rhabdolichops electrogrammus: Brazil: INPA 28863, 1, 80.6 mm LEA. Rhabdolichops lundbergi: Brazil: INPA 11406, 1, 110.2 mm LEA. Rhabdolichops nigrimans: Brazil: INPA 28862, 1, 98.1 mm LEA. Rhabdolichops troscheli: Brazil: MZUSP 57704, 2, 122.2-140.2 mm LEA. Rhabdolichops zareti: Venezuela: CAS 57444, 1, 88.9 mm LEA. Sternopygus astrabes: Brazil: MZUSP 88795, 1, 151. 0 mm LEA. Sternopygus macrurus: Brazil: MZUSP 32215, 1, 212.6 mm LEA. MPEG 22756, 2, 240.4–245.8 mm LEA. Sternopygus xingu: Brazil: MPEG 8657, 1, 230. 5 mm LEA.
RESULTS
The dorsolateral musculature of the head of Gymnotiformes: general features
Buccopalatal membrane. The buccopalatal membrane comprises the lateral limits of the anterodorsal portion of the oral cavity, which is ventrally delimited by the mandible, anteriorly by the maxilla and posteromedially by the anterodorsal margin of the suspensorium. The degree of differentiation of the membrane in Teleostei is extremely variable, ranging from prominent to weakly differentiated from surrounding connective tissues (Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). In Gymnotiformes, the membrane is usually poorly differentiated, except in representatives of Gymnotidae (Figs. 1, 2, 3), where it is thick and well differentiated. Normally, few fibers of the malaris and rictalis have a weak association with the buccopalatal membrane. However, such connections are feeble and not recognized as additional insertion points for those sub-sections.
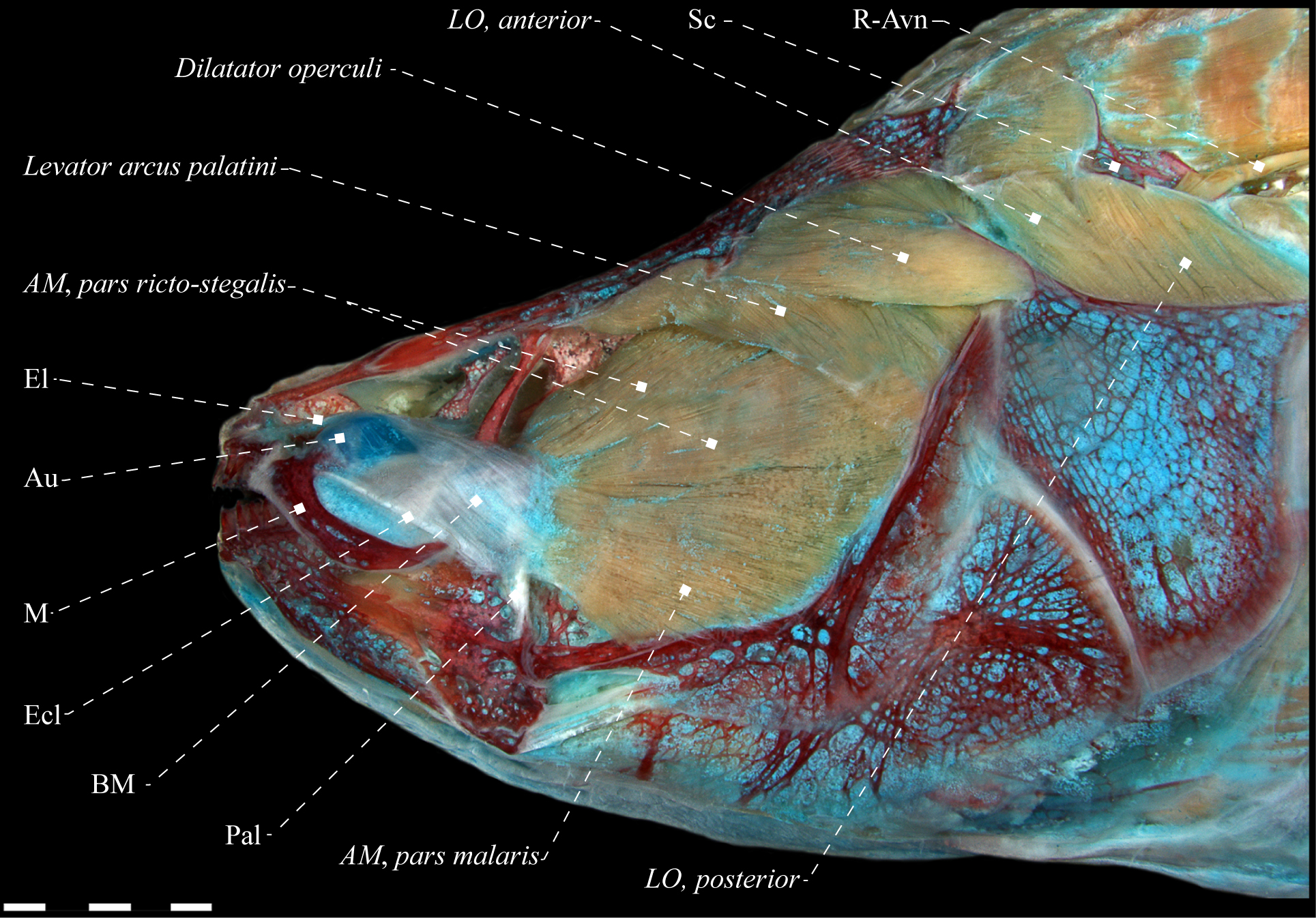
Dorsolateral head muscles of Gymnotus cylindricus (Gymnotidae), USNM 134701, 178.5 mm LEA. A. Lateral view; B. dorsal view. Anatomical abbreviations in Tab. 1. Scale bars = 4 mm.
Lateral view of dorsolateral musculature of Electrophorus cf. electricus (Gymnotidae), MZUSP 85509, 488.2 mm TL. Anatomical abbreviations in Tab. 1. Scale bar = 10 mm
Adductor mandibulae of Electrophorus cf. electricus (Gymnotidae), MZUSP 85509, 488.2 mm LEA. A. Lateral view; B. Mesial view. Anatomical abbreviations in Tab. 1. Scale bar = 10 mm.
In Gymnotidae, it is not possible to identify any ligaments associated with the buccopalatal membrane. However, in other gymnotiform subgroups the endomaxillary and ectomaxillary ligaments are often well differentiated. The endomaxillary ligament is present in representatives of some families (Hypopomidae: Fig. 4; Rhamphichthyidae: Fig. 5; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12; Sternopygidae: Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 11; and Apteronotidae: Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.). In those groups, the ligament receives the anterior fibers of the malaris and inserts directly on the jaw or on the connective tissue between the anterior margin of the premaxilla and the upper lip (in representatives of Apteronotidae; Figs. 6, 7). In Apteronotidae alone, there is a well-differentiated ectomaxillary ligament, which receives the anteroventral fibers of the malaris and inserts onto the maxilla (Fig. 7).
Mesial view of adductor mandibulae of Hypopomus artedi (Hypopomidae), USNM 408442, 202. 7 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 4 mm.
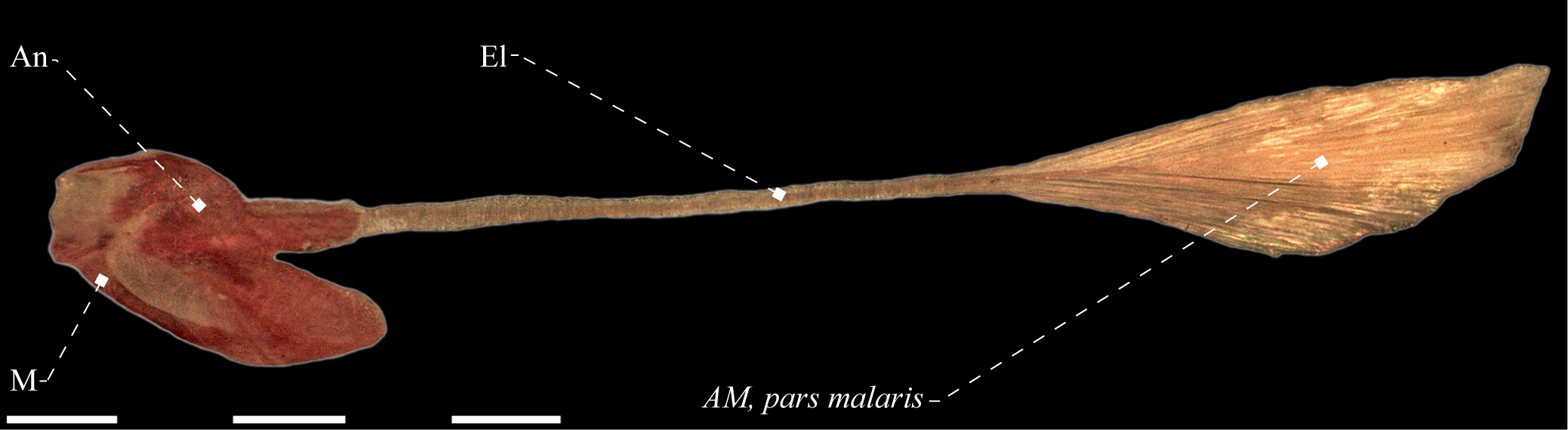
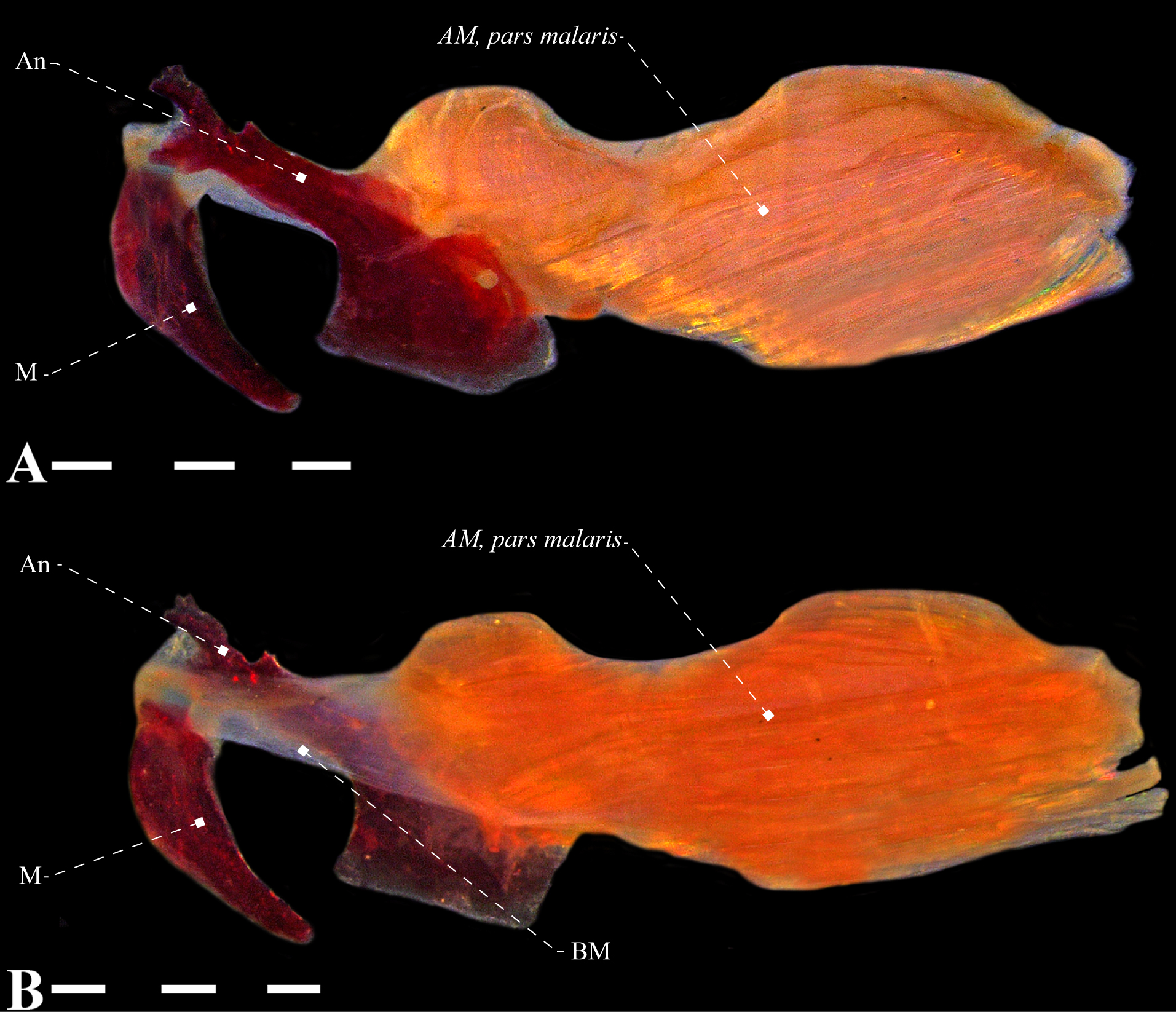
Lateral view of adductor mandibulae, pars malaris of Gymnorhamphichthys rosemariae (Rhamphichthyidae), MZUSP 56317. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
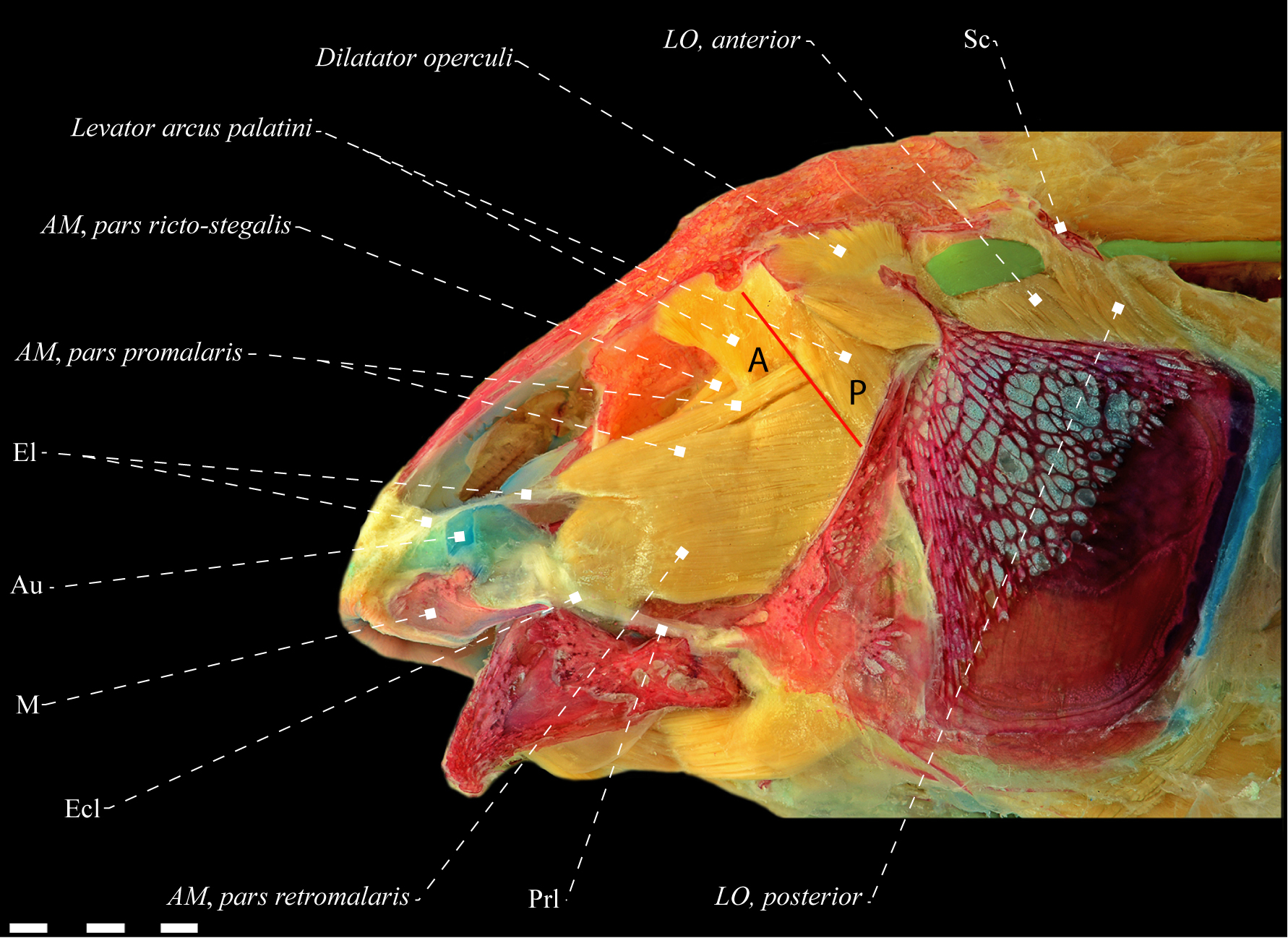
Adductor mandibulae of Tenebrosternarchus preto (Apteronotidae), MPEG 22758, 268.5 mm LEA. A. Lateral view; B. Mesial view. Median portion of the buccopalatal membrane removed. Anatomical abbreviations in Tab. 1. Scale bars = 5 mm.
Lateral view of dorsolateral musculature of Apteronotus albifrons (Apteronotidae), MZUSP 22251, 150.1 mm LEA. Green indicates the path of recurrent ramus of anteroventral part of anterior lateral line nerve. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
In Sternopygidae, there is a well-differentiated ligament located transversally in the posterior portion of the mandible and associated with the ventro-medial margin of infra-orbital 1 + 2 and posteriorly to the anguloarticular (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 40). This ligament is tentatively identified as a transverse ligament (Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
), which displays a pronounced degree of differentiation, unique to that family. Further, the vast majority of Apteronotidae, the buccopalatal membrane has two additional ligaments and their degree of differentiation is unique in the order. They are similar to the postangular and preangular ligaments (which are present in most Gymnotiformes), but contrary to the latter ligaments, they originate on the retroarticular. Such ligaments are referred to herein as pre-retroarticular and post-retroarticular ligaments (Fig. 7). The pre-retroarticular ligament differentiates anteriorly in the buccopalatal membrane towards the maxilla, and the post-retroarticular converges anteriorly on the same membrane, towards the sites of insertion of ricto-stegalis. Datovo, Vari, (2014)Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
illustrate and describe a preangular ligament in B. pinnicaudatus, however, in the majority of species analyzed this ligament is not differentiated and it was therefore not included in descriptions.
Adductor mandibulae. The adductor mandibulae of Gymnotiformes has varying configurations and its components display different degrees of differentiation, usually consisting of the adductor mandibulae, segmentum facialis and the adductor mandibulae, segmentum mandibularis (Tab. 2). Such segments are connected by an intersegmental aponeurosis, with a mandibular tendon dorsally and a meckelian tendon ventrally. These tendons are confluent along their length but still discernible because the former is located dorsally, roundish in cross section and slightly differentiated from the anterior portion of the segmentum facialis and the posterior portion of the segmentum mandibularis. In turn, the meckelian tendon is positioned ventrally, conspicuously flattened and inserted in the coronomeckelian bone. When present, the segmentum mandibularis has no subsections. It arises from the mandibular tendon, enters the mandible mesially and is located dorsally to Meckel’s cartilage (Figs. 4, 5). The segmentum mandibularis is absent in Gymnotidae (Fig. 3), Rhamphichthyidae (Fig. 8), most of the species of Archolaemus (Fig. 9) and in some representatives of Apteronotidae.
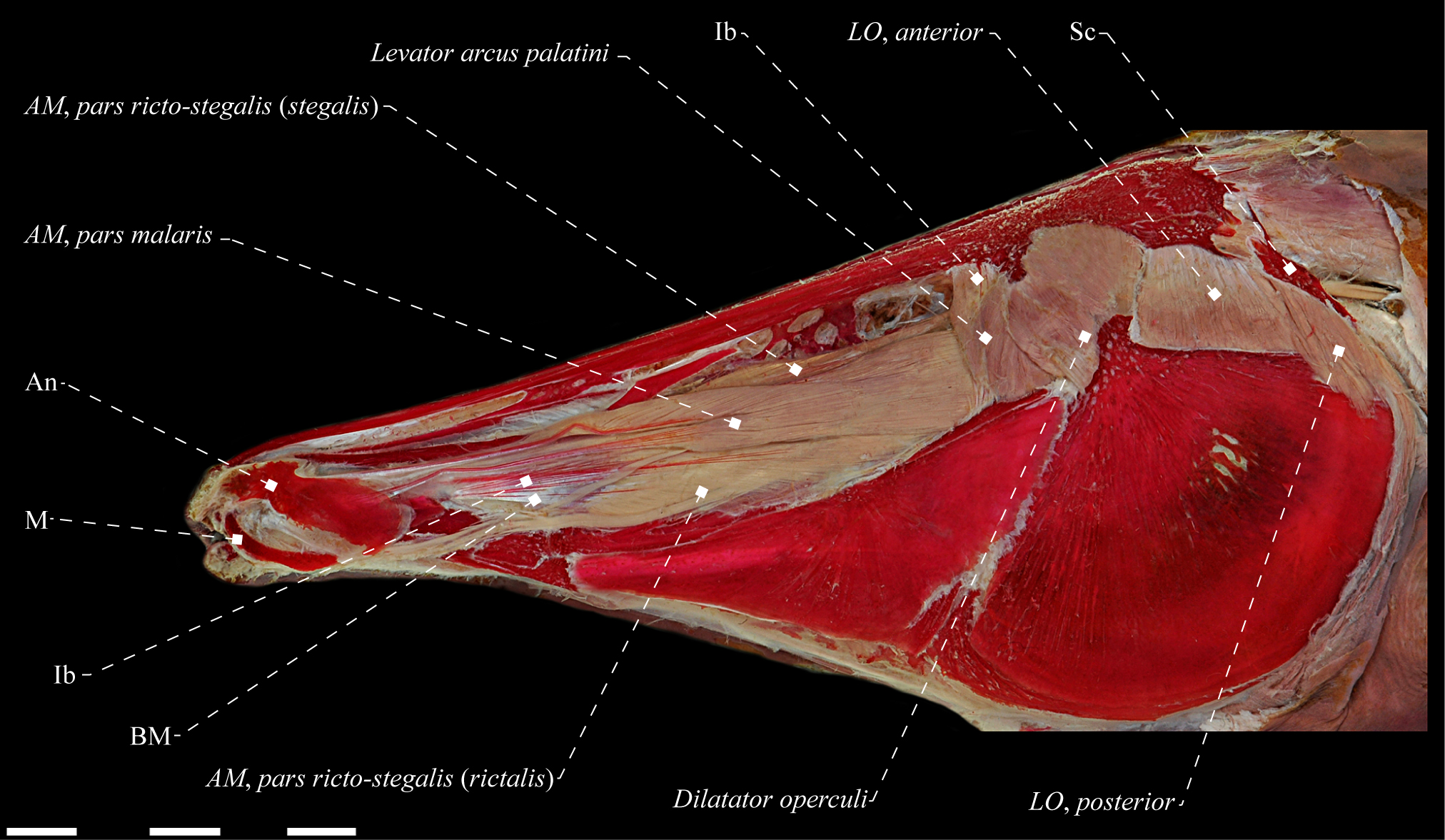
Rhamphichthys hahni (Rhamphichthyidae), MZUSP 24736, 479.5 mm TL. A. Lateral view of adductor mandibulae, pars ricto-stegalis; B. Mesial view of levator arcus palatini. Anatomical abbreviations in Tab. 1. Scale bars = 10 mm; 2 mm.
Mesial view of adductor mandibulae of Archolaemus janeae (Sternopygidae), MZUSP 97383, 171.0 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 4 mm.
The segmentum facialis is positioned mostly on the lateral surface of the suspensorium and is composed of three identifiable subsections in all Gymnotiformes: pars malaris, pars rictalis and pars stegalis. The degree of differentiation between these components is variable and ranges from a single unit, not divided into sub-sections, to a completely sectioned segment. The generalized condition in the order consists of the complete differentiation of the three sections, however, composite sub-sections can occur, as a stego-malaris (in Gymnotidae) or a ricto-stegalis (in Rhamphichthyidae and several Apteronotidae).
The malaris is commonly located immediately ventral to the orbit, usually arranged dorsolaterally to the dorsal portion of the rictalis and latero-ventrally to the mid-ventral portion of the stegalis (except for some apteronotids; see comments in “Posteroventral malaris in Apteronotidae”; Fig. 8). This section, or its corresponding fibers, arises mostly from the bony elements of the suspensorium, and may also include some components of the neurocranium (e.g., frontal, sphenotic, or parasphenoid). The insertion points are extremely variable across the order, and may involve the mandibular tendon or even in the connective tissue between the anterior margin of the premaxilla and the upper lip. The generalized pattern for gymnotiforms includes an insertion in the maxilla, and usually also involves elements of the infraorbital series (e.g., antorbital in Rhamphichthyidae and Hypopomidae, and infra-orbital 1 + 2 in Sternopygidae, Figs. 4, 10A and 11), the mandible (in Gymnotidae and Adontosternarchus) or even the mesethmoid (in Sternarchella). Commonly, the malaris consists of a single uncut section, albeit differentiated into a dorsal and a ventral sub-section (see comments in “The malaris sectioned in promalaris and retromalaris”).
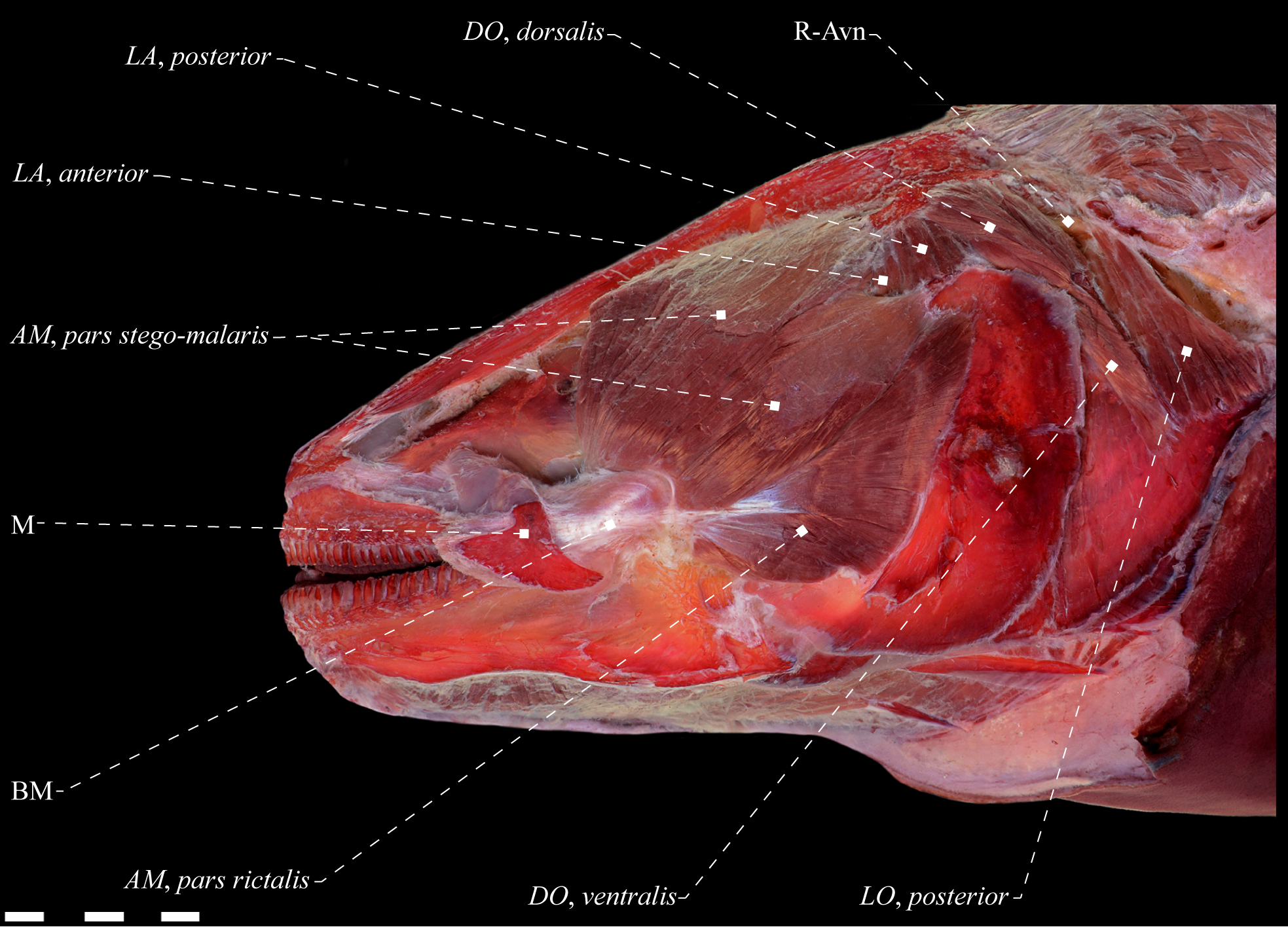
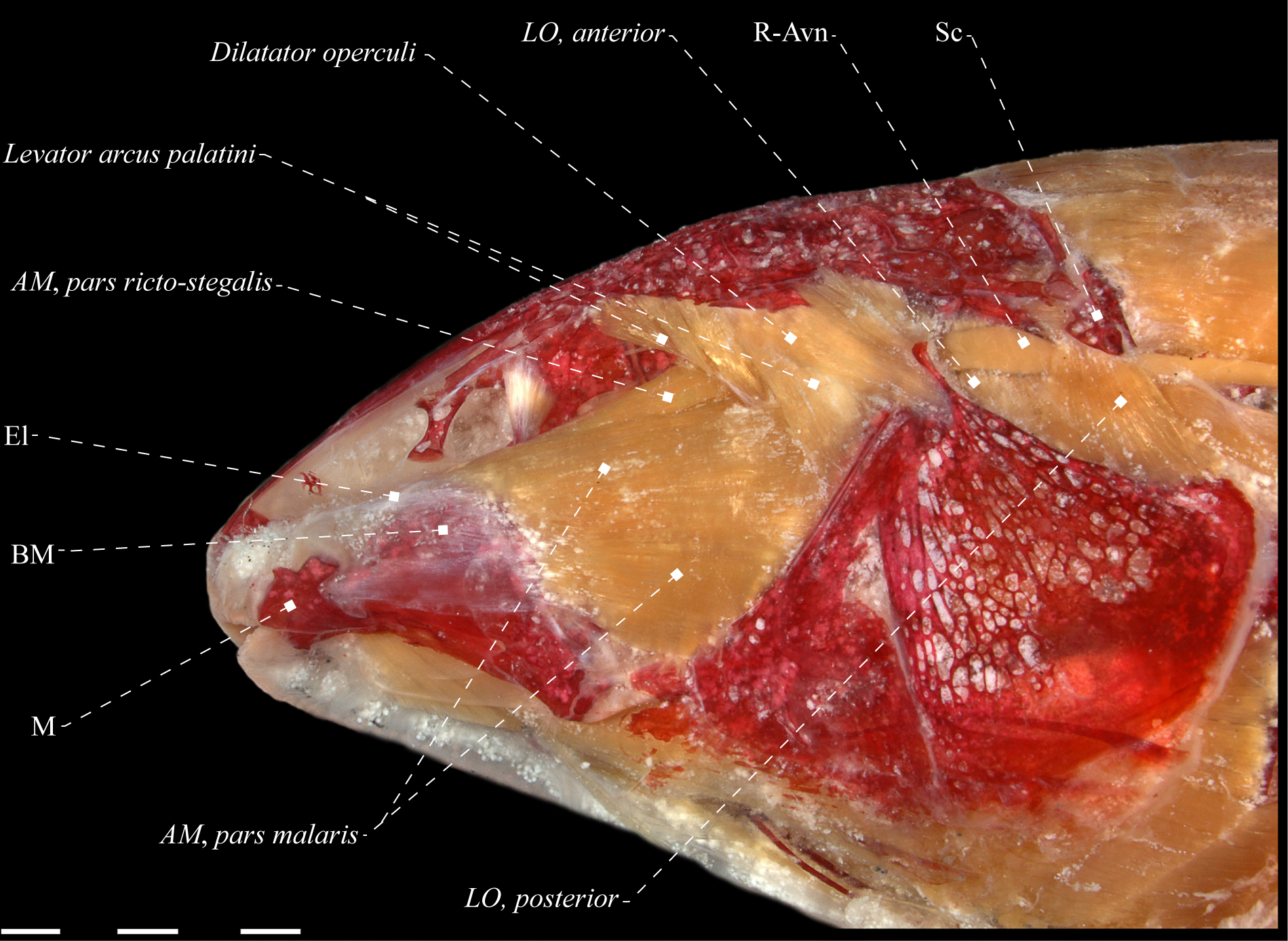
Electrophorus cf. electricus (Gymnotidae), MZUSP 85509, 488.2 mm LEA. A. Lateral view of dorsolateral head muscles; B. Posterior portion of dorsolateral head muscles. Adductor mandibulae dissected in B. LO, anterior not visible in lateral view. Anatomical abbreviations in Tab. 1. Scale bar = 10 mm.
Lateral view of dorsolateral musculature of Hypopygus lepturus (Hypopomidae), MZUSP 91426, 55.4 mm LEA. Some fibers of the LO, anterior accidentally removed during dissections. Anatomical abbreviations in Tab. 1. Scale bar = 2 mm.
Mesial view of suspensorium of Brachyhypopomus janeiroensis (Hypopomidae), MZUSP 22702, 80.9 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 4 mm.
The rictalis is located mid-ventrally in relation to the other sub-sections of the adductor mandibulae, arising strictly from the suspensorium, even in cases where it is not differentiated from the stegalis. The insertion sites commonly include the coronoid process, with some fibers attaching also on the posterolateral margin of the anguloarticular or on the intersegmental aponeurosis. The stegalis makes up the mesial-most sub-section of the segmentum facialis, arising from elements of the suspensorium, but normally also components of the neurocranium. Anteriorly, the stegalis differentiates into an intersegmental aponeurosis, dorsally entering the mandibular tendon (origin of the segmentum mandibularis) and ventrally the meckelian tendon, inserting into the coronomeckelian bone. The path of the ramus mandibularis trigeminus nerve is variable across the order (Tab. 3). This nerve is invariably mesial to the malaris and lateral to the stegalis and may be mesial or lateral to the rictalis, occasionally penetrating it. In some cases, the ramus mandibularis trigeminus may be located medially to the adductor mandibulae.
An interesting aspect of some Gymnotiformes is the presence of intermuscular bones in the adductor mandibulae. In the generalized condition of the order, the segmentum facialis composition is characterized by the absence of intermuscular bones, being essentially fibrous. However, in Gymnotus gr. carapo, Rhamphichthys, Iracema and Orthosternarchus, the subsections present ossifications of some tendons, resulting in bone filaments associated with the fibers or ligaments of this segment, named as intermuscular bones (Fig. 8A) (LAWP, pers. obs.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Hilton et al., 2007Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
; Carvalho, Albert, 2011Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
).
Intermuscular bones are present in the adductor mandibulae of species of Orthosternarchus and Rhamphichthys, and Hilton et al., (2007)Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
hypothesized that the pronounced elongation of the snout is related to the origin of those structures. Gymnotiform taxa with intermuscular bones indeed have long snouts, namely species of Rhamphichthys (snout length 46–64% HL; Carvalho, 2013Carvalho TP. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): Diversification in South American Freshwaters. [PhD Thesis]. Lafayette: University of Louisiana; 2013. Available from: https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750
https://www.proquest.com/openview/ffbdff...
), Iracema (53.8–55.4%; Carvalho, Albert, 2011Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
) and Orthosternarchus (52–60% HL; LAWP, pers. obs.) when compared to the other members of the order. However, other species with similarly elongated snouts (e.g., 34.5–68.6% HL in Gymnorhamphichthys spp., Carvalho, 2013Carvalho TP. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): Diversification in South American Freshwaters. [PhD Thesis]. Lafayette: University of Louisiana; 2013. Available from: https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750
https://www.proquest.com/openview/ffbdff...
; 44–71.2% in Sternarchorhynchus spp., de Santana, Vari, 2010de Santana CD, Vari RP. Electric fishes of the genus Sternarchorhynchus (Teleostei, Ostariophysi, Gymnotiformes); phylogenetic and revisionary studies. Zool J Linn Soc. 2010; 159(1):223–371. https://doi.org/10.1111/j.1096-3642.2009.00588.x
https://doi.org/10.1111/j.1096-3642.2009...
; 46.4–63.7% in Apteronotus acidops, Triques, 2011Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool. 2011; 61(3):299–306.), do not have any ossifications of the tendons of the adductor mandibulae sections. Additionally, such bones also occur in Gymnotus gr. carapo, a short-snout species (approx. 32–39.4% HL). Therefore, the occurrence of intermuscular bones in the adductor mandibulae apparently does not have direct correlation with the length of the snout. Later, Datovo, Vari, (2014)Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
hypothesized that ossification of internal tendons of the adductor mandibulae as a potential origin of such intermuscular bones. The commonly mesial disposition and composition of intermuscular bones, with anterior and posterior tendon portions gradually ossified towards the middle portion, agree with that hypothesis. However, the relationship between these elements and other morphofunctional traits still needs further investigation.
Levator arcus palatini. The levator arcus palatini lies posterior to the orbit, with a variable general shape ranging from roughly parallelogram, trapezoidal or inverted triangle. In Gymnotiformes, the muscle is usually in a single mass of muscle, though partial sectioning occurs in Electrophorus where two sections are recognizable (Fig. 10). The levator arcus palatini originates on the mesial part of the ventral surface of the sphenotic, commonly including also the frontal and, occasionally, the pterosphenoid. The insertion is invariably on the hyomandibula and occasionally also on the preopercle. Only the posterodorsal portion of the levator arcus palatini is positioned mesially to the dilatator operculi. However, in some representatives of Apteronotidae, in Gymnotus, and in Steatogenys, it has a mesial arrangement where the anterior margin of the dilator operculi exceeds the medial portion of the levator arcus palatini. The orientation of the anterior-most fibers is also variable, ranging from oblique to the longitudinal axis of the head (at approximately 45° angle; Fig. 1), to orthogonal relative to that axis (Figs. 2, 10).
The levator arcus palatini has a variable insertion on the hyomandibula, with four subsets of fibers commonly recognized (anterolateral, posterolateral, anteromesial and posteromesial) according to their disposition relative to the malaris or rictalis (in representatives of Apteronotidae). The most common pattern comprises a completely lateralized arrangement of the levator arcus palatini in relation to the segmentum facialis. The levator arcus palatini tends to section the malaris of the other sections, as well as the rictalis of stegalis in their respective points of origin, even when the latter are not conspicuously differentiated. The generalized pattern consists of a strictly fibrous composition of levator arcus palatini, however, some more mesial tendons ossify in Sternopygus xingu Albert & Fink, 1996 and Rhamphichthys (Fig. 8B).
Dilatator operculi. The dilatator operculi is located posterior to the levator arcus palatini, and is usually organized in a single block of mass, without sub-sections, except in Electrophorus where it is divided into dorsal and ventral component (Fig. 10). Origin is usually on the sphenotic and hyomandibula, sometimes also including the frontal and the pterotic, rarely the preopercle, orbito-sphenoid, and pteroesophoid. Insertion is invariably on the dorsal process of the opercle. The generalized pattern of the dilatator operculi in Gymnotiformes comprises a muscle strictly fibrous. Hilton et al., (2007)Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
report and illustrate the presence of intermuscular bones in the dilatator (and levator) operculi of Orthosternarchus. However, such structures are present only in the adductor mandibulae and, being absent in the dilatator and levator operculi of specimens of that taxon analyzed herein.
Levator operculi. The levator operculi is a laminar and superficial muscle, located immediately posterior to the dilatator operculi and laterally to the adductor operculi. The generalized condition in Teleostei consists of an undivided block without sub-sections, originating from the posterodorsal elements of the posterior portion of the neurocranium and inserting on the opercle (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
). The morphology of the levator operculi in Gymnotiformes departs quite markedly from the generalized teleost pattern. As previously reported (de la Hoz, Chardon, 1984de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.), the levator operculi in Gymnotiformes has two sections, here referred to as levator operculi anterior and levator operculi posterior (Figs. 1, 2, 7). Despite the recognition of such subdivisions, their delimitation and identification are still unclear and needs re-examination.
The levator operculi anterior can be differentiated from the levator operculi posterior by the following attributes: (1) origin in the pterotic (vs. postotic canal segment corresponding to the supracleithrum); (2) mesial to R-Avn nerve (vs. lateral); (3) insertion mainly on the crest of dorsal portion of the opercle (vs. insertion mainly in the posterolateral face of the opercle). Thus, the recognition of these sections is possible on the basis of their points of origin, insertion and, mainly, by the layout of the R-Avn. The levator operculi anterior originates from the lateral surface of the mid-ventral portion of the pterotic, rarely including the hyomandibula (as in Eigenmanniinae and Steatogenys) or the exoccipital (as in Electrophorus). The insertion of that subsection is invariably on a ridge in the posterodorsal portion of the opercle, with its fibers extend beyond the dorsal margin of the bone. In addition, the nerve R-Avn is positioned laterally in relation to it in the vast majority of gymnotiform species, except in members of Eigenmanniinae (Sternopygidae), Rhamphichthys (Rhamphichthyidae), Platyurosternarchus and some species of Sternarchella (Apteronotidae). Contrastingly, the levator operculi posterior originates mainly from the postotic canal segment corresponding to the supracleithrum, including also the posterior margin of the pterotic in Gymnotus (Gymnotidae), Sternopygus, R. lundbergi, R. nigrimans (Sternopygidae), Adontosternarchus, Platyurosternarchus, and Sternarchella (Apteronotidae). Its insertion site is the lateral side of the posterior portion of the opercle, with its fibers ventrally-deflected when compared to those of the levator operculi anterior. Occasionally, the posterior section inserts on the posterior portion of the dorsal crest of the opercle (e.g., Sternarchella duccius and S. raptor). Finally, the nerve R-Avn is arranged mesially to the levator operculi posterior in all species of Gymnotiformes.
Hypopygus (Hypopomidae) is the only genus that does not present the levator operculi posterior, being restricted to the presence of the anterior section (Fig. 11). In that genus, the levator operculi originates exclusively from the pterotic, is positioned mesially to the R-Avn and inserts only on the dorsal crest of the opercle. It is therefore clearly homologous to the levator operculi anterior in other Gymnotiformes. The subdivision of the levator operculi in gymnotiforms has convergent occurrences in Microgadus (Gadidae; Gadiformes) and Stephanolepis (Monacanthidae; Tetraodontiformes) as described by Winterbottom (1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
,bWinterbottom R. The familial phylogeny of the Tetraodontiformes (Acanthopterygii: Pisces) as evidenced by their comparative myology. Smithson Contrib Zool. 1974b; 155:1–201. https://doi.org/10.5479/si.00810282.155
https://doi.org/10.5479/si.00810282.155...
).
Adductor arcus palatini. The adductor arcus palatini is the mesial-most dorsolateral head muscle, located dorsally to the suspensorium and ventrally to the neurocranium. This muscle has a laminar, tapered aspect, originating mainly from the parasphenoid, but sometimes also including the prootic (several groups) and the orbitosphenoid (Japigny only). The insertion occurs mostly on the lateral side of the suspensorium, invariably involving the endopterygoid, metapterygoid and hyomandibula, with participation of the symplectic in some Hypopomidae. Some posterodorsal fibers of the adductor arcus palatini are connected with the anterodorsal fibers of the adductor hyomandibulae, however, differentiation between these two muscles is always present and thus they are considered entirely separated from each other (Fig. 12).
The generalized pattern of Gymnotiformes consists of an adductor arcus palatini totally covered by the adductor mandibulae, and its visualization requires removal of the former. In some representatives of Sternopygidae and Apteronotidae, only the posterior portion is overlapped by the segmentum facialis (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 8; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 40). Exceptions occur in the majority of Archolaemus species, where those muscles never overlap each other (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15).
Adductor hyomandibulae. The adductor hyomandibulae, along with the adductor arcus palatini, is derived from the constrictor hyoideus dorsalis (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Miyake et al., 1992Miyake T, McEachran JD, Hall BK. Edgeworth’s legacy of cranial muscle development with an analysis of muscles in the ventral gill arch region of batoid fishes (Chondrichthyes: Batoidea). J Morphol. 1992; 212(3):213–56. https://doi.org/10.1002/jmor.1052120304
https://doi.org/10.1002/jmor.1052120304...
; Datovo, Rizzato, 2018Datovo A, Rizzato PP. Evolution of the facial musculature in basal ray-finned fishes. Front Zool. 2018; 15(40):1–29. https://doi.org/10.1186/s12983-018-0285-6
https://doi.org/10.1186/s12983-018-0285-...
). This muscle differentiates from the posterior portion of the adductor arcus palatini or from fibers of the adductor operculi, and its ontogenetic origin is variable across the Teleostei (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Diogo, Vandewalle, 2003Diogo R, Vandewalle P. Review of superficial cranial musculature of catfishes, with comments on plesiomorphic states. In: Arratia G, Kapoor B, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers; 2003. p.47–70.).
In Gymnotiformes, the adductor hyomandibulae has a laminar aspect, noticeable in mesial view of the suspensorium. The muscle arises mainly from the lateral side of the prootic, sometimes also the pterotic and, more rarely, the parasphenoid and sphenotic. The insertion is invariably on the mesial surface of the posterodorsal portion of the hyomandibula. Some anterior fibers are partially continuous with the adductor arcus palatini and the adductor operculi, but the respective muscles are completely differentiated from each other. Little variation was detected in this muscle across the Gymnotiformes.
Adductor operculi.Adductor operculi is a laminar-looking muscle, completely mesially arranged to the levator operculi, resulting in a total overlap by this muscle. The points of common origins include the exoccipital, pterotic and the pro-optic, punctually being able to include the basioccipital and the epoccipital. The insertion occurs invariably on the mesial side of the opercle (Fig. 12). Some anterodorsal fibers are associated with the mid-posterior portion of adductor hyomandibulae. The R-Avn nerve is invariably disposed laterally to the adductor operculi. Little variation was detected in this muscle along the order.
General aspects of the dorsolateral head muscles of the Gymnotidae.
Adductor mandibulae. The stego-malaris is positioned dorsolaterally to the rictalis, and it originates from elements of the suspensorium and neurocranium (Figs. 1, 2). Towards their insertion, those two sub-sections become gradually differentiated, with the dorsal fibers, presumably corresponding to the malaris, converging in a thick mandibular tendon, which inserts on the mesial surface of the coronoid process; in Electrophorus such fibers converge also on the lateral side of the same spot, forming an additional insertion point. The dorsomesial fibers, corresponding to the stegalis, converge in a thick meckelian tendon inserted on the dorsal margin of the corono-meckelian bone. Invariably, the stego-malaris has some lateral fibers converging directly on the buccopalatal membrane.
The rictalis originates in bony elements of the middle-ventral portion of the suspensorium and is inserted mainly on the coronoid process, but with some lateral fibers also associated with the buccopalatal membrane. At its origin, the posterolateral fibers of the rictalis extend beyond the anterior margin of the preopercular fossa, reaching the posterior margin of the bone or its medial portion. Usually in Gymnotidae, ossification of tendons in the facial segment are absent, but in Gymnotus gr. carapo the lateral-most tendons of that segment have intermuscular bones. The trajectory of the ramus mandibularis trigeminus nerve is variable in the family. It may be disposed mesially in relation to the rictalis and lateral to the stego-malaris, trespassing the rictalis and lateral to the stego-malaris or mesial relative to all sections of adductor mandibulae.
Levator arcus palatini. Invariably in Gymnotidae, the levator arcus palatini is a roughly triangular in Gymnotus (Fig. 1) or trapezoidal in Electrophorus (Fig. 2). In Electrophorus, the origin of the levator arcus palatini is narrower than its insertion, while in Gymnotus the origin is twice as wide as its insertion. The anterior-most fibers of this muscle are straight relative to the axis of the head in Electrophorus or anteroposteriorly oblique in Gymnotus.
Electrophorus has the levator arcus palatini divided in two sections (levator arcus palatini anterior and levator arcus palatini posterior; Fig. 13), a unique condition in Gymnotiformes. The origin of the levator arcus palatini include the sphenotic, occasionally also the lateroventral portion of the frontal. Its insertion is mainly on the dorsal portion of the hyomandibula and, when divided, also on the preopercle. At the insertion point, the fibers of this muscle partially separate the stegalis from the malaris in their respective points of origin, arranged in different ways in relation to the adductor mandibulae. The levator arcus palatini has a variable disposition relative to the dilatator operculi, being mesial to it, with the anterior margin of the dilator operculi surpassing the medial portion of the levator arcus palatini in Gymnotus (Fig. 1), or with only the posterodorsal fibers being mesial to the dilatator operculi in Electrophorus. The levator arcus palatini lacks tendon ossifications in Gymnotidae (Fig. 2).
Dilatator operculi. The dilatator operculi has a roughly rectangular or conical shape. Like the latter, the dilatator operculi in Electrophorus is uniquely divided in two sections, here named dilatator operculi ventralis and dilatator operculi dorsalis (Fig. 11). The dilatator operculi ventralis differentiates as a consequence of a ventral displacement of the opercle in relation to the preopercle. The dilatator operculi dorsalis is probably homologous to the dilatator operculi of Gymnotus and other Gymnotiformes. The points of origin of this muscle include elements of the posterolateral portion of the neurocranium, hyomandibula and, when divided, also the preopercle. Its insertion, contrastingly, is always on the dorsal process of the opercle.
Levator operculi. The origin of the levator operculi anterior is on the pterotic and may include also the exoccipital in Electrophorus. The levator operculi posterior arises primarily from the postotic canal segment corresponding to the supracleithrum, with some anterodorsal fibers sometimes originating also in the pterotic in Gymnotus. The R-Avn nerve is invariably lateral to the levator operculi anterior and mesial to the levator operculi posterior. The insertion of the muscle is invariably on the opercle.
The position of the levator operculi anterior in Electrophorus deserves note. In the vast majority of species in Gymnotiformes, both levatores are clearly visible in lateral view (Figs. 1, 7). In Electrophorus, contrastingly, the anterior section is displaced mesially and as a consequence it is almost invisible in lateral view (Fig. 2), requiring dislocation of the posterior section for full view.
Adductor arcus palatini. This muscle invariably originates in the parasphenoid and prootic. Anteriorly, it inserts on the lateral face of the endopterygoid and metapterygoid; as the muscle progresses posteriorly, its insertion shifts from the lateral to the medial face of the suspensorium, finally inserting on the medial surface of the hyomandibula. Examination of the adductor arcus palatini requires dissection and partial removal of the adductor mandibulae, which completely overlaps it.
Adductor hyomandibulae. This muscle arises from the ventral region of the prootic, sphenotic and pterotic, inserting on the posteromedial margin of the hyomandibula. Little or no variation in this muscle was found throughout the Gymnotidae.
Adductor operculi. The origin of the adductor operculi is on the exoccipital, and may also include the other post-ventral elements of the neurocranium, such as the basioccipital in Electrophorus; or the pterotic and epioccipital in Gymnotus. Anteriorly, the insertion is on the dorsal margin of a dorso-mesial crest of the opercle, and posteriorly it inserts on the mesial surface of the same bone.
Detailed description of the dorsolateral musculature of the head in the genera of Gymnotidae.
Electrophorus Gill, 1864
Adductor mandibulae. The stego-malaris originates in the hyomandibula, metapterygoid, endopterygoid, quadrate, parasphenoid, frontal and sphenotic. Its component subsections are increasingly differentiated towards its insertion, where dorsolateral fibers, presumably corresponding to the malaris, converge towards the coronoid process and dorsomesial fibers converge on a thick mandibular tendon inserted on the mesial surface of the coronoid process. The middle fibers, presumably correspond to the stegalis, diverge in a thick meckelian tendon inserted on the dorsal margin of the coronomeckelian bone (Fig. 3). Dorsomesial fibers corresponding to the stegalis are disposed laterally relative to the posterolateral region of the basal portion of the endopterygoid, not completely overlapping it but totally covering laterally the adductor arcus palatini (Fig. 2).
The rictalis originates in the preopercle, quadrate and hyomandibula, with lateral fibers surpassing the anterior margin of the preopercular fossa, but not reaching the posterior margin of the bone, being restricted to its medial portion. The rictalis inserts mainly on the coronoid process, but with a few lateral fibers on the buccopalatal membrane. The dorsal portion of the rictalis is disposed mesially to the stego-malaris, with anterior fibers close to its insertion fully differentiated from the latter. The ramus mandibularis trigeminus nerve is arranged mesially to the rictalis and laterally to the stego-malaris (n = 1) or mesially to all sections of the adductor mandibulae (n = 1).
Levator arcus palatini. The levator arcus palatini anterior originates in the anteroventral margin of the sphenotic and is inserted on the hyomandibula. The levator arcus palatini posterior originates in the posteroventral margin of the sphenotic and inserts onto the hyomandibula and preopercle. In the region of origin, the two sections are partially continuous and sectioned by the truncus hyomandibularis nerve, which is lateral to the anterior section and mesial to the posterior one (Fig. 10). At the insertion, fiber bundles of the levator arcus palatini anterior are mesial to the malaris and those of the levator arcus palatini posterior are lateral to the malaris and posterolaterally to the rictalis. The origin of the levator arcus palatine is narrower than its insertion and only its posterodorsal fibers are mesial to the dilatator operculi.
Dilatator operculi. The dilatator operculi ventralis has a roughly rectangular shape, originating in the posterior margin of the preopercle and inserting on the anterodorsal portion of the dorsal process of the opercle. The dilatator operculi dorsalis is approximately conical and originates in the ventral margin of the pterotic, sphenotic and dorsal portion of the hyomandibula. The fibers of dorsal and ventral sections are partially continuous near their insertion on the anterodorsal and dorsal part of the dorsal process of the opercle (Fig. 10).
Levator operculi. The origin of the levator operculi anterior occurs in the posteroventral margin of the pterotic and exoccipital, while its insertion is into a ridge on the dorsal margin of the opercle, posterior to the anterodorsal process of that bone. The origin of the levator operculi posterior is in the postotic canal segment corresponding to the supracleithrum and it inserts along a crest at the posterolateral margin of the opercle, with fibers surpassing the dorsal margin of that bone (Fig. 2).
Gymnotus Linnaeus, 1758
Adductor mandibulae. The stego-malaris originates in the hyomandibula, metapterygoid, quadrate, parasphenoid, frontal and sphenotic. The subsections become gradually differentiated towards their insertion, where the dorsolateral fibers, corresponding to the presumed malaris, converge on a thick mandibular tendon inserted into the mesial face of the coronoid process. The middle fibers, presumably corresponding to the stegalis, diverge in the meckelian tendon and are inserted on the dorsal margin of the coronomeckelian bone. Some lateral fibers near the insertion site of the stego-malaris are inserted on the buccopalatal membrane, which in turn is associated with the posterior margin of the maxilla.
Stego-malaris is composed mainly of fibers. However, in Gymnotus gr. carapo the more lateral fibers of this sub-section, corresponding to the presumed malaris, have tendinous ossifications forming intermuscular bones. The mesial fibers, corresponding to the presumed stegalis, are laterally positioned in relation to the posterolateral region of the basal portion of the endopterygoid, not completely overlapping it, and are lateral relative to the adductor arcus palatini, totally overlapping it.
The rictalis originates in the preopercle, quadrate and hyomandibula, with lateral fibers surpassing the anterior margin of the preopercular fossa and reaching the posterior margin of that bone. This subsection is inserted mainly on the coronoid process, but with some more lateral fibers associated with the buccopalatal membrane. The rictalis is normally composed of fibers only, except in Gymnotus gr. carapo, in which some tendons ossify and form intermuscular bones. The ramus mandibularis trigeminus nerve is mesial to all sub-sections of the adductor mandibulae (in one specimen of G. coropinae, the nerve trespasses the rictalis).
Levator arcus palatini. The levator arcus palatini has the shape of an inverted triangle; with its origin twice as wide as its insertion. This muscle originates in the ventral margin of the frontal and sphenotic and is inserted on the hyomandibula. The levator arcus palatini is a non-sectioned muscle, except at the insertion, where four sub-sets of fibers are identifiable. Each of them has distinct modes at its insertion point in relation to the malaris: the anterolateral fibers, posterolateral and posteromesial is disposed laterally in relation to the malaris and dorsally to the rictalis; and the anteromesial fibers are arranged mesially in relation to the malaris. This muscle is partially mesial to the dilatator operculi, which exceeds the medial portion of the levator arcus palatini (Fig. 1).
Dilatator operculi. The dilatator operculi is roughly conical. It originates in the posterior margin of the sphenotic, pterotic, frontal and dorsal portion of the hyomandibula, and inserts on the dorsal and anterodorsal portion of the dorsal process of the opercle. In Gymnotus cylindricus its insertion includes the dorsoposterior portion of the dorsal process of the opercle. The dilatator operculi is lateral to the levator arcus palatini, overlapping approximately 2/3 of its mid-posterior portion.
Levator operculi. The levator operculi anterior originates in the ventral margin of the pterotic and is inserted in a ridge on the dorsal margin of the opercle, posterior to its anterodorsal process. The levator operculi posterior originates in the postotic canal segment corresponding to the supracleithrum and posteroventral margin of the pterotic, inserting along a ridge on the posterolateral margin of the opercle, with fibers extending beyond the dorsal margin of the bone.
General aspects of the dorsolateral head muscles of the Hypopomidae.
Adductor mandibulae. The adductor mandibulae in Hypopomidae consists of the segmentum facialis, which is connected to the segmentum mandibularis through an intersegmental aponeurosis well-differentiated in two components. The dorsal component differentiates into a mandibular tendon, which serves as the origin of the segmentum mandibularis, and the ventral component differentiates into the meckelian tendon, continuous with the stegalis for insertion into the coronomeckelian bone (Fig. 4). The segmentum facialis is composed of three subsections in all species analyzed: the adductor mandibulae, pars malaris; pars stegalis and pars rictalis.
The malaris is positioned latero-dorsally to the dorsal portion of the rictalis and the latero-ventrally to the stegalis. This sub-section originates in the mid-dorsal portion of the hyomandibula and preopercle, except in species of Brachyhypopomus, where its origin is on the hyomandibula only. Its insertion is invariably on the antorbital, where the mesial fibers differentiate into a diminutive endomaxillary ligament inserted on the posteromedial margin of the maxilla (Fig. 4). In some specimens of Brachyhypopomus, the endomaxillary ligament is only visible after complete removal of the buccopalatal membrane (Fig. 13).
Except for a few species of Brachyhypopomus, the malaris has a concavity on its dorsal margin for the allocation of the eyeball (Figs. 13, 14). This concavity is apparently present only in the representatives of Hypopomidae but its phylogenetic significance is difficult to assess due to the existence of intermediate conditions in various taxa. The rictalis originates in bony elements of the mid-ventral portion of the suspensorium and is inserted mainly on the coronoid process, with some fibers on the posterior dorsal margin of the anguloarticular in some species of Brachyhypopomus and Microsternarchus. In the region of origin, the posterolateral fibers may either extend beyond the anterior margin of the preopercular fossa, reaching the posterior margin of the bone (Fig. 11), or be restricted to the medial portion of the bone. Commonly, the origin of the stegalis includes only bony elements of the suspensorium, with its fibers converging on an intersegmental aponeurosis ventrally differentiated into the meckelian tendon and inserting on the coronomeckelian bone; and dorsally differentiated into the mandibular tendon, itself the origin of the segmentum mandibularis (Fig. 4). The adductor mandibulae, segmentum facialis is strictly fibrous, without ossifications.
Adductor mandibulae, pars malaris of Brachyhypopomus janeiroensis (Hypopomidae), MZUSP 22702, 80.9 mm LEA. A. Lateral view; B. Mesial view. Buccopalatal membrane dissected ventrally. Anatomical abbreviations in Tab. 1. Scale bars = 1 mm.
Lateral view of dorsolateral musculature of Brachyhypopomus janeiroensis (Hypopomidae), MZUSP 22702, 80.9 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 3 mm.
The segmentum mandibularis is a single section arising from the mandibular tendon and entering the mesial surface of the lower jaw, commonly involving the dentary and anguloarticular or only the latter in Microsternarchus. Invariably, this segment is restricted to the dorsal portion of Meckel´s cartilage and may either be directly associated with the dorsal margin of the cartilage or restricted to the dorsal portion of the coronomeckelian bone. This segment normally does not exceed 50% of the dorsal margin of the Meckel´s cartilage, but in B. sullivani it extends for ca. 80% of the cartilage. The course of the ramus mandibularis trigeminus nerve is variable across the family and may be either mesial to all subsections of the segmentum facialis, or lateral to the stegalis and mesial to the rictalis and malaris, or finally lateral to the rictalis and stegalis and mesial to the malaris.
Levator arcus palatini. The levator arcus palatini has roughly the shape of a parallelogram. The relative sizes of origin and insertion are variable, with the origin normally ca. one and a half times the size of the insertion, but equal in some species of Brachyhypopomus (Fig. 14). The most anterior fibers of this muscle are anteroposteriorly oblique relative to the axis of the head in all analyzed taxa (Figs. 11, 14–16). The origin of the levator arcus palatini is on the frontal and sphenotic and its insertion is on the hyomandibula. At the insertion point, anterolateral and posterolateral fibers are lateral to the malaris; while anteromesial and posteromesial fibers are mesial to the latter. In Microsternarchus, only the posteromesial fibers are mesial to the malaris. In most analyzed species, the posterodorsal fibers of the levator arcus palatini are parallel to the dilatator operculi, with no overlap between the two muscles (Fig. 11–14, 16). Some species have only the posterodorsal portion of the levator arcus palatini mesial to the dilator operculi.
Lateral view of dorsolateral musculature of Microsternarchus cf. bilineatus (Hypopomidae), MBUCV-V 7298, 59.2 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 1 mm.
Lateral view of dorsolateral musculature of Hypopomus artedi (Hypopomidae), USNM 408442, 202. 7 mm LEA. A= anterolateral fibers of the levator arcus palatini; P= posterolateral fibers of the levator arcus palatini. Remaining anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
Dilatator operculi. The dilatator operculi is commonly conical. Its origin includes the sphenotic, pterotic, frontal and hyomandibula. The origin of the anteroventral fibers of the dilatator operculi is anteroventrally displaced to the preopercle in Microsternarchus (Fig. 15) and in some species of Brachyhypopomus (Fig. 14). This muscle inserts invariably on the dorsal process of the opercle.
Levator operculi. The levator operculi is a superficial muscle located immediately posterior to the dilatator operculi and sectioned into anterior and posterior sections (Figs. 14–16). The origin of the levator operculi anterior is normally on the pterotic. The levator operculi posterior originates in the postotic canal segment corresponding to the supracleithrum, with both sections inserting onto the opercle.
Adductor arcus palatini. This muscle originates in the parasphenoid and prootic, except in Hypopomus and Microsternarchus, with the origin being restricted to the parasphenoid. Anteriorly, it inserts on the lateral face of the endopterygoid and metapterygoid; as the muscle progresses posteriorly, its insertion shifts from the lateral to the medial face of the suspensorium, finally inserting on the medial surface of the hyomandibula. The insertion includes the sympletic in Microsternarchus and B. beebei. Examination of the adductor arcus palatini requires dissection and removal of the adductor mandibulae, which completely overlaps it.
Adductor hyomandibulae. This muscle arises from the ventral region of the prootic, including the pterotic in Microsternarchus, or the posterior portion of the parasphenoid in Brachyhypopomus and Hypopomus. The insertion occurs the posteromedial margin of the hyomandibula.
Adductor operculi. The origin of the adductor operculi is on the pterotic, exoccipital, and prootic. The insertion occurs solely in the mesial surface of the opercle in the most species of Brachyhypopomus. The generalized pattern includes an insertion on the dorsal margin of a dorso-mesial crest of the opercle, and posteriorly it inserts on the mesial surface of the same bone.
Detailed description of the dorsolateral musculature of the head in the genera of Hypopomidae.
Brachyhypopomus Mago-Leccia, 1994Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.
Adductor mandibulae. The malaris arises from the mid-dorsal portion of the hyomandibula and inserts on the posteromedial portion of the antorbital bone by a fibrous attachment. Its mesialmost fibers converge onto a small endomaxillary ligament, less than one-third the length of the malaris which, in turn, inserts on the posteromedial portion of the maxilla (Fig. 13). Except for B. sullivani and B. regani, the malaris has a concavity on its dorsal margin for accommodating the eyeball.
The rictalis originates in the preopercle, quadrate and hyomandibula, or only in the preopercle and hyomandibula in B. pinnicaudatus, B. hendersoni and B. regani. The lateralmost fibers of rictalis surpass the anterior margin of the preopercular fossa and reach the mid-portion of the preopercle in most species of Brachypopomus analyzed herein. It reaches the posterior margin of that bone in B. bombilla, B. pinnicaudatus, and B. hendersoni. That section inserts mainly on the coronoid process, but with some lateral fibers on the anguloarticular in B. bombilla, B. sullivani, and B. gaudeiro. The stegalis arises from the hyomandibula, sphenotic, pterosphenoid, parasphenoid, sympletic and metapterygoid. Anteriorly, the stegalis differentiates into an intersegmental aponeurosis, dorsally entering the mandibular tendon and ventrally the meckelian tendon, inserting onto the coronomeckelian bone. The stegalis is located laterally in relation to the basal region of the endopterygoid, overlapping it completely; except in B. pinnicaudatus, in which the stegalis overlaps only the posterior portion of the basal region of the bone. Normally, the stegalis is positioned laterally to the adductor arcus palatini, overlapping it completely, except in B. pinnicaudatus, B. brevirostris, B. hendersoni, and B. regani, where the stegalis overlaps only the mid-posterior portion of the adductor arcus palatini.
Commonly, the segmentum mandibularis is located dorsally to Meckel’s cartilage and contacts it, except in B. sullivani, B. beebei, B. gaudeiro, and B. draco, where the segmentum mandibularis is restricted to the dorsal margin of the coronomeckelian bone, and does not contact the dorsal margin of Meckel’s cartilage. The path of the ramus mandibularis trigeminus nerve is invariably mesial to the malaris and rictalis, and lateral to the stegalis.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly wider than its insertion; or equal in B. draco and B. hendersoni. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris, while its anteromesial and posteromesial bundles are medial to the malaris. In most examined species, the levator arcus palatini is parallel to the dilatator operculi, with no overlap. In B. regani only, the posterodorsal fibers of the levator arcus palatini are mesial to the dilatator operculi, but without reaching the median portion of the levator arcus palatini.
Hypopomus Gill, 1864
Adductor mandibulae. The malaris originates from the mid-dorsal portion of the hyomandibula and preopercle. Its insertion occurs on the posteromedial portion of the antorbital bone by a fibrous attachment, with its mesialmost fibers converging onto a small endomaxillary ligament, less than one-third the length of the malaris which, in turn, inserts on the posteromedial portion of the maxilla (Fig. 4). The rictalis originates in the preopercle, sympletic, quadrate, and hyomandibula. The lateralmost fibers of rictalis surpass the anterior margin of the preopercular fossa and reach the mid-portion of the preopercle; with the posterodorsal fibers almost reaching the posterior margin of that bone. That subsection inserts on the coronoid process.
The stegalis arises from the sphenotic, pterosphenoid, metapterygoid and anterior margin of the hyomandibula. Towards its insertion, the fibers of stegalis differentiates into an intersegmental aponeurosis, dorsally entering the mandibular tendon and ventrally the meckelian tendon which, in turn, inserts onto the coronomeckelian bone (Fig. 4). The stegalis is located laterally in relation to the basal region of the endopterygoid and adductor arcus palatini, overlapping those structures completely.
The segmentum mandibularis is located dorsally to Meckel’s cartilage and does not contact directly the dorsal margin of Meckel’s cartilage. The path of the ramus mandibularis trigeminus nerve is lateral to the rictalis and stegalis, and mesial to the malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape (Fig. 16), originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is wider than its insertion, approximately one and a half of its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris, while its anteromesial and posteromesial bundles are medial to the malaris. Only the dorsalmost fibers of the posterior portion of the levator arcus palatini are mesial to the dilatator operculi, but without reaching the median portion of the levator arcus palatini.
Microsternarchus Fernández-Yépez, 1968
Adductor mandibulae. The malaris originates from the mid-dorsal portion of the hyomandibula and preopercle. Its insertion occurs on the posteromedial portion of the antorbital bone by a fibrous attachment. Its mesialmost fibers converge onto a small endomaxillary ligament, less than one-third the length of the malaris which, in turn, inserts on the posteromedial portion of the maxilla.
The rictalis originates in the preopercle, quadrate and hyomandibula. The lateralmost fibers of rictalis surpass the anterior margin of the preopercular fossa and reach the mid-portion of the preopercle. That subsection inserts mainly on the coronoid process, but with some lateral fibers on the anguloarticular and buccopalatal membrane. The stegalis arises from the hyomandibula, sphenotic, pterosphenoid, parasphenoid, sympletic and metapterygoid. Anteriorly, the stegalis differentiates into an intersegmental aponeurosis, dorsally entering the mandibular tendon and ventrally the meckelian tendon, inserting onto the coronomeckelian bone. The stegalis is located laterally in relation to the basal region of the endopterygoid and adductor arcus palatini, overlapping those structures completely.
The segmentum mandibularis is located dorsally to Meckel’s cartilage and contacts it by slightly more than a half of the cartilage’s extension. The path of the ramus mandibularis trigeminus nerve is mesial to all sections of the adductor mandibulae, segmentum facialis.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is wider than its insertion, approximately one and a half of its insertion (Fig. 19). At the insertion, the anterolateral, posterolateral and anteromesial fiber bundles of the levator arcus palatini are lateral to the malaris, while its posteromesial bundles are medial to the malaris. The posterodorsal fibers of the levator arcus palatini are parallel to the dilatator operculi, with no overlap between the two muscles. The truncus hyomandibularis nerve run through the lateral and mesial bundle of fibers of the levator arcus palatini.
General aspects of the dorsolateral head muscles of the Rhamphichthyidae
Adductor mandibulae. In Rhamphichthys and Gymnorhamphichthys, the adductor mandibulae is restricted to the segmentum facialis, which is composed of the pars ricto-stegalis and malaris (Figs. 5, 8; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12). In Steatogenys and Hypopygus, the adductor mandibulae consists of the segmentum facialis (composed of the pars malaris, pars rictalis, and pars stegalis), which is in turn connected to the segmentum mandibularis through an intersegmental aponeurosis well-differentiated in two components (mandibular tendon and meckelian tendon).
The malaris is positioned dorsolaterally to the dorsal portion of the rictalis and lateroventrally to the stegalis. It originates in the mid-dorsal portion of the hyomandibula and preopercle, except in species of Rhamphichthys, where its origin is on the hyomandibula only. The malaris inserts on the antorbital and maxilla, but with variations of detail within Rhamphichthyidae. In Hypopygus and Steatogenys, the malaris inserts on the posteromedial portion of the antorbital bone by a fibrous attachment, and its mesialmost fibers converge onto a small endomaxillary ligament, less than one-third the length of the malaris which, in turn, inserts on the posteromedial portion of the maxilla. In Rhamphichthys and Gymnorhamphichthys, the malaris converges anteriorly into an elongated endomaxillary ligament to an insertion on the posteromedial portion of the antorbital and maxilla (Fig. 5). In Rhamphichthys, the ventral fibers of malaris differentiate into an additional ligament, herein named “endomaxillary accessory ligament”. Some internal portions of the endomaxillary accessory ligament ossify into intermuscular bones, and this ligament inserts solely onto the posteromedial face of the antorbital. In the generalized condition of the family, the malaris lacks intermuscular bones, being essentially fibrous. However, in Rhamphichthys and Iracema (Carvalho, Albert, 2011Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
), this section has some ossified tendons, resulting in intermuscular bones (Fig. 8; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12).
In Rhamphichthys and Gymnorhamphichthys, the ricto-stegalis is mesial to the malaris, and originates from bones of the suspensorium and neurocranium. The rictalis and stegalis become gradually differentiated from each other towards their insertion, where fibers of the rictalis insert onto the coronoid process, with some fibers on the posterior margin of the anguloarticular; and the stegalis converges onto the meckelian tendon which, in turn, inserts on the coronomeckelian bone. Typically, a few fibers of the ricto-stegalis have a weak association with the buccopalatal membrane. Intermuscular bones are present in the ricto-stegalis in Rhamphichthys and Iracema. The ramus mandibularis trigeminus nerve runs through the ricto-stegalis, located mesial to the presumptive rictalis and malaris; and laterally to the presumptive stegalis.
In Hypopygus and Steatogenys, the rictalis and stegalis are completely differentiated. The rictalis originates from the suspensorium and inserts mainly onto the coronoid process, while the stegalis arises from the suspensorium and neurocranium, converging onto a poorly differentiated intersegmental aponeurosis. Towards its insertion, some lateral fibers of the stegalis and rictalis are associated with the buccopalatal membrane, which is poorly differentiated from the surrounding connective tissues. In both genera, the segmentum mandibularis is present, arising from the mandibular tendon, entering the mandible mesially and inserting on the anguloarticular. The dentary is an additional insertion site in Hypopygus.
Levator arcus palatini. The levator arcus palatini is trapezium-shaped in Rhamphichthys and Gymnorhamphichthys (Figs. 17, 18), or parallelogram-shaped in Hypopygus (Fig. 11) and Steatogenys. The relative sizes of origin and insertion are variable, with the origin and insertion equal in Rhamphichthys, with the origin ca. one and a half times the size of the insertion in Steatogenys or with the width of origin half that of its insertion in Gymnorhamphichthys and Hypopygus. The orientation of the anteriormost fibers is orthogonal relative to the longitudinal axis of the head in Rhamphichthys and Gymnorhamphichthys and oblique to the longitudinal axis of the head (at approximately 45° angle) in Hypopygus and Steatogenys.
Lateral view of dorsolateral musculature of Gymnorhamphichthys rosemariae (Rhamphichthyidae), MZUSP 56317, 116.3 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
Lateral view of dorsolateral musculature of Rhamphichthys hahni (Rhamphichthyidae), MZUSP 24736, 479.5 mm TL. Anatomical abbreviations in Tab. 1. Scale bar = 20 mm.
The origin of the levator arcus palatini is on the frontal and sphenotic and its insertion occurs on the hyomandibula, including the preopercle as an insertion site only in Gymnorhamphichthys. At the insertion point, all fibers are located laterally to the malaris in Gymnorhamphichthys and Rhamphichthys. In Hypopygus, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris, while its anteromesial and posteromesial bundles are medial to the malaris. In Steatogenys, only the posteromesial fibers of the levator arcus palatini lie medially to the malaris. The common pattern for Rhamphichthyidae is the posterodorsal fibers of the levator arcus palatini with only the posterodorsal portion of the levator arcus palatini mesial to the dilator operculi; solely in Steatogenys, the levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the medial portion of that muscle.
In the generalized condition of the family, the levator arcus palatini lacks intermuscular bones, and is entirely fibrous. However, in Rhamphichthys and Iracema (Carvalho, Albert, 2011Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
), this muscle has some ossified tendons, resulting in intermuscular bones at its anteriormost portion (Fig. 8B).
Dilatator operculi. The dilatator operculi is a conical muscle. This muscle arises from the sphenotic, pterotic and dorsal portions of the hyomandibula, plus the frontal in Rhamphichthys, Steatogenys, and Gymnorhamphichthys. The insertion is invariably on the dorsal process of the opercle. The dilatator operculi shows little variation across the Rhamphichthyidae and its only noteworthy informative condition is its insertion on the ventral portion of the dorsal process of the opercle in species of Rhamphichthys.
Levator operculi. Rhamphichthyidae generally have a levator operculi anterior and a levator operculi posterior. However, only the former is present in Hypopygus (Fig. 11). The levator operculi anterior originates from the ventral margin of the pterotic in most members of the family, but from the hyomandibula in Steatogenys. It inserts on a ridge on the dorsal margin of the opercle, posterior to its anterodorsal process. The levator operculi posterior originates in the supracleithral canal, and inserts along a ridge on the posterolateral margin of the opercle, with fibers extending beyond the dorsal margin of the bone. The R-Avn is located laterally to the levator operculi anterior and mesially to the levator operculi posterior in Gymnorhamphichthys (Fig. 17) and Steatogenys, while in Rhamphichthys it is fully mesial to both sections (Fig. 18).
Adductor arcus palatini. This muscle invariably originates on the parasphenoid, extending also onto the prootic in Hypopygus and Steatogenys. Anteriorly, it inserts on the lateral face of the endopterygoid and metapterygoid; as the muscle progresses posteriorly, its insertion shifts from the lateral to the medial face of the suspensorium, finally inserting on the medial surface of the hyomandibula. Examination of the adductor arcus palatini requires dissection and partial removal of the adductor mandibulae, which completely overlaps it.
Adductor hyomandibulae. This muscle arises from the ventral region of the prootic, including also the parasphenoid in Rhamphichthys and Gymnorhamphichthys. This muscle inserts in the posteromedial margin of the hyomandibula. Little or no variation in this muscle was found throughout the Rhamphichthyidae.
Adductor operculi. The origin of the adductor operculi is on the pterotic, exoccipital and prootics. Anteriorly, it inserts on the dorsal margin of a dorso-mesial crest of the opercle, and posteriorly on the mesial surface of the same bone.
Detailed description of the dorsolateral musculature of the head in the genera of Rhamphichthyidae.
Gymnorhamphichthys Ellis, 1912
Adductor mandibulae. The malaris is fully differentiated from the ricto-stegalis and is positioned dorsolaterally to the dorsal portion of the presumptive rictalis and the lateroventrally to the stegalis (Fig. 5; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12). The malaris has a pectinated form, originating from the mid-portion of the hyomandibula and preopercle; and converges anteriorly onto an elongated endomaxillary ligament, equivalent to twice the width of the fibrous portion of the malaris, and inserts on the posteromedial portion of the antorbital and maxilla (Fig. 5), with some fibers on the buccopalatal membrane.
The ricto-stegalis is mesial to the malaris, and originates from the parasphenoid, pterosphenoid, metapterygoid, preopercle, quadrate, sympletic and hyomandibula, with its fibers restricted to the anterior margin of the preopercle. Towards its insertion, the rictalis and stegalis become gradually differentiated from each other, with the fibers of the rictalis inserting onto the coronoid process, and the stegalis converging onto the meckelian tendon which, in turn, inserts on the coronomeckelian bone. Near the insertion, a few fibers of the ricto-stegalis have a weak association with the buccopalatal membrane and posterior margin of the anguloarticular. The bundles of fibers corresponding to the presumptive stegalis are located laterally relative to the basal region of the endopterygoid and adductor arcus palatini, overlapping those elements completely. The ramus mandibularis trigeminus nerve runs through the ricto-stegalis, located mesial to the presumptive rictalis and malaris; and laterally to the presumptive stegalis.
Levator arcus palatini. The levator arcus palatini has a trapezoidal shape, originating from the ventral margin of the frontal and sphenotics and inserting mainly in the hyomandibula, with posterolateral fibers inserting on the preopercle. The relative size of its origin is half that of its insertion, with all fibers located laterally to the malaris at insertion. Only the dorsalmost fibers of the posterior portion of the levator arcus palatini are mesial to the dilatator operculi, but without reaching the median portion of the levator arcus palatini (Fig. 17).
Hypopygus Hoedeman, 1962
Adductor mandibulae. The malaris originates from the mid-dorsal portion of the hyomandibula and preopercle. Its insertion occurs on the posteromedial portion of the antorbital bone, by fibrous attachment, with its mesialmost fibers converging onto a small endomaxillary ligament less than one-third the length of the malaris. The latter inserts on the posteromedial portion of the maxilla.
The rictalis originates in the preopercle, quadrate and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis surpass the anterior margin of the preopercular fossa and reach the mid-portion of the preopercle while posterodorsal fibers just fall short of the posterior portion of the same bone (Fig. 11); and with its insertion occurring solely on the coronoid process. The stegalis arises from the hyomandibula, sphenotic, pterosphenoid, parasphenoid, sympletic and metapterygoid. Anteriorly, the stegalis differentiates into a poorly differentiated intersegmental aponeurosis, dorsally entering the mandibular tendon and ventrally the meckelian tendon, inserting onto the coronomeckelian bone. Towards the insertion, some lateral fibers are associated with the buccopalatal membrane, which is poorly differentiated from surrounding connective tissues. The stegalis is located laterally in relation to the proximal region of the endopterygoid and the adductor arcus palatini, overlaps those structures completely.
The segmentum mandibularis is located dorsally to coronomeckelian bone, with minimum (n = 3) or no (n = 1) contact with Meckel’s cartilage, extending for ca. 20% of the dorsal portion of that cartilage. The ramus mandibularis trigeminus nerve is mesial to all sections of the adductor mandibulae, segmentum facialis.
Levator arcus palatini. The levator arcus palatini has a roughly conical shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is approximately half as wide as its insertion (Fig. 11). At insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris, while its anteromesial and posteromesial bundles are medial to the malaris. Only the dorsalmost fibers of the posterior portion of the levator arcus palatini are mesial to the dilatator operculi, but without reaching the median portion of the levator arcus palatini.
Rhamphichthys Müller & Troschel, 1846
Adductor mandibulae. The malaris is fully differentiated from ricto-stegalis and is positioned dorsolaterally to the dorsal portion of the presumably rictalis and the lateroventrally to the stegalis. This muscle arises from the mid-portion of the hyomandibula. The dorsalmost fibers differentiate into an elongated endomaxillary ligament, equal in length to the fibrous portion of the malaris, which inserts on the posteromedial portion of the antorbital and the maxilla (Fig. 5), with some lateral fibers associated with the buccopalatal membrane. The ventral fibers of the malaris converge onto the accessory endomaxillary ligament, which inserts solely on the posteromedial face of the antorbital. There are many ossified tendons in the fibrous portion of the malaris, resulting in several intermuscular bones that are coopted towards its insertion site (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12).
The ricto-stegalis is mesial to the malaris, and originates from parasphenoid, pterosphenoid, metapterygoid, preopercle, quadrate, sympletic and hyomandibula, with its fibers restricted to the anterior margin of the preopercle. In the region near the origin of the ricto-stegalis, both subsections are easily distinguished by the direction of their fibers, with those corresponding to the presumptive stegalis being posteriorly-elongated and displaced dorsomedially; and those of the presumptive rictalis are shorter and located more laterally. At its median portion, the two sections are indistinguishable, becoming slightly differentiated towards the insertion, where fibers of the rictalis insert onto the coronoid process via an elongated ligament, and those of the stegalis converge into the meckelian tendon which, in turn, inserts onto the coronomeckelian bone. The bundles of fibers corresponding to the presumptive stegalis are located laterally relative to the basal region of the endopterygoid and of the adductor arcus palatini, completely overlapping those structures (Fig. 18). The ramus mandibularis trigeminus nerve runs through the ricto-stegalis, located mesial to the presumptive rictalis and malaris; and laterally to the presumptive stegalis.
Levator arcus palatini. The levator arcus palatini has a trapezoidal shape, originating from the ventral margin of the frontal and sphenotics, inserting mainly on the hyomandibula, with posterolateral fibers inserting onto the preopercle. The size of its origin is half that of its insertion, with all fibers located laterally to the malaris at insertion. Only the dorsalmost fibers of the posterior portion of the levator arcus palatini are mesial to the dilatator operculi, but without reaching the median portion of the levator arcus palatini. The composition of the levator arcus palatini is mainly fibrous, with some ossified tendons forming intermuscular bones at its anteriormost portion (Fig. 8B).
Steatogenys Boulenger, 1898
Adductor mandibulae. The malaris arises from the mid-dorsal portion of the hyomandibula and preopercle. Its insertion occurs on the posteromedial portion of the antorbital bone by a fibrous attachment. Its mesialmost fibers converge onto a small endomaxillary ligament, less than one-third the length of the malaris which, in turn, inserts on the posteromedial portion of the maxilla.
The rictalis originates in the preopercle, sympletic, quadrate and hyomandibula. The lateralmost fibers of the rictalis surpass the anterior margin of the preopercular fossa and reach the posterior portion of the same bone. That section inserts mainly on the coronoid process, but with some lateral fibers on the anguloarticular and buccopalatal membrane. The stegalis arises from the hyomandibula, sphenotic, pterosphenoid, parasphenoid, and metapterygoid. Anteriorly, the stegalis differentiates into a poorly-differentiated intersegmental aponeurosis, dorsally entering the mandibular tendon and ventrally the meckelian tendon which, in turn, inserts onto the coronomeckelian bone. The stegalis is located laterally in relation to the basal region of the endopterygoid and adductor arcus palatini, overlapping those structures completely.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about a half of the dorsal portion of the cartilage, but not associating with it. The ramus mandibularis trigeminus nerve is mesial to all sections of the adductor mandibulae, segmentum facialis.
Levator arcus palatini. The levator arcus palatini is roughly parallelogram shaped, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is approximately 50% wider than its insertion. At the insertion, the anterolateral, posterolateral and anteromesial fiber bundles of the levator arcus palatini are lateral to the malaris, while its posteromesial bundles are medial to it. The levator arcus palatini is positioned mesially to the dilatator operculi, where the anterior margin of the latter exceeds the median portion of the former.
General aspects of the dorsolateral head muscles of the Sternopygidae.
Adductor mandibulae. In the generalized pattern found in Sternopygidae, the adductor mandibulae consists of the segmentum facialis, which is connected to the segmentum mandibularis through an intersegmental aponeurosis well-differentiated in two components. The dorsal component differentiates into a mandibular tendon, which serves as the origin of the segmentum mandibularis, and the ventral component differentiates into the meckelian tendon, continuous with the stegalis for insertion into the coronomeckelian bone (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 41A). In the most Archolaemus species, the segmentum mandibularis is absent (Fig. 9). The segmentum facialis is composed of three subsections in all species analyzed: the adductor mandibulae, pars malaris; pars stegalis and pars rictalis (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 11). These sections have some convergent fibers along their lengths, but the respective sections can be differentiated by their distinct origin and insertion points.
The malaris is positioned dorsolaterally to the dorsal portion of the rictalis and the lateroventrally to the stegalis. This section originates in the mid-dorsal portion of the hyomandibula, including the preopercle as a site of origin in Eigenmannia and Archolaemus. Its insertion is invariably on the posterodorsal expansion of the infraorbital 1+2, where the mesial fibers differentiate into a diminutive endomaxillary ligament inserted on the posteromedial margin of the maxilla (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 11). In the generalized condition found in Sternopygidae, the malaris overlaps partially the stegalis at its origin and insertions sites (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 8; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 40), however, the stegalis lies completely medial to the malaris in the most Archolaemus species (Fig. 9; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15).
The rictalis originates in bony elements of the mid-ventral portion of the suspensorium and is inserted mainly on the coronoid process, with some fibers on the posterior dorsal margin of the anguloarticular in some species of Rhabdolichops. In the region of origin, the posterolateral fibers never extend beyond the anterior margin of the preopercular fossa, being restricted to the anterior margin of this bone. At the region near to its insertion, some lateralmost fibers are associated with the buccopalatal membrane, with fibers inserted to the transverse ligament which, in turn, receives some lateroventral fibers of the malaris. The transverse ligament is well differentiated and attached in the mid-ventral portion of the infraorbital 1+2 and posterodorsal margin of the anguloarticular (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 8; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 40).
Commonly, the origin of the stegalis includes only bony elements of the suspensorium, however, its origin can include neurocranium bones in Sternopygus and Japigny. Invariably, the anteriormost fibers of stegalis converges into an intersegmental aponeurosis ventrally differentiated into the meckelian tendon and inserting on the coronomeckelian bone; and dorsally differentiated into the mandibular tendon, itself the origin of the segmentum mandibularis.
When present, the segmentum mandibularis is well developed, and restricted to a single section arising from the mandibular tendon and entering the mesial surface of the lower jaw, commonly involving the dentary and anguloarticular. Invariably, this segment is restricted to the dorsal portion of Meckel´s cartilage and may either be directly associated with the dorsal margin of the cartilage. This segment normally exceeds 40% of the dorsal margin of the Meckel´s cartilage in Archolaemus luciae or by almost the entire length in Rhabdolichops (Fig. 19). The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and mesial to the rictalis and malaris.
Lateral view of adductor mandibulae, pars malaris of Rhabdolichops eastwardi (Sternopygidae), MZUSP 81178, 188.3 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
Levator arcus palatini. The levator arcus palatini has roughly the shape of a parallelogram or inverted triangle. The relative sizes of origin and insertion are variable, with the origin normally a half size its insertion, but origin and insertion equal in some species. The orientation of the anteriormost fibers is orthogonal relative to the longitudinal axis of the head in the most sternopygids (Fig. 7; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15), however, the most anterior fibers of this muscle are anteroposteriorly oblique (at approximately 45° angle) in Japigny, R. eastwardi, and Sternopygus (Fig. 20; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 13B). The levator arcus palatini originates on the hyomandibula, commonly including also the frontal in some Eigenmannia species and Japigny, or the pterosphenoid in Archolaemus, Sternopygus, and some Eigenmannia and Rhabdolichops species. Its insertion is on the hyomandibula, with some posterodorsal fibers attached to the preopercle in Eigenmannia and Rhabdolichops.
Lateral view of dorsolateral musculature of Japigny kirshbaum (Sternopygidae), FMNH 50185, 137.2 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 4 mm.
In the common pattern found in Sternopygidae, at the insertion point, the anterolateral, anteromesial and posterolateral fibers are lateral to the malaris; while the posteromesial fibers are mesial to the latter. However, there is significant variation in the dispositions of these subsets, which lies completely lateral to the malaris in Sternopygus and Japigny. In the most analyzed species, the posterodorsal fibers of the levator arcus palatini are parallel to the dilatator operculi, with no overlap between the two muscles. Some species have only the posterodorsal portion of the levator arcus palatini mesial to the dilator operculi. The generalized pattern consists of a strictly fibrous composition of levator arcus palatini, however, some more mesial tendons ossify in S. xingu, resulting in the occurrence of intermuscular bones.
Dilatator operculi. The dilatator operculi arises from the sphenotic, pterotic and hyomandibula, sometimes including also the frontal in Eigenmannia limbata and Sternopygus. Insertion is invariably on the dorsal process of the opercle. In some Archolaemus species (A. ferreirai and A. santosi), the dilatator operculi has a tapered anteromesial extension, which passes through the sphenotic spine and originates in the anteromesial face of this bone.
Levator operculi. The levator operculi anterior originates from the lateral surface of the mid-ventral portion of the pterotic and hyomandibula, but can be restricted to the pterotic in Sternopygus, R. lundbergi and R. nigrimans. The levator operculi posterior originates mainly from the postotic canal segment corresponding to the supracleithrum, including also the posterior margin of the pterotic only in Sternopygus, R. lundbergi and R. nigrimans. This muscle inserts on the posterodorsal portion of the opercle. In the generalized condition, the nerve R-Avn is positioned medially in relation to the entire levator operculi (Fig. 20; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 13A; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15). In a juvenile specimen of E. microstoma, and mature specimens of R. lundbergi, R. nigrimans, and Sternopygus, the R-Avn lies laterally to the levator operculi anterior and medially to the levator operculi posterior (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 13B).
Adductor arcus palatini. The adductor arcus palatini arises mainly from the parasphenoid and prootic, sometimes also including the orbitosphenoid in Japigny (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 42). At its anterior portion, the insertion occurs mostly on the lateral side of the endopterygoid, metapterygoid and hyomandibula. In A. luciae, the insertion of the adductor arcus palatini includes the posterior margin of the ascendant process of the endopterygoid, a similar condition found in Japigny, however, without insertion on its dorsalmost portion near the attachment with the neurocranium in the latter. In the remaining sternopygids, the insertion is restricted to the basal portion of the ascendant process of the endopterygoid. Only the posterior portion of the adductor arcus palatini is overlapped by the adductor mandibulae, segmentum facialis in the most species; and can be totally covered by this muscle in Sternopygus. In the majority of Archolaemus species, the segmentum facialis does not overlap the adductor mandibulae (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15).
Adductor hyomandibulae. This muscle arises from the ventral region of the prootic and pterotic, inserting on the posteromedial margin of the hyomandibula. Little or no variation in this muscle was found throughout the Sternopygidae.
Adductor operculi. The origin of the adductor operculi is on the exoccipital, pterotic, and prootic. In Archolaemus, the origin does not occur in the prootic. Anteriorly, the insertion is on the dorsal margin of a dorso-mesial crest of the opercle, and posteriorly it inserts on the mesial surface of the same bone.
Detailed description of the dorsolateral musculature of the head in the genera of Sternopygidae.
Archolaemus Korringa, 1970
Adductor mandibulae. The malaris is positioned dorsolaterally to the dorsal portion of the rictalis and completely lateral to the stegalis (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15), except for Archolaemus orientalis in which the malaris overlaps partially the stegalis.
The rictalis originates in the preopercle, sympletic, quadrate and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis is restricted to the anterior margin of the preopercular fossa. That section inserts solely on the coronoid process, with some lateralmost fibers associated with the buccopalatal membrane and transverse ligament. The stegalis arises from the hyomandibula, pterosphenoid, metapterygoid, and quadrate; and converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone. Usually, the stegalis overlaps only the ventral portion of the adductor arcus palatini, except in A. orientalis, where the stegalis is positioned laterally only to the mid-posterior portion of the adductor arcus palatini, overlapping it partially.
The segmentum mandibularis occurs only in A. luciae and arises from the mandibular tendon, enters the mandible mesially and inserts on the anguloarticular and dentary. The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 40% of the dorsal portion of this cartilage. The segmentum mandibularis is absent in the remaining species (Fig. 9). The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a triangular shape, originating from the ventral margin of the pterosphenoid and sphenotic, inserting in the hyomandibula. The relative size of origin is equal to a half of its insertion. At the insertion point, the anterolateral, anteromesial and posterolateral fibers are lateral to the malaris; while and posteromesial fibers are mesial to the latter. The posterodorsal fibers of the levator arcus palatini are parallel to the dilatator operculi, with no overlap between the two muscles.
Lateral view of dorsolateral musculature of Distocyclus conirostris (Sternopygidae), MZUSP 23316, 242.2 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 4 mm.
Distocyclus Mago-Leccia, 1978Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51.
Adductor mandibulae. The rictalis originates in the preopercle, sympletic, quadrate and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis is restricted to the anterior margin of the preopercular fossa. That section inserts solely on the coronoid process, with some lateralmost fibers associated with the buccopalatal membrane and transverse ligament. The stegalis arises from the hyomandibula, metapterygoid, and quadrate; and converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone, with some anterodorsal fibers converging to the mandibular tendon.
The segmentum mandibularis arises from the mandibular tendon, enters the mandible mesially and inserts on the anguloarticular and dentary. The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 40% of the dorsal portion of this cartilage. The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a triangular shape, originating from the sphenotic and inserting in the hyomandibula (Fig. 21). The relative size of origin is equal to a half of its insertion. At the insertion point, the anterolateral, anteromesial and posterolateral fibers are lateral to the malaris; while and posteromesial fibers are mesial to the latter. The posterodorsal fibers of the levator arcus palatini are parallel to the dilatator operculi, with no overlap between the two muscles.
Eigenmannia Jordan & Evermann, 1896
Adductor mandibulae. The rictalis originates in the preopercle, sympletic, quadrate and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis is restricted to the anterior margin of the preopercular fossa. That section inserts solely on the coronoid process, with some lateralmost fibers associated with the buccopalatal membrane and posterior margin of the anguloarticular. The stegalis arises from the hyomandibula, metapterygoid, and quadrate; and converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone, with some anterodorsal fibers converging to the mandibular tendon (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 41A). In E. humboldtii, E. meeki, E. macrops, E. sayona, E. trilineata, E. besouro, E. matintaperera, E. waiwai, E. nigra, and E. oradens, the stegalis overlaps only the posterior portion of the basal region of the endopterygoid not overlapping it completely; in E. microstoma, E. pavulagem, E. antonioi, E. virescens, E. guairaca, E. desantanai, E. muirapinima, E. limbata, and E. vicentespelaea, the stegalis is located laterally in relation to the basal region of the endopterygoid, overlapping it completely.
The segmentum mandibularis arises from the mandibular tendon, enters the mandible mesially and inserts on the anguloarticular and dentary; except for E. humboldtii, with the insertion restricted to the anguloarticular. The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 50-60% of the dorsal portion of this cartilage. The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly triangular shape. Within Eigenmannia, the generalized condition is the origin of the levator arcus palatini arising from the sphenotic, but including the pterosphenoid in E. muirapinima and E. vicestespelaea, or the frontal in E. humboldtii, E. pavulagem, E. nigra, E. oradens and E. limbata. The insertion occurs on the hyomandibula, and includes the preopercle in E. muirapinima and E. vicentespelaea. The relative size of origin is equal to a half of its insertion. At the insertion point, the anterolateral, anteromesial and posterolateral fibers are lateral to the malaris; while and posteromesial fibers are mesial to the latter. Among the analyzed species, the anteromesial fibers lies mesially to the malaris only in E. limbata. The posterodorsal fibers of the levator arcus palatini are mesial to the dilatator operculi, but without reaching the median portion of the levator arcus palatini in E. humboldtii, E. limbata and E. vicentespeleae. In E. pavulagem, E. antonioi, E. besouro and E. guairaca, the posterodorsal fibers of the levator arcus palatini is lateral to the dilatator operculi. In the remaining species, the levator arcus palatini is parallel to the dilatator operculi, with no overlap.
Japigny Meunier, Jégu & Keith, 2011
Adductor mandibulae. The rictalis originates in the preopercle, sympletic, and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis is restricted to the anterior margin of the preopercular fossa. That section inserts solely on the coronoid process, with some lateralmost fibers associated with the buccopalatal membrane. The stegalis arises from the hyomandibula, pterosphenoid, parasphenoid, metapterygoid, and sympletic; and converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone, with some anterodorsal fibers converging to the mandibular tendon. The stegalis overlaps only the posterior portion of the basal region of the endopterygoid not overlapping it completely.
The segmentum mandibularis arises from the mandibular tendon, enters the mandible mesially and inserts on the anguloarticular. The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 60% of the dorsal portion of this cartilage. The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly narrower than its insertion (Fig. 20). At the insertion of the levator arcus palatini, all fibers are located laterally to the malaris; and with only the posterodorsal fibers being mesial to the dilatator operculi, where the anterior margin of the dilator operculi does not exceeds the medial portion of the levator arcus palatini.
Rhabdolichops Eigenmann & Allen, 1942
Adductor mandibulae. The rictalis originates in the quadrate, sympletic, preopercle, and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis is restricted to the anterior margin of the preopercular fossa. That section inserts solely on the coronoid process and anguloarticular, with some lateralmost fibers associated with the buccopalatal membrane. The stegalis arises from the hyomandibula, metapterygoid, and quadrate; and converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone, with some anterodorsal fibers converging to the mandibular tendon. The stegalis is located laterally in relation to the basal region of the endopterygoid, overlapping it completely; except in R. zareti, R. lundbergi, and R. nigrimans, in which the stegalis overlaps only the posterior portion of the basal region of the bone.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about its entire length of the dorsal portion of this cartilage (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 41B); or extending about 70% of the dorsal portion of this cartilage in R. lundbergi and R. nigrimans. The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and rictalis, and mesial to the malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape. Within Rhabdolichops, the generalized condition is the origin of the levator arcus palatini arising from the sphenotic in R. lundbergi and R. nigrimans, including the pterosphenoid in the remaining species, or the frontal solely in R. troscheli. The insertion occurs on the hyomandibula, and includes the preopercle in R. zareti. The origin of the levator arcus palatini is equal to a half of its insertion in R. zareti, R. lundbergi, and R. nigrimans; or equal to its insertion in the remaining species. At the insertion point, the anterolateral, anteromesial and posterolateral fibers are lateral to the malaris; while and posteromesial fibers are mesial to the latter, except for the R. zareti, with all fibers lateral to the malaris.
Sternopygus Müller & Troschel, 1849
Adductor mandibulae. The rictalis originates in the quadrate, sympletic, and hyomandibula. The lateralmost fibers of the ventral portion of the rictalis is restricted to the anterior margin of the preopercular fossa. That section inserts solely on the coronoid process, with some lateralmost fibers associated with the buccopalatal membrane. The stegalis arises from the hyomandibula, sphenotic, pterosphenoid, metapterygoid, and quadrate; and converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone, with some anterodorsal fibers converging to the mandibular tendon. The stegalis is located laterally in relation to the basal region of the endopterygoid, overlapping it completely; except in S. xingu, in which the stegalis overlaps only the posterior portion of the basal region of the bone. Normally, the stegalis overlaps only the ventral portion of the adductor arcus palatini, except in S. xingu, where the stegalis is positioned laterally only to the mid-posterior portion of the adductor arcus palatini, overlapping it partially.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 80% of the dorsal portion of this cartilage. The course of the ramus mandibularis trigeminus nerve is lateral to the stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid, and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to its insertion (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 13B). At the insertion of the levator arcus palatini, all fibers are located laterally to the malaris (except for one specimen of S. macrurus, with the posteromedial fibers mesial to the malaris); and with only the posterodorsal fibers being mesial to the dilatator operculi, where the anterior margin of the dilator operculi does not exceeds the medial portion of the levator arcus palatini. The generalized pattern consists of a strictly fibrous composition of levator arcus palatini, however, some more mesial tendons ossify in S. xingu, resulting in the occurrence of intermuscular bones.
General aspects of the dorsolateral head muscles of the Apteronotidae.
Adductor mandibulae. The adductor mandibulae of Apteronotidae presents the most variable configuration when compared to the other representatives of Gymnotiformes. The morphological pattern varies from components of the segmentum facialis undifferentiated from each other and thereby forming a largely continuous muscle mass with a partial differentiation at its insertion sites in Platyurosternarchus (Fig. 22) to a fully differentiation of the three primary sections of this segment (malaris, rictalis, and stegalis) in Adontosternarchus. However, the common pattern for Apteronotidae subgroups is the segmentum mandibularis composed of a clearly differentiated malaris and the remaining sections undifferentiated, equivalent to the ricto-stegalis.
Platyurosternarchus macrostomus (Apteronotidae), MZUSP 57686, 189.5 mm LEA. A. Lateral view of dorsolateral musculature; B. Mesial view of the adductor mandibulae. Anatomical abbreviations in Tab. 1. Scale bars = 10 mm; 5 mm.
The position of the malaris is variable among apteronotids, and can be located dorsolaterally (Fig. 23) or lateroventrally (Fig. 8) in relation to the dorsal portion of the rictalis and midventral portion of the stegalis (or ricto-stegalis, when undifferentiated). The malaris originates solely from suspensorium bones and usually inserts on the maxilla by means of elongated ligaments, and can include additional sites of insertions, as the connective tissues between the premaxilla and upper lip (Fig. 6), the mesethmoid and premaxilla (Fig. 24); or the posterior margin of the anguloarticular. This section is composed of a single mass of fibers in the most Apteronotidae genera. However, the malaris can be differentiated into promalaris and retromalaris in “A” gr. bonapartii, Sternarchogiton, and Porotergus (Figs. 6, 25) (see “Additional comments on adductor mandibulae, pars malaris of Apteronotidae”).
Lateral view of dorsolateral musculature of Adontosternarchus clarkae (Apteronotidae), MZUSP 30072, 79.3 mm LEA. Green indicates the path of the recurrent ramus of anteroventral part of anterior lateral line nerve. Anatomical abbreviations in Tab. 1. Scale bar = 2 mm.
Sternarchella terminalis (Apteronotidae), MPEG 3481, 155.3 mm TL [regenerated]. A. Detail of lateral view of anterior portion of dorsolateral head muscles; B. Detail of lateral view of posterior portion of dorsolateral head muscles. Anatomical abbreviations in Tab. 1. Scale bars = 5 mm.
Lateral view of dorsolateral musculature of Apteronotus bonapartii (Apteronotidae), MPEG 3038, 217.5 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
The rictalis, or its corresponding fibers, arises mostly from the bony elements of the suspensorium. The insertion sites commonly include the coronoid process, with some fibers attaching also on the posterolateral margin of the anguloarticular in some species of Adontosternarchus, Apteronotus, Parapteronotus, and Sternarchorhamphus. The intersegmental aponeurosis corresponding to the mandibular tendon is coopted as an additional insertion site for rictalis solely in Adontosternarchus. In the taxa with rictalis arising from the preopercle, the posterolateral fibers never extend beyond the anterior margin of the preopercular fossa, being restricted to the anterior margin of this bone.
The stegalis arises from elements of the suspensorium, but normally also from components of the neurocranium, as the sphenotics, pterosphenoid, and parasphenoid. Anteriorly, the stegalis converges into an intersegmental aponeurosis with several degrees of differentiation, where its anterodorsal portion diverges into the mandibular tendon, which serves as the origin for the segmentum mandibularis (when present); and an anteroventral meckelian tendon, in turn, inserts on the coronomeckelian bone. The coronomeckelian bone is absent in Sternarchorhynchus, and the stegalis attaches via meckelian tendon to the mesial face of the posterior margin of the dentary. In the generalized condition, the adductor mandibulae composition is characterized by the absence of intermuscular bones, being essentially fibrous. However, the intermuscular bones occur solely in Orthosternarchus.
When present, the segmentum mandibularis has no subsections. It arises from the mandibular tendon, enters the mandible mesially to an insertion into the anguloarticular and dentary. Invariably, the segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 20–60% of the dorsal portion of this cartilage. The segmentum mandibularis is absent in Platyurosternarchus, Orthosternarchus, Sternarchorhynchus and Sternarchorhamphus. In the vast majority of Apteronotidae, the buccopalatal membrane has two ligaments well differentiated, the pre-retroarticular and post-retroarticular ligaments (Figs. 7, 25). Such ligaments are not clearly differentiated in Adontosternarchus, Sternarchella, Platyurosternarchus, Sternarchorhynchus, Orthosternarchus, and Sternarchorhamphus, thus, were considered absent in those groups.
The path of the ramus mandibularis trigeminus nerve is variable across the family. This nerve is invariably mesial to the malaris and lateral to the stegalis and may be mesial or lateral to the rictalis (or to ricto-stegalis when not differentiated). In some taxa, the ramus mandibularis trigeminus may be located medially to the adductor mandibulae, segmentum facialis.
Levator arcus palatini. The levator arcus palatini has roughly the shape of a parallelogram or triangle. The relative sizes of origin and insertion are variable, with the origin typically equal to its insertion in the most species. The orientation of the anteriormost fibers is anteroposteriorly oblique (at approximately 45° angle) to the longitudinal axis of the head (Figs. 7, 24, 25), except for Platyurosternarchus, Sternarchorhynchus, Orthosternarchus, and Sternarchorhamphus, which presents the anteriormost fibers orthogonal to this axis (Figs. 26, 27). The common sites of origin of the levator arcus palatini are the frontal and sphenotics, commonly including also the pterosphenoid in some taxa. Its insertion is invariably on the hyomandibula.
Lateral view of dorsolateral musculature of Sternarchorhamphus mulleri (Apteronotidae), USNM 373030, 222.2 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 10 mm.
Sternarchorhynchus goeldii (Apteronotidae), MPEG 1193, 1, 148.3 mm LEA. A. Lateral view of dorsolateral musculature; B. Mesial view of the adductor mandibulae. Anatomical abbreviations in Tab. 1. Scale bars = 5 mm.
In the generalized pattern found in Apteronotidae, at the insertion point, the anterolateral and posterolateral fibers of the levator arcus palatini are lateral to the segmentum facialis; while the anteromesial and posteromesial fibers are mesial to the latter. However, there is significant variation in the disposition of these subsets, ranging from a pattern with only the posterolateral fibers located laterally to the segmentum facialis to a completely lateral position of the levator arcus palatini in relation to this segment. In the generalized condition found in Apteronotidae, the posterodorsal portion of the levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle in the most apteronotid species. Some species have only the posterodorsal portion of the levator arcus palatini mesial to the dilatator operculi (e.g., Compsaraia, Orthosternarchus and Sternarchogiton). In Sternarchorhynchus species, an extreme condition occurs with the anterior margin of the dilatator operculi reaching the anterodorsal margin of the levator arcus palatini (Fig. 27). The generalized pattern consists of a strictly fibrous composition of levator arcus palatini.
Dilatator operculi. The dilatator operculi is located posterior to the levator arcus palatini, and is organized in a single conical block of mass. Origin is usually on the sphenotic, frontal and hyomandibula, sometimes including also the pterotic, except in Adontosternarchus, Sternarchogiton, Porotergus and Compsaraia. The preopercle is included as a site of origin only in Platyurosternarchus. In Sternarchorhynchus, the dilatator operculi is medially displaced and includes the orbitosphenoid and pterosphenoid as its origin sites. Insertion is invariably on the dorsal process of the opercle.
Levator operculi. The levator operculi anterior originates from the lateral surface of the mid-ventral portion of the pterotic, and can be restricted to a fascia located at the pterotic canal in Platyurosternarchus (Fig. 30) and Sternarchella (Fig. 24). The levator operculi posterior originates mainly from the postotic canal segment corresponding to the supracleithrum, including also the posterior margin of the pterotic or a fascia between this point and pterotics (Fig. 24B). This muscle inserts on the posterodorsal portion of the opercle. The R-Avn is located laterally to the levator operculi anterior and mesially to the levator operculi posterior; or mesially to both sections in Sternarchella (Fig. 28) and Platyurosternarchus.
Lateral view of dorsolateral musculature of Sternarchella terminalis (Apteronotidae), MPEG 3481, 155.3 mm TL [regenerated]. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
Adductor arcus palatini. The adductor arcus palatini arises mainly from the parasphenoid, sometimes also including the prootic in Apteronotus, Adontosternarchus, Platyurosternarchus, Sternarchogiton, Compsaraia, Sternarchorhynchus, and Parapteronotus. Anteriorly, it inserts on the lateral face of the endopterygoid and metapterygoid; as the muscle progresses posteriorly, its insertion shifts from the lateral to the medial face of the suspensorium, finally inserting on the medial surface of the hyomandibula. The generalized pattern of the family consists of an adductor arcus palatini totally covered by the segmentum facialis, and its visualization requires removal of the latter. Only the posterior portion of the adductor arcus palatini is overlain by the segmentum facialis in Adontosternarchus, Orthosternarchus and Sternarchorhamphus.
Adductor hyomandibulae. This muscle arises from the ventral region of the prootic, including the parasphenoid solely in Adontosternarchus, and inserting on the posteromedial margin of the hyomandibula. Little or no variation in this muscle was found throughout the Apteronotidae.
Adductor operculi. The origin of the adductor operculi is on the exoccipital, pterotic, and prootic. Anteriorly, the insertion is on the mesial face of a dorso-mesial crest of the opercle, and posteriorly it inserts on the mesial surface of the same bone.
Additional comments on adductor mandibulae , pars malaris in Apteronotidae
The anatomical complexity of the head in Apteronotidae results in more variation in the morphology of theadductor mandibulae than in any other gymnotiform family and the homology and nomenclature of the name “adductor mandibulae, pars malaris” in Apteronotidae needs discussion.
Generally, in Teleostei, the malaris is positioned dorsolaterally relative to other subsections of the adductor mandibulae and immediately ventral to the eyeball, originating on the dorsal portion (dorsal arm) of the preopercle and on the dorsoposterior region of the hyomandibula (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). Such configuration is also present in the vast majority of Gymnotiformes. However, in several apteronotid subgroups (Apteronotus, Sternarchella, Platyurosternarchus, Sternarchorhynchus, Parapteronotus and Sternarchorhamphus), the section herein identified as the malaris is ventrally displaced relative to the eyeball, resulting in a ventrolateral position relative to the rictalis and stegalis (Figs. 22, 26–29). Such hypothesis is based on various lines of evidence.
Lateral view of dorsolateral musculature of Sternarchella raptor (Apteronotidae), USNM 374014, 71.9 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
The malaris (or the corresponding set of fibers when not differentiated from the stegalis - e.g., in Gymnotidae), is invariably the most lateral subsection of the adductor mandibulae. Likewise, the subsection identified here as the malaris in Apteronotidae is also the most lateral one. This applies even in those cases where the muscle is subdivided into promalaris and retromalaris (“Apteronotus” gr. bonapartii, Sternarchogiton and Porotergus). Additionally, the generalized pattern for the order, as for other teleosts (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
), has the malaris originating on the preopercle and hyomandibula, the same origins as the ventrolateral subsection (sometimes including also the quadrate as in Sternarchella). Also, the insertion of the malaris is directly or indirectly on the maxilla in all species of Gymnotiformes (except Gymnotidae), similarly to the ventrolateral malaris in Apteronotidae. The malaris is associated with the buccopalatal membrane in all gymnotiform except Gymnotidae, where its anterior fibers diverge onto an endomaxillary ligament, itself inserting onto the maxilla. The same situation is again present in the ventrolateral subsection of the adductor mandibulae in most apteronotids further corroborating its identity with the malaris.
The path of the ramus mandibularis trigeminus has been recognized as an unreliable landmark for determining homologies among sections of the adductor mandibulae (Dietz, 1914Dietz PA. Beiträge zur Kenntnis der Kiefer - und Kiemenbogenmuskulatur der Teleostier. I, Die Kiefer und Kiemenbogenmuskeln der Acanthopterygier. Mitt Zool Sta Neapel. 1914; 22:99–162.; Edgeworth, 1935Edgeworth FH. The cranial muscles of the vertebrates. Cambridge: Cambridge University Press; 1935.; Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). The path of that nerve is extremely variable across different taxa, sometimes even on different sides of the same specimen (LAWP, pers. obs.; Geerinckx et al., 2009Geerinckx T, Huysentruyt F, Adriaens D. Ontogeny of the jaw and maxillary barbel musculature in the armoured catfish families Loricariidae and Callichthyidae (Loricarioidea, Siluriformes), with a discussion on muscle homologies. Zool J Linn Soc. 2009; 155(1):76–96. https://doi.org/10.1111/j.1096-3642.2008.00434.x
https://doi.org/10.1111/j.1096-3642.2008...
). Despite such caveats, its trajectory can be conserved in certain groups (Tab. 1; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
) and in combination with other attributes (e.g., place of origin, form and position), the ramus mandibularis trigeminus can sometimes be useful in determining homologies of subsections of the adductor mandibulae. In all gymnotiform taxa with a well-differentiated malaris, the ramus mandibularis trigeminus is always positioned mesially to the malaris, independently of the latter´s position. This provides additional evidence for the equivalence between the ventrolateral subsection of apteronotids with the dorsolateral subsection of other Gymnotiformes.
In sum, various lines of evidence related to topology, sites of origin and insertion, association with buccopalatal membrane and position of the ramus mandibularis trigeminus nerve corroborate the ventrolateral subsection of the adductor mandibulae in some apteronotids as homologous with the dorsolateral subsection of the adductor mandibulae in other gymnotiforms and remaining teleosts, i.e., the adductor mandibulae, pars malaris.
The hypothesis above implies that the ventrolateral position of the malaris in some taxa is the result of a ventral displacement of its origin. An alternative explanation is that the anomalous position of the adductor mandibulae in apteronotids is the result of a migration in the insertion of both the malaris and rictalis. In this case, the insertion of the malaris shifts from the maxilla to the coronoid process, with a simultaneous shift of the insertion of the rictalis from the coronoid process to the buccopalatal membrane and the maxilla. Concomitantly, this scenario requires a midlateral migration of the rictalis and a parasagittal displacement of the malaris. The latter hypothesis requires a far more complex set of modifications than its alternative, none of which required by data available, and is therefore rejected. Interestingly, a similar set of conclusions can be apprehended indirectly from the illustrations and descriptions in Aguilera, (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23., although without explicit discussion.
Detailed description of the dorsolateral musculature of the head in the genera of Apteronotidae.
Adontosternarchus Ellis, 1912
Adductor mandibulae. The malaris is composed of a single mass of fibers, originating from the mid-dorsal portion of the hyomandibula, with its anterodorsal fibers differentiate into an elongated endomaxillary ligament, equal to the fibrous portion of the malaris, inserting on the posteromedial margin of the maxilla; and the its anteroventral fibers inserted to the posterior margin of the anguloarticular and dentary. The malaris is positioned dorsolaterally to the dorsal portion of the rictalis and lateroventrally to the stegalis (Fig. 23).
The rictalis originates in the metapterygoid, preopercle and hyomandibula, with its lateralmost fibers restricted to the anterior margin of the preopercular fossa. The mesialmost fibers of the rictalis converge into the intersegmental aponeurosis, with its lateralmost fibers inserting into the lateral margin of the anguloarticular. The stegalis arises from the hyomandibula, sphenotic, pterosphenoid, parasphenoid, and metapterygoid. Towards its anterior portion, the stegalis converges into the meckelian tendon which, in turn, inserts on the coronomeckelian bone; and with anterodorsal fibers converging to the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 60% of the dorsal portion of this cartilage. The course of the ramus mandibularis trigeminus nerve is lateral to the rictalis and stegalis; and mesial to the malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid, and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is narrower than its insertion, approximately a half of its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris, while its anteromesial and posteromesial bundles are medial to the malaris. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
Apteronotusgr.albifrons (Linnaeus, 1766)
Adductor mandibulae. The malaris is composed of a single mass of fibers, originating from the mid-dorsal portion of the hyomandibula and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to the fibrous portion of the malaris, to a insertion at the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla (Fig. 8). The malaris is positioned lateroventrally to the ricto-stegalis.
The ricto-stegalis originates in the pterosphenoid, parasphenoid, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process and to the posterodorsal margin of the anguloarticular; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 20% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly wider than its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the presumptive rictalis, while its anteromesial and posteromesial bundles are medial to the rictalis. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
“Apteronotus” gr.bonapartii (Castelnau, 1855)
Adductor mandibulae. The malaris is sectioned into a dorsal subsection promalaris and a ventral subsection retromalaris, which are well-differentiated, except for some set of fibers associated with the ricto-stegalis. The promalaris is positioned dorsolaterally to the dorsal portion of the ricto-stegalis, and arises from the mid-dorsal portion of the hyomandibula. This subsection converges to the buccopalatal membrane, with its anterodorsal fibers differentiate into a moderate endomaxillary ligament, equal to 2/3 of its fibrous portion, to an insertion at the connective tissue between the anterior margin of the premaxilla and upper lip. The retromalaris is positioned lateroventrally to the ricto-stegalis, arising from the anteroventral portion of the preopercle and hyomandibula, with its fibers converging into an elongated ectomaxillary ligament, equal to one and a half of its fibrous portion, that inserts at the posterolateral face of the maxilla (Fig. 25).
The ricto-stegalis originates in the pterosphenoid, parasphenoid, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 30% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly wider than its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the promalaris and ricto-stegalis, while its anteromesial and posteromesial bundles are medial to the promalaris and ricto-stegalis (specifically to the presumed rictalis). The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
Apteronotus gr. leptorhynchus (Ellis, 1912)
Adductor mandibulae. The malaris is composed of a single mass of fibers, originating from the mid-dorsal portion of the hyomandibula and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an elongated endomaxilar ligament, twice to the fibrous portion of the malaris, to a insertion at the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla. The malaris is positioned lateroventrally to the ricto-stegalis.
The ricto-stegalis originates in the preopercle, quadrate, pterosphenoid, hyomandibula, and sphenotic. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon (Fig. 30).
Apteronotus rostratus (Apteronotidae), USNM 317229, 142.3 mm LEA. A. Lateral view of dorsolateral musculature; B. Mesial view of the adductor mandibulae. Green indicates the path of the recurrent ramus of anteroventral part of anterior lateral line nerve. Anatomical abbreviations in Tab. 1. Scale bars = 5 mm; 4 mm.
The segmentum mandibularis extends posteriorly for a significant distance, with the major portion of the segment localized beyond the posterior limit of the lower jaw, and arising along the anterior part of the intersegmental aponeurosis and from the mandibular raphe that is shared with the anterior portion of the segmentum facialis. The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 60% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly wider than its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the presumptive rictalis, while its anteromesial and posteromesial bundles are medial to the rictalis. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
Compsaraia Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.
Adductor mandibulae. The malaris is composed of a wide single mass of fibers, with a more concentrated bundle of fibers at its dorsalmost portion than its midventral portion, and located laterally to the ricto-stegalis, overlapping it almost completely. The malaris arises from hyomandibula and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to 2/3 to the fibrous portion of the malaris, to an insertion at the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla (Fig. 31).
Lateral view of dorsolateral musculature of Compsaraia compsa (Apteronotidae), MZUSP 56206, 123.4 mm LEA. Anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
The ricto-stegalis originates in the pterosphenoid, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 30% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to half of its insertion. At the insertion, the posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris and to the presumed rictalis, while its anteromesial, anterolateral, and posteromesial bundles are medial to those sections. The anteriormost fibers of the levator arcus palatini presents an aponeurotic aspect; and its posterodorsal fibers lies mesially to the dilatator operculi, but without reaching the median portion of this muscle.
Orthosternarchus Ellis, 1912
Adductor mandibulae. The malaris is composed of a single mass of fibers, originating from the mid-dorsal portion of the hyomandibula, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to three times of the fibrous portion of the malaris, to a insertion at the posteromesial margin of the premaxilla; and the anteroventral fibers converges into an ectomaxillary ligament poorly differentiated that inserts at the posterolateral face of the maxilla.
The ricto-stegalis originates in the quadrate, pterosphenoid, parasphenoid, hyomandibula, sphenotic, and metapterygoid. At its origin, the ricto-stegalis is a single mass of fibers, and partially differentiated towards their insertion sites. The fibers corresponding to the presumed rictalis inserts mainly into the coronoid process throught a ligament equal to the fibrous portion of the ricto-stegalis; and the presumed stegalis converges into the meckelian tendon to an insertion to the coronomeckelian bone.
The pattern consists of a fibrous composition of adductor mandibulae, segmentum facialis, however, some more mesial tendons ossify, resulting in the occurrence of intermuscular bones. The ramus mandibularis trigeminus nerve lies mesial to the segmentum facialis.
Levator arcus palatini. The levator arcus palatini has a trapezoidal shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini corresponds to the 2/3 of its insertion. At the insertion of the levator arcus palatini, all fibers are located laterally to the malaris; and with only the posterodorsal fibers being mesial to the dilatator operculi, where the anterior margin of the dilator operculi does not exceeds the medial portion of the levator arcus palatini.
Parapteronotus Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.
Adductor mandibulae. The malaris is composed of a single mass of fibers, originating from the mid-dorsal portion of the hyomandibula and preopercle, converging anteriorly to a well differentiated buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to the fibrous portion of the malaris, to a insertion at the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla. The malaris is positioned lateroventrally to the ricto-stegalis.
The ricto-stegalis originates in the hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process and to the posterodorsal margin of the anguloarticular; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 50% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly wider than its insertion. At the insertion, posterolateral fiber bundles of the levator arcus palatini are lateral to the presumptive rictalis, while its anterolateral, anteromesial and posteromesial bundles are medial to the rictalis. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
Pariosternarchus Albert & Crampton, 2006
Adductor mandibulae. The malaris arises from hyomandibula and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to 2/3 to the fibrous portion of the malaris, to an insertion at the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla.
The ricto-stegalis originates in the parasphenoid, hyomandibula, sphenotic, quadrate, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 60% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the malaris and dorsolaterally to the presumed rictalis; while its anteromesial and posteromesial bundles are medial to the malaris and to the presumed rictalis. The anteriormost fibers of the levator arcus palatini presents an aponeurotic aspect. This muscle has a mesial arrangement where the anterior margin of the dilator operculi exceeds its median portion.
Platyurosternarchus Mago-Leccia, 1994Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.
Adductor mandibulae. The adductor mandibulae, segmentum facialis lacks any subdivisions, however, at its origin, the segment is partially sectioned posterodorsally by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The segmentum facialis originates from the hyomandibula, quadrate, simpletic, pterosphenoid, parasphenoid, sphenotic, metapterygoid, and preopercle. The lateralmost fibers converge anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to the fibrous portion of this portion of the segmentum facialis, to a insertion at the connective tissue between the anterior margin of the premaxilla and upper lip, and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla; and, based on positional correspondence, this muscle portion presumably corresponds to the malaris.
The rictalis and stegalis differentiates towards their insertion sites, with the lateroventral fibers homologous, at least in part, with the rictalis, inserting into the coronoid process; and the medialmost set of fibers of the segmentum facialis, presumably homologous with the stegalis, converging to the meckelian tendon that, in turn, inserts on the coronomeckelian (Fig. 22). The ramus mandibularis trigeminus nerve trespasses the presumed rictalis and stegalis, and lies lateral to both sections and mesial to the malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is slightly wider than its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the presumed rictalis and dorsolaterally to malaris; while its anteromesial and posteromesial bundles are medial to the presumed rictalis. This muscle has a mesial arrangement where the anterior margin of the dilatator operculi reaching the anterodorsal margin of the levator arcus palatini.
Porotergus Ellis, 1912
Adductor mandibulae. The malaris is partially differentiated into a dorsal subsection promalaris and a ventral subsection retromalaris, which are well-differentiated from the remaining segmentum facialis, except for some set of fibers associated with the ricto-stegalis. The promalaris is positioned dorsolaterally to the dorsal portion of the ricto-stegalis, and arises from the mid-dorsal portion of the hyomandibula. This subsection converges to the buccopalatal membrane, with its anterodorsal fibers differentiate into an endomaxillary ligament equal to its fibrous portion; and inserting to the connective tissue between the anterior margin of the premaxilla and upper lip. The retromalaris is positioned lateroventrally to the ricto-stegalis, arising from the anteroventral portion of the preopercle and hyomandibula, with its fibers converging into an elongated ectomaxillary ligament, equal to one and a half of its fibrous portion, that inserts at the posterolateral face of the maxilla.
The ricto-stegalis originates in the hyomandibula, quadrate, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 30% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and subsections of malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid, and sphenotic; and inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to its insertion. At the insertion, the posterolateral fiber bundles of the levator arcus palatini are lateral to the promalaris and ricto-stegalis, while its anterolateral, anteromesial and posteromesial bundles are medial to the promalaris and ricto-stegalis (specifically to the presumed rictalis). The anteriormost fibers of the levator arcus palatini presents an aponeurotic aspect. This muscle has a mesial arrangement where the anterior margin of the dilator operculi exceeds its median portion.
Sternarchogiton Eigenmann, 1905 and Tenebrosternarchus Bernt, Fronk, Evans & Albert, 2020
Adductor mandibulae. The promalaris and retromalaris subsections are continuous with each other at their origin and differentiated towards their insertion sites (Figs. 6–32). The posterodorsal portion of the promalaris is sectioned by the posterolateral fibers of the levator arcus palatini, being medial to the posterolateral fibers of that muscle; and with the remaining fibers located laterally to the levator arcus palatini.
Lateral view of dorsolateral musculature of Tenebrosternarchus preto (Apteronotidae), MPEG 22758, 268.5 mm LEA. A= anterolateral fibers of the levator arcus palatini; P= posterolateral fibers of the levator arcus palatini. Remaining anatomical abbreviations in Tab. 1. Scale bar = 5 mm.
The promalaris is positioned dorsolaterally to the dorsal portion of the ricto-stegalis, and arises from the mid-dorsal portion of the hyomandibula. This subsection converges to the buccopalatal membrane, with its anterodorsal fibers differentiate into an endomaxillary ligament equal to its fibrous portion; and inserting to the connective tissue between the anterior margin of the premaxilla and upper lip. The retromalaris is positioned lateroventrally to the ricto-stegalis, arising from the anteroventral portion of the preopercle and hyomandibula, with its fibers converging to the buccopalatal membrane and its ventralmost portion converging into an elongated ectomaxillary ligament, equal to one and a half of its fibrous portion, that inserts at the posterolateral face of the maxilla.
The ricto-stegalis originates in the parasphenoid, pterosphenoid, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 30% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and subsections of malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid, and sphenotic; and inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to its insertion. At the insertion, the posterolateral fiber bundles of the levator arcus palatini are lateral to the promalaris and ricto-stegalis, while its anterolateral, anteromesial and posteromesial bundles are medial to the promalaris and ricto-stegalis (specifically to the presumed rictalis). The anteriormost fibers of the levator arcus palatini presents an aponeurotic aspect. Only the posterodorsal fibers of the levator arcus palatini located mesially to the dilatator operculi, where the anterior margin of the dilator operculi does not exceeds the median portion of the levator arcus palatini.
Sternarchella Eigenmann, 1905
Adductor mandibulae. The malaris arises from the mid-dorsal portion of the hyomandibula, quadrate, and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to the fibrous portion of the malaris, to an insertion at the mesethmoid, premaxilla and the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla (Figs. 24, 28).
The ricto-stegalis originates in the pterosphenoid, parasphenoid, sympletic, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process and to the posterodorsal margin of the anguloarticular; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 20% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal, pterosphenoid, and sphenotic; inserting onto the hyomandibula. The origin of the levator arcus palatini is wider than its insertion, equal to one and a half of its insertion (Fig. 28). At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the presumptive rictalis, while its anteromesial and posteromesial bundles are medial to the rictalis. The anteromesial fibers are inserted into the hyomandibula through an aponeurotic attachment, with the remaining subsets of fibers being essentially fibrous. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
S. duccis and S. raptor [“S. duccis clade” sensuEvans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
]
Adductor mandibulae. The malaris arises from the mid-dorsal portion of the hyomandibula, quadrate, and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to the fibrous portion of the malaris, to a insertion at the mesethmoid, premaxilla and the connective tissue between the anterior margin of the premaxilla and upper lip; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the maxilla. Some medialmost fibers attaches to the posterior margin of the anguloarticular. The malaris is positioned lateroventrally to the ricto-stegalis (Fig. 29).
The ricto-stegalis originates in the pterosphenoid, parasphenoid, sympletic, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers corresponding to the rictalis inserts mainly into the coronoid process; and the presumed stegalis converges into an intersegmental aponeurosis weakly differentiated, where its ventral portion differentiates into the meckelian tendon to an insertion to the coronomeckelian bone, and the anterodorsal fibers converges into the mandibular tendon.
The segmentum mandibularis is located dorsally to Meckel’s cartilage, extending about 60% of the dorsal portion of this cartilage. The ramus mandibularis trigeminus nerve trespasses the ricto-stegalis, and lies lateral to the presumptive stegalis and mesial to the rictalis and malaris.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic; inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to its insertion. At the insertion, the anterolateral, anteromesial and posterolateral fiber bundles of the levator arcus palatini are lateral to the presumptive rictalis, while its posteromesial bundles are medial to the rictalis. The anterolateral fibers are inserted into the hyomandibula through an aponeurotic attachment, with the remaining subsets with a fibrous aspect. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the median portion of this muscle.
Sternarchorhamphus Eigenmann, 1905
Adductor mandibulae. The malaris arises from the mid-dorsal portion of the hyomandibula, preopercle, and simpletic; converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into an endomaxilar ligament, equal to a half of the fibrous portion of the malaris, to an insertion at the posteromesial margin of the premaxilla; and the anteroventral fibers converges to the same membrane which, in turn, attaches to the dorsal and posterior margins of the maxilla (Fig. 26).
The ricto-stegalis originates in the hyomandibula, quadrate, metapterygoid, sphenotic, parasphenoid, pterosphenoid, and sympletic. At its origin, the ricto-stegalis is a single mass of fibers, and partially differentiated towards their insertion sites. The fibers corresponding to the presumed rictalis inserts mainly into the posterior margin of the anguloarticular; and the presumed stegalis converges into the meckelian tendon to an insertion to the coronomeckelian bone. The ramus mandibularis trigeminus nerve lies mesial to the segmentum facialis.
Levator arcus palatini. The levator arcus palatini has a trapezoidal shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini corresponds to its insertion. At the insertion of the levator arcus palatini, all fibers are located laterally to the malaris; and with only the posterodorsal fibers being mesial to the dilatator operculi, where the anterior margin of the dilator operculi exceeds the median portion of the levator arcus palatini.
Sternarchorhynchus Castelnau, 1855
Adductor mandibulae. The malaris arises from the mid-dorsal portion of the hyomandibula and preopercle, converging anteriorly to the buccopalatal membrane, where the anterodorsal portion differentiates into a poorly differentiated endomaxilar ligament, equal to three time of the fibrous portion of the malaris, to an insertion at the posterodorsal face of the maxilla; and the anteroventral fibers converges into an ectomaxillary ligament that inserts at the posterolateral face of the same bone (Fig. 27). The malaris is positioned lateroventrally to the ricto-stegalis.
The ricto-stegalis originates in the quadrate, sympletic, endopterygoid, pterosphenoid, parasphenoid, hyomandibula, sphenotic, and metapterygoid. At its origin, the lateralmost portion of the ricto-stegalis, presumably corresponding to the rictalis, is separated dorsally from the fibers of the presumed stegalis by the levator arcus palatini, becoming continuous at their mid-portion and partially differentiated towards their insertion sites. The fibers presumably corresponding to the rictalis inserts mainly into the coronoid process throught a ligament equal to the fibrous portion of this section; and the presumed stegalis converges into the meckelian tendon which, in turn, inserts on the posterodorsal margin of the dentary. The segmentum mandibularis is absent (Fig. 27B). The ramus mandibularis trigeminus nerve lies mesial to the segmentum facialis.
Levator arcus palatini. The levator arcus palatini has a roughly parallelogram shape, originating from the ventral margin of the frontal and sphenotic and inserting onto the hyomandibula. The origin of the levator arcus palatini is equal to its insertion. At the insertion, the anterolateral and posterolateral fiber bundles of the levator arcus palatini are lateral to the presumptive rictalis, while its anteromesial and posteromesial bundles are medial to the rictalis. The levator arcus palatini has a mesial arrangement where the anterior margin of the dilator operculi exceeds the anterodorsal margin of this muscle.
Synonymy of the dorsolateral musculature of the head of Gymnotiformes.
The list below includes all muscle elements treated in this work and synthetizes conclusions about homology and valid names, organizing information in previous publications. The 15 different muscles recognized in this study have received 33 names in the literature. The main criterion for nomenclatural identity of each myological component is phylogenetic homology, in some cases with topological qualifiers, such as the levator operculi anterior and levator operculi posterior. The names employed with priority are highlighted in bold face and are properly referenced. Senior names are followed by their respective junior synonyms and after that their authors organized chronologically, with respective examined taxa on which their observations were based. Muscles which are newly named herein are indicated as “[n. nom.]”.
A large portion of available names refers to the adductor mandibulae, which comprises 20 previous names for its nine configurations herein identified. Remaining names are available for six muscles of the suspensorium, three each for the levator operculi posterior and the dilatator operculi, two each for the levator arcus palatini and levator operculi posterior. Other muscles have received a single name.
Adductor mandibulae,segmentum facialisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
Adducteur de la mandibulae.
Chardon, de La Hoz (1973): Sternopygus.
Adductores mandibulares.
de La Hoz, Chardon (1984): Sternopygus.
Adductor mandibula.
Albert, Campos-da-Paz (1998): Adontosternarchus, Archolaemus, Apteronotus, “Apteronotus” anas (= Parapteronotus hasemani), “Apteronotus” hasemani (= Parapteronotus hasemani), Brachyhypopomus, Distocyclus, Eigenmannia, †Ellisella kirschbaumi (= †Humboldtichthys kirschbaumi), Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Magosternarchus (= Sternarchella), Microsternarchus, Microsternarchus fimbriipinnus (= Racenisia fimbriipinna), Oedemognathus exodon (= Sternarchogiton nattereri), Orthosternarchus, Platyurosternarchus, “Porotergus” compsus (= Compsaraia compsa), Porotergus, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton. Albert, (2001)Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.: Adontosternarchus, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Magosternarchus (= Sternarchella), Microsternarchus, Orthosternarchus, Parapteronotus, Platyurosternarchus, Porotergus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton. Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: Adontosternarchus, Akawaio, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Japigny, Magosternarchus (= Sternarchella), Megadontognathus, Microsternarchus, Orthosternarchus, Parapteronotus, Pariosternarchus, Platyurosternarchus, Porotergus, Procerusternarchus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton.
Adductor mandibulae
,
pars malaris
Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
A1.
Fink, Fink (1981)Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
: Adontosternarchus, Apteronotus, Eigenmannia, Gymnotus, Rhabdolichops, Sternarchorhamphus, Sternarchorhamphus macrostomus (= Platyurosternarchus macrostomus?), Sternopygus. Aguilera, (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Adontosternarchus, Apteronotus, Eigenmannia, Gymnorhamphichthys, Rhamphichthys, Rhabdolichops, Steatogenys, Sternarchella, Sternarchorhamphus, Sternarchorhynchus, Sternopygus.
A1a or m.ad.m.I (a).
de La Hoz, Chardon (1984): Sternopygus.
m.ad.m.I or 1er faisceaux des muscles adducteurs de la mandibulae.
Chardon, de La Hoz (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.: Sternopygus.
Outer segment of the adductor muscle or outer segment of the adductor.
Howes (1983)Howes JG. Cranial muscles of loricarioid catfishes, their homologies and value as taxonomic characters (Teleostei: Siluroidei). Bull Br Mus Nat Hist Zool. 1983; 45:309–45. https://doi.org/10.5962/bhl.part.28003
https://doi.org/10.5962/bhl.part.28003...
: Eigenmannia, Gymnotus, Rhamphichthys, Sternopygus.
Ventrolateral branch of m. Adductor mandibulae (ostariophysan A1).
Albert (2001): Adontosternarchus, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Magosternarchus (= Sternarchella), Microsternarchus, Orthosternarchus, Parapteronotus, Platyurosternarchus, Porotergus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton. Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: Adontosternarchus, Akawaio, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Japigny, Magosternarchus (= Sternarchella), Megadontognathus, Microsternarchus, Orthosternarchus, Parapteronotus, Pariosternarchus, Platyurosternarchus, Porotergus, Procerusternarchus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton.
Adductor mandibulae,pars promalarisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
A1α.
Aguilera (1986): Apteronotus bonaparti (= Apteronotus bonapartii).
Adductor mandibulae,pars retromalarisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
A1β.
Aguilera (1986): Apteronotus bonaparti (= Apteronotus bonapartii).
Adductor mandibulae,pars rictalisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
A2.
Aguilera (1986): Adontosternarchus, Apteronotus, Eigenmannia, Rhabdolichops, Steatogenys, Sternarchella, Sternarchorhamphus, Sternarchorhynchus, Sternopygus. Albert, (2001)Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.: Adontosternarchus, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Magosternarchus (= Sternarchella), Microsternarchus, Orthosternarchus, Parapteronotus, Platyurosternarchus, Porotergus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton. Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: Adontosternarchus, Akawaio, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Japigny, Magosternarchus (= Sternarchella), Megadontognathus, Microsternarchus, Orthosternarchus, Parapteronotus, Pariosternarchus, Platyurosternarchus, Porotergus, Procerusternarchus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton.
A1b or m.ad.m.I (b).
de La Hoz, Chardon (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
m.ad.m.II or 2e faisceaux des muscles adducteurs de la mandibulae.
Chardon, de La Hoz (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.: Sternopygus.
Adductor mandibulae,pars ricto-stegalisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
A2-3.
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Gymnorhamphichthys, Rhamphichthys, Sternarchorhamphus, Sternarchorhynchus.
Adductor mandibulae,pars stegalisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
A3.
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Adontosternarchus, Eigenmannia, Rhabdolichops, Steatogenys, Sternarchorhamphus, Sternarchorhynchus, Sternopygus.
M. ad. m. II and m. ad. m. III [?- muscle identify with two subsections]
de La Hoz, Chardon (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
m.ad.m.III or 3e faisceaux des muscles adducteurs de la mandibulae.
Chardon, de La Hoz (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.: Sternopygus.
Adductor mandibulae,pars stego-malarisandpars rictalisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
Complejo adductor mandibulae [?- muscle identify with two subsections].
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Electrophorus and Gymnotus.
Adductor mandibulae,segmentum mandibularisDatovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
.
Aw.
de La Hoz, Chardon (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
Levator arcus palatini sensuWinterbottom, (1974a)Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
.
m.le.hm or muscle élévateur de l’arc palatin et de l’hyomandibulaire.
Chardon, de La Hoz (1973)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
Levator hyomandibulae [?- unidentified muscle].
de La Hoz, Chardon (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
Dilatator operculi sensuWinterbottom, (1974a)Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
.
Dilator operculi.
Carvalho, Albert (2011)Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
: Gymnorhamphichthys, Iracema e Rhamphichthys.
Dilator operculi or dilator opérculi.
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Adontosternarchus, Apteronotus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Rhamphichthys, Rhabdolichops, Steatogenys, Sternarchella, Sternarchorhamphus, Sternarchorhynchus, Sternopygus.
m.dil.op or muscle dilatateur de l’opercule.
Chardon, de La Hoz (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.: Sternopygus.
Levator operculi sensuWinterbottom, (1974a)Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
.
Levator opercularis.
Freihofer (1963)Freihofer WC. Patterns of the ramus lateralis accessorius and their systematic significance in teleostean fishes. Stanf Ichthyol Bull; 1963(8):81–189.: Gymnotus, Sternopygus.
Levator operculi anteriorAguilera, (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23..
Levator operculi anterior or Levator opérculi anterior.
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Adontosternarchus, Apteronotus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Rhamphichthys, Rhabdolichops, Steatogenys, Sternarchella, Sternarchorhamphus, Sternarchorhynchus, Sternopygus.
m.le.op.a or muscle élévateur antérieur de l’opercule.
Chardon, de La Hoz (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.: Sternopygus.
Levator operculi posteriorAguilera, (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23..
Levator operculi posterior or levator opérculi posterior.
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: Adontosternarchus, Apteronotus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Rhamphichthys, Rhabdolichops, Steatogenys, Sternarchella, Sternarchorhamphus, Sternarchorhynchus, Sternopygus.
Levator posterior.
Albert, Campos-da-Paz (1998)Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.: Adontosternarchus, Archolaemus, Apteronotus, “Apteronotus” anas (= Parapteronotus hasemani), “Apteronotus” hasemani (= Parapteronotus hasemani), Brachyhypopomus, Distocyclus, Eigenmannia, †Ellisella kirschbaumi (= †Humboldtichthys kirschbaumi), Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Magosternarchus (= Sternarchella), Microsternarchus, Microsternarchus fimbriipinnus (= Racenisia fimbriipinna), Oedemognathus exodon (= Sternarchogiton nattereri), Orthosternarchus, Platyurosternarchus, “Porotergus” compsus (= Compsaraia compsa), Porotergus, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton. Albert, (2001)Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.: Adontosternarchus, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, †Ellisella kirschbaumi (= †Humboldtichthys kirschbaumi), Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Magosternarchus (= Sternarchella), Microsternarchus, Orthosternarchus, Parapteronotus, Platyurosternarchus, Porotergus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton. Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: Adontosternarchus, Akawaio, Archolaemus, Apteronotus, Brachyhypopomus, Compsaraia, Distocyclus, Eigenmannia, Electrophorus, Gymnotus, Gymnorhamphichthys, Hypopygus, Hypopomus, Iracema, Japigny, Magosternarchus (= Sternarchella), Megadontognathus, Microsternarchus, Orthosternarchus, Parapteronotus, Pariosternarchus, Platyurosternarchus, Porotergus, Procerusternarchus, Racenisia, Rhamphichthys, Rhabdolichops, Sternarchorhamphus, Sternarchella, Steatogenys, Sternopygus, Sternarchorhynchus, Sternarchogiton.
m.le.op.p or muscle élévateur postérieur de l’opercule.
Chardon, de La Hoz (1973)Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.: Sternopygus.
Adductor arcus palatini sensuWinterbottom, (1974a)Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
.
Adductor hyomandibulae anterior [?- name supposedly designated to the posterior portion of the adductor arcus palatini].
de La Hoz, Chardon (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
Adductor hyomandibulae sensuWinterbottom, (1974a)Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
.
Adductor hyomandibulae posterior.
de La Hoz, Chardon (1984)de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.: Sternopygus.
Phylogenetic inference
The comparative analysis of the dorsolateral head muscles of Gymnotiformes yielded 56 morphological characters listed below, which were organized into a data matrix (Tab. S1). This matrix was analyzed according to the methods described in the Material and Methods section above (Analysis 1). Subsequently, the myological matrix was concatenated with phenotypic characters from Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
with the modifications of Peixoto et al., (2019)Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
(Analysis 2). Results are discussed and further analyzed by means of a partitioned Bremer support as to their significance for the phylogeny of Gymnotiformes and for elucidating the impact of myology on phylogenetic hypotheses. Descriptions of phylogenetically-informative variation identified in the present study are organized by morphological complex. Each character is presented with a short title followed by recognized states, original source when not the present paper, respective CI and RI and any needed explanation.
Characters of the dorsolateral musculature of the head of Gymnotiformes: Description and Analysis 1. A parsimony analysis containing only characters from dorsolateral head miology for 87 terminals (4 outgroups and 83 Gymnotiformes) and 56 characters (Tab. S1), resulted in 40 equally parsimonious trees with 192 steps (CI: 0.37, RI: 0.87). A strict consensus tree (Fig. 33) is the basis for referring to clades mentioned below. Support indices and mapping of synapomorphies are presented in Fig. 34.
Strict consensus of MPT’s resulting from parsimony analysis of character matrix in S1 [Score: 192; RI: 0.87; CI: 0.37]. Abbreviations below each branch are those used in text for respective clades. Taxon legends: Brachyhypopomus spp.1 (B. beebei, B. bombilla, B. draco, B. gaudeiro, B. janeiroensis, and B. sullivani); Brachyhypopomus spp.2 (B. brevirostris, B. hendersoni, B. pinnicaudatus and B. regani); Sternarchella spp.1 (S. duccis and S. raptor); Sternarchella spp.2 (S. schotti and S. terminalis); Eigenmannia spp.1 (E. muirapinima and E. vicentespelaea); Eigenmannia spp.2 (E. humboldtii, E. limbata, E. nigra, E. pavulagem, and E. sp. “ventuari”); Eigenmannia spp.3 (E. antonioi, E. besouro, E. desantanai, E. guairaca, E. matintaperera, E. macrops, E. meeki, E. microstoma, E. sayona, E. trilineata, E. virescens, and E. waiwai); Rhabdolichops spp.1 (R. nigrimans and R. lundbergi); Rhabdolichops spp.2 (R. caviceps, R. electrogrammus, and R. zareti); Rhabdolichops spp.3 (R. eastwardi and R. troscheli).
Same tree as in Fig. 33, with numbered dorsolateral head musculature characters (below branches) and respective character states (above branches). Black squares indicate homoplasy-free characters and white squares indicate homoplastic characters. Table on left indicates support values for each node: BR (Relative Bremer support), BST (Bootstrap), and JCK (Jackknife). Taxon legends as in Fig. 33.
1) Degree of separation betweenmalarisandstegalis: (0) completely differentiated; (1) partially continuous, forming stego-malaris [CI: 0.33–0.66; RI: 0.66].
The generalized condition in Ostariophysi consists of a completely differentiated malaris and stegalis, although cases of non-differentiation have been recorded (Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
).
The apomorphic condition (State 1) is present in basal Siluriformes and Gymnotiformes and interpreted as a synapomorphy for Siluriphysi (sensu Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Siluriformes + Gymnotiformes), with a reversal in the ST clade and subsequent reacquisition in Platyurosternarchus.
2) Degree of separation betweenrictalisandstegalis: (0) completely differentiated; (1) partially continuous, forming ricto-stegalis [CI: 0.50; RI: 0.95].
The generalized pattern for Gymnotiformes and all outgroup representatives, consists of a total differentiation of the rictalis and the stegalis.
According to our analysis, the differentiation between rictalis and stegalis is plesiomorphic among gymnotiforms, whereas a continuous ricto-stegalis is an apomorphy for Ramphichthyidae and some Apteronotidae, convergent in Clades R and PC (Fig. 33).
3) Origin ofmalarison suspensorium: (0) on hyomandibula, preopercle and other elements of suspensorium; (1) origin on hyomandibula only [CI: 1; RI: 0.71].
The origin of the malaris in all representatives of outgroups and in some gymnotiforms includes the hyomandibula and preopercle, in addition to other elements of the suspensorium. In some cases, the malaris may originate on the hyomandibula only.
The condition described in State 1 is a synapomorphy for the Gymnotiformes (clade GY), with reversals in the clade PC (and subsequent redevelopment in Orthosternarchus) and in the clade AE (and redevelopment in the clade DR).
4) Origin ofmalarison skull: (0) not including sphenotic; (1) including sphenotic (modified from Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
: ch. 8) [CI: 0.50-0.80; RI: 0.80].
The generalized condition in Teleostei is to have the origin of the malaris limited to the elements of the suspensorium, not including the sphenotic (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). Contrastingly, in the vast majority of Siluriformes (including basal lineages such as Diplomystidae and Cetopsidae), and basal members of Gymnotiformes, the origin of the malaris extends dorsally and includes the sphenotic. Datovo, Vari (2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
: 60) proposed the “origin of the malaris extending dorsally and connecting with the neurocranium” as synapomorphic for the Siluriphysi. Herein, the sphenotic is identified as the exact reference point of attachment of the malaris on the neurocranium.
The malaris with origin in the sphenotic is a synapomorphy for the Siluriphysi, with a reversal in the clade ST.
5) Insertion of themalarison the mandibular tendon: (0) absent; (1) present [CI: 0.50-0.80; RI: 0.80].
Insertion sites of the malaris are variable (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
). In Ostariophysi, it ranges from an inter-segmental aponeurosis (subocular tendon sensuDatovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
) in Chanos (LAWP, pers. obs.; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
), to a layer of connective tissue between the upper lip and the premaxilla in some Apteronotidae. In basal Siluriformes and Gymnotiformes, the fibers of the malaris converge onto a thick mandibular tendon inserted on the mesial surface of the coronoid region (Fig. 3).
In Gymnotidae there is no evident differentiation between the malaris and the stegalis, which are thus jointly referred to as the stego-malaris. The topological inference about the insertion site referable to the malaris follows the same reasoning explained in Character 3 above. Some publications (e.g.,Diogo, Chardon, 2000Diogo R, Chardon M. Homologies among different adductor mandibulae sections of teleostean fishes, with special regard to catfishes (Teleostei: Siluriformes). J Morphol. 2000; 243(2):193–208. https://doi.org/10.1002/(SICI)1097-4687(200002)243:2<193::AID-JMOR8>3.0.CO;2-2
https://doi.org/10.1002/(SICI)1097-4687(...
; Diogo, 2004Diogo R. Morphological evolution, adaptations, homoplasies, constraints, and evolutionary trends: catfishes as a case study on general phylogeny and macroevolution. Enfield: Science Publishers; 2004.) reported insertion sites for the malaris different from those observed here in outgroups. The codification of states included in the data matrix utilized here corresponds to the states directly observed in the material examined.
The insertion of the malaris on the mandibular tendon is herein listed as a synapomorphy for Siluriphysi, with a reversal in the clade ST.
6) Insertion ofmalarison antorbital: (0) absent; (1) present [CI: 1; RI: 1].
With exception of Gymnotidae, the malaris of Gymnotiformes converges onto an endomaxillary ligament, itself inserted on different sites in various taxa across order.
In representatives of the Rhamphichthyoidea (Rhamphichthyidae + “Hypopomidae”; clade RH) that muscle - by means of its endomaxillary ligament - inserts on the mesial surface of the antorbital and maxilla (Figs. 4, 5, 8, 13), a synapomorphy for that clade.
7) Insertion of themalarison infraorbital 1+2: (0) absent; (1) present (modified from Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.: ch. 46; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.: ch. 45; Albert et al., 2005Albert JS, Crampton WGR, Thorsen DH, Lovejoy NR. Phylogenetic systematics and historical biogeography of the Neotropical electric fish Gymnotus (Teleostei: Gymnotidae). Syst Biodivers. 2005; 2(4):375–417. https://doi.org/10.1017/S1477200004001574
https://doi.org/10.1017/S147720000400157...
: ch. 82; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: ch. 57; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 115) [CI: 1; RI: 1].
As described on characters 5 and 6, there is ample variation in the insertion of the malaris among gymnotiforms. Uniquely in Sternopygidae, that muscle inserts on the compound infraorbital 1+2 (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 11), with mesial fibers differentiated into a minute endomaxillary ligament inserted on the posteromesial surface of the maxilla. For historical overview of this character see Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
.
The condition described above is unique among Ostariophysi, and is an exclusive synapomorphy for the Sternopygidae (clade S).
8) Insertion ofmalaristhrough an endomaxillary ligament on connective tissue between premaxilla and upper lip: (0) absent; (1) present [CI: 0.50; RI: 0.92].
The most conspicuous variation relative to the insertion of the malaris among gymnotiforms is seen in some Apteronotidae. In those species, the dorsal fibers of the malaris diverge onto a long endomaxillary ligament while ventral fibers attach to a medium-sized ectomaxillary ligament. As a result, this muscle has two insertions (Figs. 6, 7, 28–30, 31, 32).
The insertion of the malaris through a long endomaxillary on the connective tissue between the anterior margin of the premaxilla and the upper lip is an unusual condition and it is recovered as a synapomorphy for the clade PC with a reversal in the clade PS.
9) Insertion of ventrolateral fibers ofmalarison posterior margin of dentary and anguloarticular: (0) absent; (1) present (Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.) [CI: 1; RI: 1].
In Gymnotiformes in general, the anterior fibers of the malaris are differentiated into ligaments with variable insertion sites across the order. Species of Adontosternarchus have an additional macroscopically fibrous insertion directly on the posterodorsal margin of the dentary.
The condition described in the State 1 is synapomorphic for Adontosternarchus (clade AD).
10) Insertion of mesial fibers ofmalarison posterior margin of anguloarticular: (0) absent; (1) present [CI: 1; RI: 1].
Plesiomorphically in Gymnotiformes, all fibers of the malaris converge to respective insertions sites, be they single or multiple (e.g., clade PC). In species of Sternarchella, only the mesialmost fibers of the malaris diverge in the parasagittal region and insert on the posterior margin of the anguloarticular.
The condition of the State 1 is an exclusive synapomorphy for Sternarchella (clade MS).
11) Insertion ofmalarison mesethmoid and premaxilla: (0) absent; (1) present [CI: 1; RI: 1].
As explained in Character 8 above, some Apteronotidae (clade PC) have the malaris inserting on the connective tissue between the anterior margin of the premaxilla and the upper lip by means of the endomaxillary ligament.
Species of Sternarchella have an additional attachment site for the ligament at the dorsal region of the mesethmoid and posterodorsal surface of the premaxilla (Fig. 24), a synapomorphy for the genus (clade MS).
12) Attachement type ofmalaris: (0) mostly tendinous; (1) fibrous [CI: 1; RI: 1].
In the vast majority of Gymnotiformes and outgroups, the insertion of the malaris is mostly or exclusively by aponeurotic or ligamentous connections. In Sternopygidae, contrastingly, the insertion is mostly through muscle fibers (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 11), with only a few medial fibers differentiated in an endomaxillary ligament.
The fibrous attachement of malaris is synapomorphic for the Sternopygidae (clade S).
13) Composition ofmalaris: (0) malaris undivided, as a single muscle bundle; (1) malaris partly divided into promalaris and retromalaris towards insertion; (2) median and dorsoposterior regions of promalaris completely differentiated from its ventromedial region and from retromalaris; (3) promalaris and retromalaris entirely separated, from origin to insertion (ordered multistate) [CI: 1; RI: 1].
The general pattern in Gymnotiformes, as well as in outgroup taxa, is to have a consolidated malaris, not divided in subsections. A different situation occurs in Porotergus, Sternarchogiton and “Apteronotus” gr. bonapartii, where the malaris has some degree of subdivision, ranging from partial sectioning to fully differentiated subsections (Figs. 6, 25, 32). Hypothetically, the division into promalaris and retromalaris results from a gradual and apparently sequential set of modifications. That sequence ranges from a broad malaris positioned laterally to remaining sections of the adductor mandibulae, to a total differentiation into promalaris and retromalaris.
Species of Porotergus exemplify the initial extreme of the series, with a wide malaris having a dorsal portion incipiently differentiated from the ventral one, each corresponding to promalaris and retromalaris, continuous at the origin and middle portion but gradually diverging towards the buccopalatal membrane. The situation is more conspicuous in Sternarchogiton, where the malaris is partly divided into promalaris and retromalaris. The posterodorsal region of the promalaris is completely differentiated from the remaining fibers of that section, and are oriented medially relative to the fibers of the levator arcus palatini. The subdivision of the malaris is complete in “Apteronotus” gr. bonapartii, where the muscle is entirely separated into dorsal (promalaris) and ventral (retromalaris) subsections.
Clearly, there is a clinal sequence starting from a broad malaris located almost totally laterally to the ricto-stegalis and with dorsal-ventral differentiation mostly indicated by differential fiber densities. A subsequent condition has the dorsal portion of the promalaris differentiated from the rest of the muscle. Finally, there is complete separation of the malaris into promalaris and retromalaris. Such sequence of similarity supports a natural ordering of States 0, 1, 2 and 3.
In the hypothesis presented here, State 1 is a synapomorphy exclusive to clade PAS, with subsequent transition to State 2 in the clade AS and to State 3 in “Apteronotus” gr. bonapartii.
14)Malarisand buccopalatal membrane: (0) malaris not entirely continous to the lateral membrane to buccopalatal membrane; (1) malaris entirely continous to the lateral layer of buccopalatal membrane [CI: 1; RI: 1].
Plesiomorphically in Gymnotiformes, the malaris is continuous to ligaments of the buccopalatal membrane, which in turn serve as insertion elements on various sites (Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
). In Apteronotidae, all lateral fibers of the malaris are directly associated with the external layer of the buccopalatal membrane (Figs. 8, 25).
The latter state is obtained as a synapomorphy for Apteronotidae (clade A).
15) Origin ofrictalisin relation to the preopercle: (0) including preopercle; (1) not including preopercle [CI: 0.33-0.95; RI: 0.90].
In the general condition found in Gymnotiformes, the origin of the rictalis includes the preopercle, among other elements of the suspensorium. In the vast majority of apteronotids and some sternopygids, the muscle is mesially and dorsoposteriorly displaced. As a result, the origin of the rictalis no longer includes the preopercle.
The State 1 is synapomorphic for Sternopygoidea (clade SI; Apteronotidae + Sternopygidae; “Sinusoidea” of Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), with reversals in Apteronotus gr. leptorhynchus and in clade EI.
16) Origin of ventrolateral fibers ofrictalisin relation to the preopercle: (0) extending posteriorly beyond middle portion of preopercle; (1) restricted to anterior portion of preopercle, not extending beyond preopercular fossa [CI: 0.50; RI: 0.95].
In the primitive ostariophysan and in some Gymnotiformes, the rictalis has a posteriorly-displaced origin where its lateral fibers originate from the lateral surface of the preopercle and extend beyond the preopercular fossa (Figs. 1, 11). Sternopygoidea and Rhamphichthyidae are different in that regard and the origin of their rictalis is restricted to the anteromesial margin of the preopercle (Figs. 17, 18; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 8; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: figs. 15, 40).
State 1 is convergent in Rhamphichthyidae (clade R) and Sternopygoidea (clade SI). This character was coded as inapplicable in Sternopygus and all members of clade PC (except Apteronotus gr. leptorhynchus), because they lack an association between the rictalis and the preopercle.
17) Insertion of lateral fibers of therictalison the posterior margin of the anguloarticular: (0) absent; (1) present [CI: 0.83; RI: 0.52].
Primitively in Gymnotiformes, the rictalis inserts on the coronoid process, which is formed by the posterodorsal portion of the dentary and the dorsal margin of the anguloarticular. In some Gymnotiformes, that insertion is broader and also includes a considerable portion of the posterior margin of the anguloarticular.
State 1 is convergent in Adontosternarchus (clade AD), Sternarchorhamphus, and in Rhabdolichops (Clade RL).
18) Origin of thestegalisin relation to the sphenotic: (0) absent; (1) present (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 117) [CI: 0.16; RI: 0.87].
Primitively in Teleostei, the origin of the stegalis is restricted the suspensorium, not including elements of the neurocranium (e.g., Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). In basal Siluriformes and in the vast majority of Gymnotiformes, the origin of the stegalis is extended dorsoposteriorly and also includes the sphenotic.
The origin of the stegalis in the sphenotic is interpreted herein as a synapomorphy for Siluriphysi, with reversals in Apteronotus gr. leptorhynchus, Porotergus, Pariosternarchus, and in clades EI and R.
19) Origin of thestegalisin relation to the parasphenoid: (0) absent; (1) present [CI: 0.12-0.85; RI: 0.82-0.85].
As explained in the preceding character, the origin of the stegalis in teleosts is restricted to the suspensorium. In Gymnotiformes, the vast majority of species has a mesial displacement of the dorsoposterior part of the stegalis, so that its origin comprises also the posteromedial part of the parasphenoid.
The condition described in the State 1 is a synapomorhy for Gymnotiformes (clade GY), with reversals at Sternopygidae (clade S; and subsequent reacquisitions in Japigny) Hypopomus, Compsaraia, Porotergus, Parapteronotus, Apteronotus gr. leptorhynchus.
20) Origin of thestegalisin relation to the frontal: (0) absent; (1) present [CI: 1; RI: 1].
The origin of the stegalis is dorsally expanded in Gymnotidae, where it includes not only the suspensorium but also the frontal.
State 1 represents an exclusive synapomorphy for Gymnotidae (clade GY).
21) Origin of thestegalisin relation to the pterosphenoid: (0) absent; (1) present (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 118) [CI: 0.14; RI: 0.85].
Plesiomorphically in Teleostei (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
), the origin of the stegalis is limited to the suspensorium, not including neurocranial components. In most Gymnotiformes, however, the origin of the muscle is displaced posteromesially to also include the ventroposterior margin of the pterosphenoid.
The State 1 is a synapomorphy for clade ST, with reversals in Gymnorhamphichthys (clade GR), Platyurosternarchus, Porotergus, Parapteronotus, Pariosternarchus, and clade EE.
22) Insertion ofstegalison posteromesial margin of dentary: (0) absent; (1) present [CI: 1; RI: 1].
Generally, in teleosts, the stegalis differentiates into a meckelian tendon, connected to the dorsoposterior margin of the coronomeckelian bone (Diogo, Chardon, 2000Diogo R, Chardon M. Homologies among different adductor mandibulae sections of teleostean fishes, with special regard to catfishes (Teleostei: Siluriformes). J Morphol. 2000; 243(2):193–208. https://doi.org/10.1002/(SICI)1097-4687(200002)243:2<193::AID-JMOR8>3.0.CO;2-2
https://doi.org/10.1002/(SICI)1097-4687(...
; Wu, Shen, 2004Wu KY, Shen SC. Review of the teleostean adductor mandibulae and its significance to the systematic positions of the Polymixiiformes, Lampridiformes, and Triacanthoidei. Zool Stud. 2004; 43(4):712–36.; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
; 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). The same configuration happens in Gymnotiformes (LAWP, pers. obs.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). In fact, the coronomeckelian bone is considered an ossification of the meckelian tendon (= “sesamoid articular” in Ridewood, 1904Ridewood WG. On the cranial osteology of the clupeoid fishes. J Zoo. 1904; 74:448–93.; Starks, 1916Starks EC. The sesamoid articular: a bone in the mandible of fishes. Stanford: Stanford University Press; 1916.; Haines, 1937Haines RW. The posterior end of Meckel’s cartilage and related ossifications in bony fishes. J Cell Sci. 1937; 80(317):1–38. https://doi.org/10.1242/jcs.s2-80.317.1
https://doi.org/10.1242/jcs.s2-80.317.1...
), and thus it is invariably associated with the insertion of the stegalis.
The stegalis inserting on the posteromesial surface of the dorsoposterior process of the dentary are exclusive for the Sternarchorhynchus (Fig. 27).
23) Relative position of thestegalisin relation to theadductor arcus palatini: (0) stegalis not overlapping the adductor arcus palatini; (1) stegalis completely overlapping the adductor arcus palatini; (2) stegalis partially overlapping the adductor arcus palatini (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 119) [CI: 0.28; RI: 0.88].
In outgroup taxa, the stegalis is always ventral to the insertion of the adductor arcus palatini, and thus never overlaps it. In most Gymnotiformes, the stegalis is lateral to the adductor arcus palatini and overlaps it completely (Figs. 1, 2). In some species of the order, only the posteromedial and posterior portions of the adductor arcus palatini are positioned mesially to the stegalis (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 13A). This character is based on a relational landmark (position of a muscle relative to another) and as such cannot be ordered by simple similarity because a single primary variable cannot be determined. It is thus treated as unordered.
According to our analysis, State 1 is a synapomorphy for Gymnotiformes (clade GY), with convergent instances to State 2 in Adontosternarchus and clades B, SO and EI (with subsequent transformations to State 0 in clade AR and in Distocyclus).
24) Relative position ofmalarisandstegalis: (0) malaris partly overlapping the stegalis; (1) malaris completely overlapping the stegalis (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 116) [CI: 1; RI: 1].
In Gymnotiformes, the malaris is usually located laterally and partially overlaps the ventromedial portion of the stegalis. In species of Archolaemus (except A. orientalis), the stegalis is dorsoventrally compacted and completely mesial to the malaris, thus not visible in lateral view (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 15).
State 1 is synapomorphic for clade AR.
25) Position of themalarisin relation to therictalis: (0) malaris positioned dorsolaterally to dorsal portion of rictalis; (1) malaris lateral to dorsal portion of rictalis, overlapping it almost entirely; (2) malaris ventral relative to dorsal portion of rictalis; (3) dorsal portion of malaris (promalaris) dorsolateral to dorsal portion of rictalis, with its ventral region (retromalaris) ventrolateral to dorsal portion of rictalis [CI: 0.60; RI: 0.87].
The most common configuration among gymnotiforms is of a malaris dorsolaterally to the dorsal portion of the rictalis and ventrolateral to the ventromedial portion of the stegalis, occupying the region immediately ventral to the eyeball (State 0). The majority of species of Apteronotidae, however, diverge from that configuration. For a discussion on the homology of the malaris in Apteronotidae, especially in species of the clade AP, cf. section “Additional comments on the adductor mandibulae, pars malaris in Apteronotidae”. This character is based on a relational landmark (position of a muscle relative to another) and as such cannot be ordered by simple similarity because a single primary variable cannot be determined. It is thus treated as unordered.
State 1 is hypothesized as a synapomorphy for the clade PC (convergent in Brycon among outgroups), with subsequent transition to State 2 in clade AP (with reversal to state 0 in Orthosternarchus) and to 3 in “Apteronotus” bonapartii.
26) Intermuscular bones inadductor mandibulae,segmentum facialis: (0) absent; (1) present (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.: ch. 45; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.: ch. 44; Albert et al., 2005Albert JS, Crampton WGR, Thorsen DH, Lovejoy NR. Phylogenetic systematics and historical biogeography of the Neotropical electric fish Gymnotus (Teleostei: Gymnotidae). Syst Biodivers. 2005; 2(4):375–417. https://doi.org/10.1017/S1477200004001574
https://doi.org/10.1017/S147720000400157...
: ch. 83; Hilton et al., 2007Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: ch. 56) [CI: 0.33; RI: 0.50].
The most common condition of the adductor mandibulae, segmentum facialis in gymnotiforms and other ostariophysans is to lack any internal ossifications. In a few taxa, like Gymnotus gr. carapo, Rhamphichthys and Orthosternarchus, the segmentum facialis exhibits bony filaments resulting from tendinous or ligamentous ossifications (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12). Additional discussion on such ossifications is presented section “General aspects of the dorsolateral head musculature in Gymnotiformes”, subsection “Adductor mandibulae”.
The presence of such ossifications is convergently developed in Gymnotus gr. carapo, Rhamphichthys and Orthosternarchus.
27)Segmentum mandibularis: (0) present; (1) absent (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 120) [CI: 0.16; RI: 0.75].
The adductor mandibulae segment inserted medially on the mandible is called segmentum mandibularis (Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
) and it originates from the mandibular tendon. The segmentum mandibularis is present in the vast majority of Ostariophysi, as well as in Gymnotiformes. However, this segment is absent in Labeo (Cypriniformes), Gymnotidae (clade G), Rhamphichthyidae (clade R), clade SS and Archolaemus (clade AC, with reversal in A. luciae).
All cases describe above represent convergent losses.
28) Length ofsegmentum mandibularisin relation to Meckel´s cartilage: (0) segmentum mandibularis contacting up to 2/3 of the dorsal margin of Meckel´s cartilage; (1) segmentum mandibularis contacting the entire dorsal margin of Meckel´s cartilage (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 121) [CI: 0.33; RI: 0.66].
When present in Gymnotiformes, the segmentum mandibularis usually contacts up to two-thirds of the dorsal margin of Mecke´s cartilage. Contrastingly, the segmentum mandibularis in Parapteronotus and Rhabdolichops (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 41B) contacts practically the entire dorsal margin of Meckel´s cartilage.
This character is inapplicable in Labeo (Cypriniformes), Gymnotidae (clade G), Rhamphichthyidae (clade R), remaining Archolaemus and speies on Clade SS, because the segmentum mandibularis is absent in those taxa.
In Analysis 1, State 1 is convergent in Brycon, clade RC and Parapteronotus.
29) Insertion ofsegmentum mandibularis: (0) restricted to dentary; (1) including anguloarticular and dentary; (2) restricted to anguloarticular (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 122) [CI: 0.33; RI: 0.20].
In primitive members of Ostariophysi, the segmentum mandibularis inserts on the dentary only (State 0). In other taxa of the ingroup, the insertion can be on the dentary and anguloarticular (State 1) or restricted to the anguloarticular (State 2).
The segmentum mandibularis is absent in Labeo (Cypriniformes), Gymnotidae (clade G), Rhamphichthyidae (clade R), Archolaemus (except A. luciae) and species in clade SS. This character is therefore coded as inapplicable in those taxa.
State 1 is hypothesized as a synapomorphy for Characiphysi, with convergent transitions to state 2 in Microsternarchus, Steatogenys (clade SY), Japigny and E. humboldtii, and a reversal to State 0 in A. luciae.
30) Posterior extension ofsegmentum mandibularisbeyond posterior margin of mandible: (0) absent; (1) present [CI: AUT; RI: AUT].
In the primitive condition in Gymnotiformes and basal representatives of other Ostariophysi, the segmentum mandibularis is limited to the mesial face of the dentary and is not visible in lateral view. In Apteronotus gr. leptorhynchus, the segmentum mandibularis originates from a mandibular raphe on the anteromedial portion of the adductor mandibulae, segmentum facialis, emerging posteriorly relative to the posterior margin of the anguloarticular and visible laterally (Fig. 30).
The segmentum mandibularis is absent in Labeo (Cypriniformes), Gymnotidae (clade G), Rhamphichthyidae (clade R), Archolaemus (except A. luciae) and species in clade SS. This character is therefore coded as inapplicable in those taxa.
The condition described in the State 1 is synapomorphic for Apteronotus gr. leptorhynchus.
31) Transverse ligament: (0) undifferentiated; (1) well differentiated [CI: 1; RI: 1].
Tendinous or ligamentous structures associated to the buccopalatal membrane usually result from mechanical stress associated with forces during opening and closing of the mouth. Collagen strips concentrate in regions of greater tension, differentiating into ligaments associated with the buccopalatal membrane (Osse, 1969Osse JWM. Functional morphology of the head of the perch (Perca fluviatilis L.): an electromyographic study. Neth J Zool. 1969; 19(3):289–392. https://doi.org/10.1163/002829669X00134
https://doi.org/10.1163/002829669X00134...
; Gosline, 1986Gosline WA. Jaw muscle configuration in some higher teleostean fishes. Copeia. 1986; 1986(3):705–13. https://doi.org/10.2307/1444953
https://doi.org/10.2307/1444953...
; Datovo, Castro, 2012Datovo A, Castro RMC. Anatomy and evolution of the mandibular, hyopalatine, and opercular muscles in characiform fishes (Teleostei: Ostariophysi). Zoology. 2012; 115(2):84–116. https://doi.org/10.1016/j.zool.2011.09.008
https://doi.org/10.1016/j.zool.2011.09.0...
). One of those ligaments is called transverse ligament (Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
) and it arises from the inter-segmental aponeurosis and runs along the posterior margin of the retrojugal lamina. The transverse ligament is not differentiated in the majority of Gymnotiformes, except in species of Sternopygidae, where it is modified into a conspicuous ligament transversely oriented relative to the anterior margin of the segmentum facialis (Fig. 7).
State 1 is a synapomorphy for Sternopygidae (clade S).
32) Endomaxilar ligament, lenght relative to fibrous portion ofmalaris: (0) shorter than 2/3; (1) equal; (2) twice; (3) triple. [CI: 0.37; RI: 0.73].
Several ligaments are associated with the buccopalatal membrane. Among those, the endomaxilar ligament is present in the vast majority of Gymnotiformes, always associated with the lateral subsection of the adductor mandibulae, pars malaris.
The endomaxillary ligament is variable in size, with the most common situation in gymnotiforms being a minute condition, shorter than 2/3 the length of the fibrous portion of the malaris. That ligament, however, can be variably elongated in different taxa, reaching up to three times the length of the fibrous portion of the malaris. This character is based on a relational landmark (length of a ligament vs. that of fibrous portion of a muscle) and as such cannot be ordered by simple similarity because a single primary variable cannot be determined. It is thus treated as unordered.
There is no endomaxilary ligament in Chanos and Gymnotidae (clado G), which are thus coded as inapplicable.
In the Analysis 1, State 1 (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12) is convergent in Rhamphichthyidae (clade R), with a modification to State 2 (Fig. 5) in Gymnorhamphichthys (clado GR), Adontosternarchus and clade PA. In the latter, there is a reversal to State 0 in “Apteronotus” bonapartii, a transition to State 2 in Apteronontus gr. leptorhynchus to State 3 in clade SS (reversed to State 0 in Sternarchorhamphus).
33) Endomaxillary ligament position in relation to the autopalatine: (0) ventrolateral; (1) dorsolateral [CI: 0.33; RI: 0.81].
The endomaxillary ligament in Gymnotiformes is usually located ventrolaterally to the autopalatine. Unusually, in some Apteronotidae the ligament is dorsomedially displaced to a dorsolateral position relative to the autopalatine (Figs. 7, 32).
The State 1 is a synapomorphy for clade CP, with independent reversals in clade PS and Parapteronotus.
34) Post-retroarticular and pre-retroarticular ligaments: (0) undifferentiated; (1) differentiated [CI: 0.50; RI: 0.88].
Buccopalatal ligaments in gymnotiforms are usually poorly differentiated, except for those involved in the connection with the adductor mandibulae or with bony elements (e.g., endomaxillary and ectomaxillary ligaments). In some Apteronotidae, two ligaments are differentiated from the posterior portion of the buccopalatal membrane, originating from the retroarticular and diverging anteriorly to the dorsoposterior portion of the membrane. They are here called post-retroarticular and pre-retroarticular ligaments (Figs. 7, 25), and not considered homologous to the preangular and postangular ligaments of Datovo, Vari, (2013)Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
because all ligaments are present in some taxa, thus failing the conjunction test of homology (Patterson, 1982Patterson C. Morphological characters and homology. In: Joysey KA, Friday AE, editors. Problems of phylogenetic reconstruction. London: Academic Press; 1982. p.21–74.; de Pinna, 1991de Pinna MCC. Concepts and tests of homology in the cladistic paradigm. Cladistics. 1991; 7(4):367–94. https://doi.org/10.1111/j.1096-0031.1991.tb00045.x
https://doi.org/10.1111/j.1096-0031.1991...
).
The presence of such ligaments is synapomorphic for the clade PC, with a reversal in clade MP.
35) Origin ofadductor arcus palatini: (0) not including orbitosphenoid; (1) including orbitosphenoid (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 123) [CI: AUT; RI: AUT].
The adductor arcus palatini in Gymnotiformes usually originates on the ventrolateral surface of the parasphenoid and anteroventral part of the prootic (LAWP, pers. obs.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.). The origin of that muscle is anterodorsally displaced in Japigny, resulting in the incorporation of the ventrolateral surface of the orbitosphenoid (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 42).
This condition is an autapomorphy for Japigny.
36) Origin ofadductor arcus palatiniin relation to the parasphenoid: (0) restricted to posterior half of orbit; (1) extending along entire orbit [CI: 1; RI: 1].
The origin of the adductor arcus palatini is normally in the parasphenoid and prootic but can also involve other bones (Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
). Its trajectory is also variable among teleosts. In Ostariophysi, the generalized pattern is an adductor arcus palatini originating on the posterior half of the parasphenoid and extending along the posterior half of the orbit. Contrastingly, in the majority of taxa examined for this study, both ingroup and outgroup, the origin of the adductor arcus palatini is on the anterior half of the parasphenoid and the muscle extends along the orbit.
In the present analysis, the orign of this muscle along the entire orbial region is synapomorphic for Characiphysi (Characiformes + Siluriphysi; sensuFink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; 1996Fink S, Fink WL. Interrelationships of Ostariophysan fishes (Teleostei). In: Stiassny ML, Parenti LR, Johnson D, editors. Interrelationships of fishes. San Diego: Academic Press; 1996. p.209–49.).
37) Insertion ofadductor arcus palatinion endopterygoid: (0) limited to dorsolateral portion of the bone, not extending beyond the horizontal line through the midline of this bone; (1) on dorsolateral and median portion of bone, reaching the horizontal line through the midline of this bone (modified from Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
: Character 140) [CI: 0.25; RI: 0.87].
Diogo et al. (2008) proposed the insertion of a significant part of the adductor arcus palatini on the lateral surface of the suspensorium as a synapomorphy for the Gymnotiformes (character 140; state 1). Herein, we provide a more detailed description of this condition and describe the insertion to be specifically at the endopterygoid, a configuration shared among all gymnotiforms. Despite that general pattern, there is still phylogenetically informative variation in this complex.
Primitively in Ostariophysi, the insertion of the adductor arcus palatini is restricted to the dorsal portion of the lateral surface of the endopterygoid. However, in most Gymnotiformes, the insertion of the adductor arcus palatini is ventrally displaced, surpassing the horizontal line through the middle portion of the endopterygoid of the lateral surface (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 42).
The latter condition is a synapomorphy for clade ST, with reversals in Gymnorhamphichthys (clade GR) and in clade CP (with subsequent reacquisition in clade MS).
38) Origin oflevator arcus palatiniin relation to the frontal: (0) absent; (1) present (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 125) [CI: 0.20; RI: 0.85].
Generally, in Ostariophysi, the levator arcus palatini originates on the sphenotic (LAWP, pers. obs.; Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
). In basal members of the Siluriformes and in the great majority of Gymnotiformes, the muscle has an anterior displacement so that its origin includes the frontal.
The State 1 is a synapomorphy for Siluriphysi, with reversals in Electrophorus, Archolaemus (clade AC), and in clade ER (with reacquisition in R. troscheli).
39) Origin oflevator arcus palatiniin the pterosphenoid: (0) absent; (1) present (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 126) [CI: 0.14; RI: 0.75].
As with the preceding character, basal representatives of Ostariophysi have the origin of the levator arcus palatini is mostly restricted to the sphenotic, sometimes also involving elements of the posterior part of the neurocranium (LAWP, pers. obs.; Winterbottom, 1974aWinterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
https://www.jstor.org/stable/4064691...
). Within Gymnotiformes, some species of Apteronotidae and Sternopygidae have the levator arcus palatini in a more mesial position, resulting in a connection with the pterosphenoid.
The latter condition is apomorphic for SI, with independent reversals in “Apteronotus” bonapartii, clade AP (reacquired in clade SN), Japigny, and clade ED (reacquired in Rhabdolichops, clade RL).
40) Relative width of origin and insertion oflevator arcus palatini: (0) origin narrower than insertion; (1) origin wider than insertion, up to 150% width of insertion; (2) width of origin equal to that of insertion; (3) width of origin twice that of insertion (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 129) [CI: 0.21; RI: 0.75].
The levator arcus palatini in ostariophysians a roughly triangular shape, with its origin narrower than its insertion. In Gymnotiformes, the relation between the width of the origin and that of the insertion is variable, ranging from an origin narrower than the insertion to cases in which the origin is twice as wide as the insertion. This character is based on a relational landmark (origin vs. insertion) and as such cannot be ordered by simple similarity because a single primary variable cannot be determined. It is thus treated as unordered.
In the hypothesis here advanced, State 1 is convergent in Diplomystes (Siluriformes) and clade SN. State 2 is synapomorphic for clade RC and State 3 for Gymnotus (clade GM).
41) Structure oflevator arcus palatini: (0) as a single muscle; (1) subdivided into two sections [CI: AUT; RI: AUT].
In nearly all examined taxa, the levator arcus palatini is a single muscle, without subdivisions. Uniquely in Electrophorus, this muscle is differentiated in two well-defined sections, herein named levator arcus palatini anterior and levator arcus palatini posterior (Figs. 2, 10). The two sections have a single origin on the ventral margin of the sphenotic, but their insertions differ with the anterior section converging entirely on the hyomandibula and the posterior one inserting mostly on the preopercle, with a few fibers on the hyomandibula.
Aguilera (1986)Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23. described the levator arcus palatini as a single muscle in gymnotiforms, including Electrophorus (Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.: fig. 10). Our own observations on the dual structure of the muscle is based on two sides of two specimens of E. electricus.
The division of the levator arcus palatini into anterior and posterior sections is retrieved as a synapomorphy for Electrophorus.
42) Insertion of the anterolateral fibers oflevator arcus palatiniat relative toadductor mandibulae,pars malaris: (0) mesial; (1) lateral; (2) dorsal (modified from Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
: ch. 130; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
: ch. 9; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 123) [CI: 0.50; RI: 0.88].
At its insertion, the levator arcus palatini is completely mesial in relation to the adductor mandibulae (Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). In the Gymnotiformes, the levator arcus palatini has a different configuration, and is generally lateral to the malaris (LAWP, pers. obs.; Chardon, de la Hoz, 1973Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.), a condition listed as synapomorphic for the order (Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
; ch. 130; Datovo, Vari, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
; ch. 9).
However, fibers of the levator arcus palatini at its insertion are variable in relation to the malaris of Gymnotiformes. This requires the recognition of four subsets of fibers (Figs. 16-32; anterolateral, posterolateral, anteromesial and posteromesial). Those different muscle regions are anatomically distinct and were therefore treated as independent characters herein. This character is based on a relational landmark (position of two separate muscles) and as such cannot be ordered by simple similarity because a single primary variable cannot be determined. It is thus treated as unordered.
Under this new interpretation, the lateral position of the anterolateral fibers of the levator arcus palatini relative to the malaris (State 1) is a synapomorphy for Gymnotiformes, with reversal in Compsaria and further transition to State 2 in Clade PA, (within the latter with reversal to State 1 in Clade SO).
43) Insertion of the posterolateral fibers oflevator arcus palatiniin relation to theadductor mandibulae, pars malaris: (0) mesial; (1) lateral; (2) dorsal [CI: 0.66; RI: 0.94].
In outgroup representatives, the levator arcus palatini is entirely mesial to the malaris. In Gymnotiformes, the posterolateral fibers of the levator arcus palatini are positioned completely laterally (Fig. 16) or dorsal (Fig. 30) to the malaris. This character is treated as unordered for the same reason as the previous one.
The condition described as State 1 is a synapomorphy for Gymnotiformes (clade Gy), with transition to State 2 in clade PA (therein with reversal to State 1 in clade SO).
44) Insertion of the anteromesial fibers oflevator arcus palatiniin relation to theadductor mandibulae, pars malaris: (0) mesial; (1) lateral; (2) dorsal [CI: 0.28-0.76; RI: 0.82-0.85].
As mentioned under characters 42 and 43, the generalized pattern found in teleosts consists of the levator arcus palatini being positioned mesially to the malaris. In Gymnotiformes, the anteromesial fibers of the levator arcus palatini are positioned laterally or dorsal to the malaris. This character is treated as unordered for the same reason as the previous one.
The lateral position of anteromesial fibers of the levator arcus palatini at insertion, relative to the malaris (State 1), is abundantly convergent, occurring in Electrophorus, Rhamphichthyidae (clade R) and Sternopygidae (clade S, with a reversal in clade AE). The dorsolateral position of the same fibers (State 2), on the other hand, is synapomorphic for clade PA, with subsequent transition to State 1 in clade SO.
45) Position ofdilatator operculiin relation to thelevator arcus palatini: (0) dilatator operculi covering anterior half of the levator arcus palatini; (1) dilatator operculi covering less than anterior half of the levator arcus palatini; (2) dilatator operculi mesial to levator arcus palatini [CI: 0.25-0.76; RI: 0.76].
In basal members of Ostariophysi, including some Gymnotiformes, the dilatator operculi is located laterally to the levator arcus palatini, with the anterior margin of the former covering half or more of the latter. In a majority of Gymnotiformes, the dilatator operculi overlaps only the posterior margin of the levator arcus palatini (Figs. 11, 15–18, 20; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 8, 13; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: fig. 40). This character is treated as unordered for the same reason as the previous one.
The latter condition (State 1) is synapomorphic for the order, with reversals in Gymnotus (clade GM) and Apteronotidae (clade A, with independent transitions back to State 1 in Compsaraia, Orthosternarchus and Sternarchogiton). State 2 is exclusive to Diplomystes among examined taxa.
46) Orientation of anterior margin oflevator arcus palatini: (0) anterolateral fibers of levator arcus palatini approximately straight relative to the horizontal arm of preopercle, forming an angle of ca. 90° relative to the longitudinal axis of head; (1) anterolateral fibers of levator arcus palatini oriented obliquely relative to the horizontal arm of preopercle, forming a 45° angle relative to longitudinal axis of head (modified from Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 128) [CI: 0.16; RI: 0.87].
Primitively in ostariophysans, the levator arcus palatini has its anterolateral fibers of levator arcus palatini approximately straight relative to the horizontal arm of preopercle, forming an angle of ca. 90° relative to the longitudinal axis of head. On a different configuration, in basal representatives of Siluriformes and in the majority of Gymnotiformes, that muscle is oblique relative to the horizontal arm of the preopercle, forming with it an angle of approximately 45° (Figs. 1, 7, 15, 16, 28, 30).
State 1 is a synapomorphy for Siluriphysi, with reversals in Electrophorus, Rhamphichthyidae (clade R), clades PS and AE (here with a return to State 1 in R. eastwardi).
47) Relative position oflevator arcus palatini: (0) levator arcus palatini overlapping posterior margin of pterosphenoid only; (1) levator arcus palatini overlapping pterosphenoid almost entirely, except for its anterior region; (2) levator arcus palatini overlapping half of pterosphenoid; (3) levator arcus palatini overlapping posterior third of pterosphenoid [CI: 0.50; RI: 0.92].
Primitively in teleosts, the levator arcus palatini is located posteriorly to the pterosphenoid, not covering it and leaving the bone visible in lateral view. In most Siluriformes and Gymnotiformes, the muscle has a more anterior origin, with a horizontally expanded anterodorsal portion. As a result, the levator arcus palatini is lateral to the pterosphenoid, covering it and making it invisible in lateral view. In some taxa the degree to which the levator arcus palatini covers the pterosphenoid is variable, for example it covers the posterior half of the bone in Hypopygus, and the posterior third in some Sternopygidae. This character is based on a relational landmark (relative size or shape of a muscle relative to that of a bone) and as such cannot be ordered by simple similarity because a single primary variable cannot be determined. It is thus treated as unordered.
State 1 is a synapomorphy for Siluriphysi, com reversal to State 0 in Electrophorus, transition to State 2 in Hypopygus and to State 3 in clade EI (with transformation to State 1 in clade RB).
48) Intermuscular bones in the anterior portion oflevator arcus palatini: (0) absent; (1) present [CI: 0.50; RI: 0.66].
Ostariophysi in general lack ossifications in the levator arcus palatini. In species of Rhamphichthys and S. xingu, however, the levator arcus palatini has some ossified tendons, forming intermuscular bones (Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
: fig. 12).
The presence of such ossifications is a synapomorphy for Rhamphichthys (clade RY), with a convergent occurrence in S. xingu.
49)Dilatator operculi: (0) single; (1) subdivided [CI: AUT; RI: AUT].
The dilatator operculi is a single muscle bundle in nearly all gymnotiforms, with no subdivisions (LAWP, pers. obs.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.).
Uniquely in Electrophorus, the muscle is subdivided in two sections, here named dilatator operculi ventralis and dilatator operculi dorsalis (Fig. 10), an autapomorphy for the genus.
50) Origin ofdilatator operculion sphenotic: (0) origin of dorsal fibers on sphenotic and pterotic not overlapping the bones entirely and not reaching suture with parietal; (1) origin of dorsal fibers on sphenotic and pterotic overlapping the bones completely and extending to suture with parietal [CI: 1; RI: 1].
Normally in gymnotiforms, the dilatator operculi originates on the ventromedial portion of the sphenotic and pterotic, some distance from the suture with the parietal, so that all those bones are visible in lateral view. Contrastingly, o dilatator operculi in Sternarchella is more robust, with its origin dorsally displaced relative towards the suture of the pterotic and sphenotic with the parietal.
State 1 is synapomorphic for clade MS.
51) Origin oflevator operculi anterior: (0) not including hyomandibula; (1) including hyomandibula (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 131) [CI: 0.25; RI: 0.91].
The anterior fibers of the levator operculi, called levator operculi anterior in Gymnotiformes (see Section “The dorsolateral musculature of the head of Gymnotiformes: General features- levator operculi”), origin on the pterotic (LAWP, pers. obs.; Chardon, de la Hoz, 1973Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.).
In some members of the order, the origin of the levator operculi is anteriorly expanded to include also the hyomandibula, a convergent condition in Labeo (Cypriniformes), Steatogenys (clade SY) and clade EI (with reversal in clade RD).
52)Levator operculi posterior: (0) absent; (1) present (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.: Character 47; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.: ch. 46 [?]; Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
: Character 151) [CI: 0.50; RI: 0.75].
Albert (2001) proposed the absence of the muscle “levator posterior” as a synapomorphy for Gymnotiformes (their character 46). There are no descriptions or illustrations associated the character in that publication, but we presume the reference is to the same muscle here called “levator operculi posterior”. Similarly, Diogo et al., (2008)Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
suggested that the division of the levator operculi (their character 151) as a synapomorphy for Sternopygidae + Gymnotidae (clade 51 of Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
), reporting the absence of such subdivision in Hypopomidae. Such observations and proposals are in stark contrast to those made herein, which indicate that the posterior section of the levator operculi is present in the vast majority of Gymnotiformes (except in Hypopygus).
The levator operculi posterior, apparently results from a secondary differentiation of the posterior fibers of the levator operculi, on the basis of its posterior and more superficial relative position. Such division of the levator operculi in a posterior section (State 1) is a synapomorphy for Gymnotiformes (with a reversal in Hypopygus).
53) Insertion oflevator operculi: (0) on the mesial surface of dorsal region of opercle; (1) on a dorsal crest on the lateral surface of opercle [CI: 1; RI: 1].
Generally in Ostariophysi, the levator operculi inserts on the mesial surface (sometimes on a crest) of the opercle (LAWP, pers. obs.; Takahasi, 1925Takahasi N. On the homology of the cranial muscles of the cypriniform fishes. J Morphol. 1925; 40(1):1–109. https://doi.org/10.1002/jmor.1050400102
https://doi.org/10.1002/jmor.1050400102...
; Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
). Contrastingly, the levator operculi in gymnotiforms inserts on a crest on the lateral surface of the opercle, surpassing the dorsal margin of the opercle.
State 1 is a synapomorphy for the Gymnotiformes.
54) Position of nerve R-Avn in relation tolevator operculi anterior: (0) mesial; (1) lateral (Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
: ch. 130) [CI: 0.16; RI: 0.87].
The nerve ramus R-Avn originates on the electrosensory lobe of the lateral line and innervates electroreceptors of the trunk (Carr et al., 1982Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory nerves in gymnotiform fish. J Comp Neurol. 1982; 211(2):139–53. https://doi.org/10.1002/cne.902110204
https://doi.org/10.1002/cne.902110204...
; Vischer et al., 1989Vischer HA, Lannoo MJ, Heiligenberg W. Development of the electrosensory nervous system in Eigenmannia (Gymnotiformes): I. The peripheral nervous system. J Comp Neurol. 1989; 290:16–40. https://doi.org/10.1002/cne.902900103
https://doi.org/10.1002/cne.902900103...
). Generally in teleosts, it is positioned medially relative to the levator operculi. However, the widespread condition in Gymnotiformes is to have the nerve positioned laterally relative to the levator operculi anterior and mesially to the levator operculi posterior (State 1) (Fig. 1).
This character is polymorphic in Eigenmannia microstoma, because one juvenile specimen (80.0 mm LEA) of the species has the right-side nerve disposed laterally to the muscle, differing from the usual condition found in all specimens with the both sides presenting the condition described in the State 0.
The condition in State 1 is listed as a synapomorphy for the order, with independent reversals in Rhamphichthys (clade RY), clade SN, Platyurosternarchus and clade EI (in the later with reacquisition of State 1 in Clade RD).
55) Origin ofadductor hyomandibulae: (0) not including the sphenotic; (1) including the sphenotic [CI: 1; RI: 1].
In Gymnotiformes, the adductor hyomandibulae is a laminar muscle visible only in a mesial view of the suspensorium. It originates from the lateral surface of the prootic and sometimes the pterotic and parasphenoid (LAWP, pers. obs.; de la Hoz, Chardon, 1984de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.). In Gymnotidae, the muscle has a posteromesially displaced origin, also including the sphenotic.
State 1 is synapomorphic for Gymnotidae.
56) Origin ofadductor operculi: (0) including prootic; (1) not including prootic [CI: 0.33-0.81; RI: 0.81].
The pterotic, prootic and exoccipital are usual points of origin of the adductor operculi in some Gymnotiformes and outgroup representatives (LAWP, pers. obs.; Aguilera, 1986Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.; Diogo et al., 2008Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
http://dx.doi.org/10.4067/S0717-95022008...
). In basal members of Siluriformes and subgroups of Gymnotiformes, the origin of the adductor operculi is posteromesially displaced and does not include the prootic as part of its.
State 1 is a synapomorphy for Siluriphysi, with a reversal in clade ST (reacquired in Archolaemus, clade AC).
DISCUSSION
ANALYSIS 1 - Dorsolateral head musculature as phylogenetic characters in Gymnotiformes: comparisons with previous studies
Analyses of characters from the dorsolateral head musculature strongly support the monophyly of Gymnotiformes (Fig. 33). This further corroborates a long list of studies based on morphology (Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Gayet et al., 1994; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Crampton, 2005aAlbert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005a. p.360–409. https://doi.org/10.1007/0-387-28275-0_13
https://doi.org/10.1007/0-387-28275-0_13...
; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
), on molecular data combined with electrophysiological and phenotypic characters (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
), on molecular data combined with morphological data (Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
), and on molecular data (Elbassiouny et al., 2016Elbassiouny AA, Schott RK, Waddell JC, Kolmann MA, Lehmberg ES, Nynatten AV, Crampton WGR, Chang BSW, Lovejoy NR. Mitochondrial genomes of the South American electric knifefishes (Order Gymnotiformes). Mitochondrial DNA B Resour. 2016; 1(1):401–03. https://doi.org/10.1080/23802359.2016.1174090
https://doi.org/10.1080/23802359.2016.11...
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
). Beyond that, the hypothesis presented here differs in some important points relative to previous proposals.
In our hypothesis of relationship based solely on myological data, a monophyletic Gymnotidae is sister group to all other Gymnotiformes, which is in agreement with various other previous proposals, both morphological and/or molecular (Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Crampton, 2005aAlbert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005a. p.360–409. https://doi.org/10.1007/0-387-28275-0_13
https://doi.org/10.1007/0-387-28275-0_13...
; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). Some other studies based on phenotypic data have proposed Apteronotidae occupying a basal position in gymnotiforms, mostly due to a putatively plesiomorphic presence of a caudal fin and skeleton (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Elbassiouny et al., 2016Elbassiouny AA, Schott RK, Waddell JC, Kolmann MA, Lehmberg ES, Nynatten AV, Crampton WGR, Chang BSW, Lovejoy NR. Mitochondrial genomes of the South American electric knifefishes (Order Gymnotiformes). Mitochondrial DNA B Resour. 2016; 1(1):401–03. https://doi.org/10.1080/23802359.2016.1174090
https://doi.org/10.1080/23802359.2016.11...
; with the latter study based on an extremely limited taxonomic representation of only eight taxa). However, Santana et al., (2013) demonstrated the presence of a well-developed caudal skeleton also in the gymnotid Electrophorus, thus rendering the distribution of this character ambiguous in the order. The evolution of the caudal skeleton in the group remains uncertain.
The clade named Sternopygoidei by Mago-Leccia (1978; clade E of Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), composed of all families of Gymnotiformes except Gymnotidae (i.e., including Rhamphichthyidae, Hypopomidae, Apteronotidae and Sternopygidae), is corroborated here, as in various other studies (Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Crampton, 2005aAlbert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005a. p.360–409. https://doi.org/10.1007/0-387-28275-0_13
https://doi.org/10.1007/0-387-28275-0_13...
; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). However, the monophyly of Sternopygoidei was not corroborated in some other hypotheses (seeTriques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Elbassiouny et al., 2016Elbassiouny AA, Schott RK, Waddell JC, Kolmann MA, Lehmberg ES, Nynatten AV, Crampton WGR, Chang BSW, Lovejoy NR. Mitochondrial genomes of the South American electric knifefishes (Order Gymnotiformes). Mitochondrial DNA B Resour. 2016; 1(1):401–03. https://doi.org/10.1080/23802359.2016.1174090
https://doi.org/10.1080/23802359.2016.11...
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
). A monophyletic Rhamphichthyoidea is recovered by myological data, and has also previously been found in other morphological (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130., 2007; Gayet et al., 1994Gayet M, Meunier FJ, Kirschbaum F. Ellisella kirschbaumi Gayet & Meunier, 1991, gymnotiforme fossile de Bolivie et ses relations phylogénétiques au sein des formes actuelles. Cybium. 1994; 18(3):273–306.; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
) molecular (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Maldonado-Ocampo et al., 2014Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR. Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
http://dx.doi.org/10.1111/zsc.12035...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
) and total evidence (Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
) studies. However, composition and relationships between and among constituent families remain controversial, as discussed below.
Analyses based on morphology (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
), molecular (Alves-Gomes, 1998Alves-Gomes JA. The phylogenetic position of the South American electric fish genus Sternopygus and Archolaemus (Ostariophysi: Gymnotiformes) according to 12S e 16S Mitochondrial DNA Sequences. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.447–59.; Maldonado-Ocampo et al., 2014Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR. Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
http://dx.doi.org/10.1111/zsc.12035...
) and total evidence (Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Crampton et al., 2016Crampton WGR, de Santana CD, Waddell JC, Lovejoy NR. A taxonomic revision of the Neotropical electric fish genus Brachyhypopomus (Ostariophysi: Gymnotiformes: Hypopomidae), with descriptions of 15 new species. Neotrop Ichthyol. 2016; 14(4):e150146. https://doi.org/10.1590/1982-0224-20150146
https://doi.org/10.1590/1982-0224-201501...
) supported a clade composed of Hypopygus and Steatogenys. The two genera, traditionally allocated in Hypopomidae (sensu Mago-Leccia, 1994Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), have recently been proposed as more closely related to Rhamphichthyidae (Maldonado-Ocampo et al., 2014Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR. Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
http://dx.doi.org/10.1111/zsc.12035...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Crampton et al., 2016Crampton WGR, de Santana CD, Waddell JC, Lovejoy NR. A taxonomic revision of the Neotropical electric fish genus Brachyhypopomus (Ostariophysi: Gymnotiformes: Hypopomidae), with descriptions of 15 new species. Neotrop Ichthyol. 2016; 14(4):e150146. https://doi.org/10.1590/1982-0224-20150146
https://doi.org/10.1590/1982-0224-201501...
). As a result, the family is nowadays composed of Rhamphichthys, Iracema, Gymnorhamphichthys and the clade called “Steatogenae” (Steatogenys + Hypopygus; sensuTagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). Alternatively, Hypopomus, Microsternarchus, Brachyhypopomus, Procerusternarchus and Racenisia are part of Hypopomidae (Maldonado-Ocampo et al., 2014Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR. Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
http://dx.doi.org/10.1111/zsc.12035...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
).
Myological data did not recover any of the aforementioned clades, resulting in a polytomy involving Hypopomus, Hypopygus, Microsternarchus, Brachyhypopomus, Steatogenys and the genera of Rhamphichthyidae (sensuMago-Leccia, 1994Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
). Such result may be due to the lack of specimens of Iracema, Procerusternarchus and Racenisia for study. Despite discrepancies in the internal relationships of Rhamphichthyoidea, its position as sister group of Sternopygoidea (Sternopygidae + Apteronotidae; Albert’s 2001 “Sinusoidea”) is repeatedly corroborated in other studies (Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
), including the present one.
Some molecular analyses have indicated a non-monophyletic Sternopygidae. Such results prompted suggestions to restrict the family to the genus Sternopygus and to allocate the remaining Sternopygidae in a separate Eigenmanniidae (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
, 1998). However, the monophyly of Sternopygidae has been well corroborated in various other studies, both previous and subsequent, and on both morphological and molecular data (Mago-Leccia, 1978Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51., 1994; Mago-Leccia, Zaret, 1978Mago-Leccia F, Zaret TM. The taxonomic status of Rhabdolichops troscheli (Kaup, 1856), and speculations on gymnotiform evolution. Environ Biol Fishes. 1978; 3:379–84. https://doi.org/10.1007/BF00000530
https://doi.org/10.1007/BF00000530...
; Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
).
Myological data decisively support the monophyly of Sternopygidae and of its two subfamilies, Sternopyginae and Eigenmanniinae (sensuAlbert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; clade EI). The latter point disagrees with Triques, (1993)Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130., where Rhabdolichops was sister group to all other Sternopygidae and Sternopygus was in a polytomy with Eigenmannia and a clade formed by Archolaemus plus Distocyclus (thus resulting in a paraphyletic Eigenmanniinae). Five characters from dorsolateral head musculature support the monophyly of Eigenmanniinae, corroborating previous proposals based on molecular data and total evidence (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). Our data also strongly support Sternopygus as sister group to all the rest of the family. Beyond that, relationships among general of Eigenmanniinae remain uncertain.
Conflicting ideas about the most basal taxon in Eigenmanniinae have been recurrent, regardless of the kind of data employed. Some analyses place Rhabdolichops in that position (Mago-Leccia, 1978Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51.; Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
, 1998; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
), others allocate instead Archolaemus as sister group to all other Eigenmanniinae (Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Lundberg, Mago-Leccia, 1986Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
; Albert, Fink, 1996Albert JS, Fink WL. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
https://doi.org/10.2307/1446944...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.). Myological data presented herein support Japigny as sister group to remaining eigenmanniins, corroborating Vari et al., (2012)Vari RP, de Santana CD, Wosiacki WB. South American electric knifefishes of the genus Archolaemus (Ostariophysi, Gymnotiformes): undetected diversity in a clade of rheophiles. Zool J Linn Soc. 2012; 165(3):670–99. https://doi.org/10.1111/j.1096-3642.2012.00827.x
https://doi.org/10.1111/j.1096-3642.2012...
and Dutra et al., (2021)Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
.
Leaving aside Japigny, a clade formed by Archolaemus, Eigenmannia, Distocyclus and Rhabdolichops is supported by Analysis 1 (clade AE), in agreement with a number of previous morphological studies (Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Lundberg, Mago-Leccia, 1986Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
; Albert, Fink, 1996Albert JS, Fink WL. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
https://doi.org/10.2307/1446944...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
). Our results also corroborate the monophyly of Archolaemus, with A. orientalis as the sister group to the rest of the genus (in agreement with Vari et al., 2012Vari RP, de Santana CD, Wosiacki WB. South American electric knifefishes of the genus Archolaemus (Ostariophysi, Gymnotiformes): undetected diversity in a clade of rheophiles. Zool J Linn Soc. 2012; 165(3):670–99. https://doi.org/10.1111/j.1096-3642.2012.00827.x
https://doi.org/10.1111/j.1096-3642.2012...
) but no resolution beyond that (clade AR). Archolaemus is sister group to clade EE, composed of Eigenmannia, Distocyclus and Rhabdolichops, again a grouping previously supported by independent evidence both morphological (Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Lundberg, Mago-Leccia, 1986Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
; Albert, Fink, 1996Albert JS, Fink WL. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
https://doi.org/10.2307/1446944...
; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.) and molecular (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
). Species of Eigenmannia do not form a monophyletic group, a possibility already suggested in other studies (Mago-Leccia, 1978Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51., 1994Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.; Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Alves-Gomes, 1998Alves-Gomes JA. The phylogenetic position of the South American electric fish genus Sternopygus and Archolaemus (Ostariophysi: Gymnotiformes) according to 12S e 16S Mitochondrial DNA Sequences. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.447–59.; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). However, the monophyly of Eigenmannia was recently recovered in Dutra et al., (2021)Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
.
The clade composed of Distocyclus and Rhabdolichops (clade DR) corroborates Lundberg, Mago-Leccia, (1986)Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
, and not other proposals where the former genus is sister to either Archolaemus (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
), or to Eigenmannia (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Alves-Gomes, 1998Alves-Gomes JA. The phylogenetic position of the South American electric fish genus Sternopygus and Archolaemus (Ostariophysi: Gymnotiformes) according to 12S e 16S Mitochondrial DNA Sequences. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.447–59.; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
), or to Eigenmannia plus Rhabdolichops (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.). Other suggestions have positioned Distocyclus in a polytomy with Eigenmannia and Archolaemus (Mago-Leccia, 1978Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51.) or in a polytomy with Rhabdolichops and Eigenmannia (Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Lundberg, Mago-Leccia, 1986Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
; Albert, Fink, 1996Albert JS, Fink WL. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
https://doi.org/10.2307/1446944...
). Our support of the monophyly of Rhabdolichops agrees with Lundberg, Mago-Leccia, (1986)Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
and Correa et al., (2006)Correa SB, Crampton WGR, Albert JS. Three new species of the neotropical electric fish Rhabdolichops (Gymnotiformes: Sternopygidae) from the central Amazon, with a new diagnosis of the genus. Copeia. 2006; 2006(1):27–42. https://doi.org/10.1643/0045-8511(2006)006[0027:TNSOTN]2.0.CO;2
https://doi.org/10.1643/0045-8511(2006)0...
, and our result showing the clade R. nigrimans + R. lundbergi (clade RD) as sister group to remaining species of Rhabdolichops is in line with Correa et al., (2006)Correa SB, Crampton WGR, Albert JS. Three new species of the neotropical electric fish Rhabdolichops (Gymnotiformes: Sternopygidae) from the central Amazon, with a new diagnosis of the genus. Copeia. 2006; 2006(1):27–42. https://doi.org/10.1643/0045-8511(2006)006[0027:TNSOTN]2.0.CO;2
https://doi.org/10.1643/0045-8511(2006)0...
.
Myology data analyzed separately support the monophyly of Apteronotidae (clade A). Despite considerable effort in untangling the phylogenetic relationships of the family, there is still little consensus on the subject (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130., 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Ivanyisky III, Albert, 2014Ivanyisky III SJ, Albert JS. Systematics and biogeography of Sternarchellini (Gymnotiformes: Apteronotidae): Diversification of electric fishes in large Amazonian rivers. Neotrop Ichthyol. 2014; 12(3):565–84. http://dx.doi.org/10.1590/1982-0224-20130159
http://dx.doi.org/10.1590/1982-0224-2013...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Evans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
). Our results show Adontosternarchus as monophyletic (clade AD), in agreement with numerous previous contributions (e.g., Lundberg, Cox Fernandes, 2007Lundberg JG, Cox Fernandes C. A new species of South American ghost knifefish (Apteronotidae: Adontosternarchus) from the Amazon Basin. Proc Acad Nat Sci Phila. 2007; 156(1):27–37. https://doi.org/10.1635/0097-3157(2007)156[27:ANSOSA]2.0.CO;2
https://doi.org/10.1635/0097-3157(2007)1...
; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; de Santana, Vari, 2012de Santana CD, Vari RP. New species of Adontosternarchus (Gymnotiformes, Apteronotidae) from the Rio Purus Basin, Brazil. Copeia. 2012; 2012(3):535–40. https://doi.org/10.1643/CI-11-135
https://doi.org/10.1643/CI-11-135...
; Bernt et al., 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
). However, our placement of the genus as sister group to all other Apteronotidae is new. As sister group to all other Apteronotidae, previous proposals have placed either the clade formed by Platyurosternarchus, Sternarchorhynchus, Sternarchorhamphus and Orthosternarchus (Sternarchorhynchinae sensuAlbert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
, Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
) or alternatively the group composed of Sternarchorhamphus + Orthosternarchus (i.e., Sternarchorhamphinae sensuTagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
).
Analysis 1 places Pariosternarchus as the sister to all other genera of Apteronotidae except Adontosternarchus (clade PC). Such placement for Pariosternarchus also has no precedent in the literature. Previous studies have placed the genus as close to Sternarchella (Sternarchellini sensuIvanyisky III, Albert, 2014Ivanyisky III SJ, Albert JS. Systematics and biogeography of Sternarchellini (Gymnotiformes: Apteronotidae): Diversification of electric fishes in large Amazonian rivers. Neotrop Ichthyol. 2014; 12(3):565–84. http://dx.doi.org/10.1590/1982-0224-20130159
http://dx.doi.org/10.1590/1982-0224-2013...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Evans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
) or to a clade composed of Compsaraia + Melanosternarchus (Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
). Again, at odds with previous proposals, our analysis places Compsaraia as sister to clade PA. Formerly, the genus was considered as sister group either to a clade composed of Porotergus, Adontosternarchus and Sternarchogiton (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), or of Apteronotus anas (= Parapteronotus hasemani; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.), or of “Apteronotus” gr. bonapartii + Porotergus (Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
), or still of Melanosternarchus (Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
).
The clade here denominated PA is split in two subgroups, PAS and AP. Clade PAS includes Porotergus + Sternarchogiton + “Apteronotus” gr. bonapartii, and is supported by a unique subdivision of the adductor mandibulae, pars malaris into retromalaris and promalaris (see Character 13 above). The close proximity among the genera in clade PAS was partly retrieved by Tagliacollo et al. (2016), except for their inclusion of Compsaraia therein. Within clade AP, the most relevant result is the non-monophyly of Apteronotus sensu stricto, a finding that disagrees with previous studies (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
).
Clade MP is composed of species of Sternarchella (clade MS) and clade PS, and is equivalent to Sternarchorhynchinae (sensuAlbert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.). Clade MS includes species traditionally allocated in Sternarchella (S. schotti and S. orthos) and species previously in Magosternarchus (M. raptor and M. duccis; the genus was recently considered as a junior synonym of Sternarchella by Ferraris et al., 2017Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
https://doi.org/10.1590/1982-0224-201600...
and Evans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
). Clade MS is one of the few groups unanimously supported in all previous hypotheses of relationships among Apteronotidae (e.g., Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Ivanyisky III, Albert, 2014Ivanyisky III SJ, Albert JS. Systematics and biogeography of Sternarchellini (Gymnotiformes: Apteronotidae): Diversification of electric fishes in large Amazonian rivers. Neotrop Ichthyol. 2014; 12(3):565–84. http://dx.doi.org/10.1590/1982-0224-20130159
http://dx.doi.org/10.1590/1982-0224-2013...
; Evans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
) and is also supported here.
Our results on the monophyly of Sternarchorhynchinae (clade PS) corroborates the ideas of Albert, Campos-da-Paz, (1998)Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60. and Albert (1998, 2001), who placed Platyurosternarchus, Sternarchorhynchus, Sternarchorhamphus, Orthosternarchus as a clade, with the two former genera forming a group which is sister to the two latter ones. Despite the agreement about the entire clade, our hypothesis of intrarelationships differs somewhat, placing Platyurosternarchus as sister group to the remaining three genera (clade SO). Of course, differences exist also about the position of Sternarchorhynchinae within Apteronotidae, with cited authors placing it in a basal position in the family, while our myological data place it more deeply internested therein. Clade SO has also been routinely supported in previous studies (Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Bernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
).
All things considered, there is substantial agreement between the phylogenetic signal inferred from myological characters studied herein and other hypotheses based on other natural partitions, as external anatomy, DNA sequences, electrophysiology, neuroanatomy and osteology. Some of the most relevant parallels are: (1) Gymnotidae as sister group to remaining Gymnotiformes, (2) monophyly of Rhamphichthyoidea, (3) monophyly of Sternopygoidea, (4) monophyly of Sternopygidae, including Eigenmanniinae, and (5) monophyly of Apteronotidae and of its subclades.
Such congruence with other data sources obviously suggests confidence that reiterated components reflect actual features of the phylogenetic history of Gymnotiformes. Still, despite the effort invested here in detecting all phylogenetically-informative variation in dorsolateral head musculature, it is unavoidable that any single data source is but a tiny sample of the rich phenotypic variation observed in gymnotiforms. The next Section explores this topic by quantifying the influence of myological characters against a broader sample of morphological characters (Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
).
ANALYSIS 2 - Influence of myological characters on the relationships of Gymnotiformes
A parsimony analysis of the entire set of dorsolateral head myology data, concatenated with phenotypic characters from Tagliacolloet al., (2016; subsequently modified by Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
), resulted in 99,999 MPT’s (score: 795; CI: 0.37; RI: 0.92), which served as a basis for a strict consensus tree and for mapping synapomorphies from the dorsolateral head musculature (Fig. 35; general synapomorphies listed in Tab. S3).
Strict consensus of MPT’s resulting from parsimony analysis of character matrix in Peixoto et al., (2019)Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022... , concatenated with dorsolateral head musculature characters of this study [Score: 795; RI: 0.92; CI: 0.37], with numbered dorsolateral head musculature characters (below branches) and respective characters states (above branches. Black circles indicate homoplasy-free characters and white circles indicate homoplastic characters. Taxon legends: Gymnotus spp.1 (G. panamensis, G. maculosus, and G. cylindricus); Gymnotus spp.2 (G. varzea, G. pantanal, G. obscurus, G. chaviro); Gymnotus spp.3 (G. ucamara, G. sylvius, G. sp, G. omamorum, G. mamiraua, G. bahianus); Gymnotus spp.4 (G. choco and G. ardilai); Gymnotus spp.5 (G. carapo and G. arapaima); Gymnotus spp.6 (G. tigre and G. hehni); Gymnotus spp.7 (G. stenoleucus, G. pedanopterus, G. pantherinus, G. jonasi, G. javari, G. coropinae, G. coatesi, G. cf. anguilaris, and G. cataniapo); Brachyhypopomus spp.1 (B. sp., B. accidentalis, and B. diazi); Brachyhypopomus spp.2 (B. pinnicaudatus; B. sp. 2); Brachyhypopomus spp.3 (B. bullocki and B. brevirostris); Rhabdolichops spp. (R. jegui and R. cf. stewarti); Adontosternarchus spp. (A. nebulosus, A. devenanzii, A. clarkae, A. balaenops); Apteronorus spp.1 (A. caudimaculosus and A. albifrons); Apteronorus spp.2 (A. magdalenensis and A. cuchillo); Apteronorus spp.3 (A. leptorhynchus and A. eschemeyeri); Sternarchorhynchus spp. (S. starski, S. hagedornae, S. galibi, S. sp.).
Results from the analysis 2 are reasonably consistent with most previous hypotheses of gymnotiform relationships. Seven new myological synapomorphies are listed for Gymnotiformes (Chs. 19, 23, 42, 43, 52, 53, 54), including a condition unique among Ostariophysi, the presence of the secondary section of the levator operculi, herein named levator operculi posterior (Ch. 52; reversed in Hypopygus). A similar division in the levator operculi is only seen homoplastically in unrelated percomorphs, such as tetraodontiforms, stromateiforms and pleuronectiforms (Pastana et al., 2021Pastana MNL, Johnson GD, Datovo A. Comprehensive phenotypic phylogenetic analysis supports the monophyly of stromateiform fishes (Teleostei: Percomorphacea). Zool J Linn Soc. 2021; zlab058. https://doi.org/10.1093/zoolinnean/zlab058
https://doi.org/10.1093/zoolinnean/zlab0...
).
The Gymnotidae is corroborated as monophyletic (in line with all previous hypotheses, e.g., Alves-Gomes et al., 1998Alves-Gomes JA. The phylogenetic position of the South American electric fish genus Sternopygus and Archolaemus (Ostariophysi: Gymnotiformes) according to 12S e 16S Mitochondrial DNA Sequences. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.447–59.; Albert et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
) and sister group to Sternopygoidea. The absence of the adductor mandibulae, segmentum mandibularis is a new synapomorphic condition for the family (Ch. 27). Within Gymnotidae, Electrophorus alone has both the levator arcus palatini and dilatator operculi each divided in two sections, conditions recovered as unique synapomorphies for that genus (Chs. 41 and 49, respectively). The monophyly of Sternopygoidei, although often corroborated in different studies (Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Albert, Crampton, 2005aAlbert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005a. p.360–409. https://doi.org/10.1007/0-387-28275-0_13
https://doi.org/10.1007/0-387-28275-0_13...
; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
), is not without controversy, mainly because of the position of Apteronotidae (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Gayet et al., 1994Gayet M, Meunier FJ, Kirschbaum F. Ellisella kirschbaumi Gayet & Meunier, 1991, gymnotiforme fossile de Bolivie et ses relations phylogénétiques au sein des formes actuelles. Cybium. 1994; 18(3):273–306.; Elbassiouny et al., 2016Elbassiouny AA, Schott RK, Waddell JC, Kolmann MA, Lehmberg ES, Nynatten AV, Crampton WGR, Chang BSW, Lovejoy NR. Mitochondrial genomes of the South American electric knifefishes (Order Gymnotiformes). Mitochondrial DNA B Resour. 2016; 1(1):401–03. https://doi.org/10.1080/23802359.2016.1174090
https://doi.org/10.1080/23802359.2016.11...
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
). In this study, a monophyletic Sternopygoidei is corroborated, with two new myological synapomorphies (Chs. 21 and 37).
Rhamphichthyoidea and Sternopygoidea are both monophyletic, a hypothesis previously supported by both morphological and/or molecular data (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49., 2007; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Carvalho, 2013Carvalho TP. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): Diversification in South American Freshwaters. [PhD Thesis]. Lafayette: University of Louisiana; 2013. Available from: https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750
https://www.proquest.com/openview/ffbdff...
; Maldonado-Ocampo et al., 2014Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR. Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
http://dx.doi.org/10.1111/zsc.12035...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
).
The taxonomic composition of Rhamphichthyidae corroborates traditional classifications of the family (including Rhamphichthys, Iracema and Gymnorhamphichthys; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), thus contradicting results based on molecular data, which also allocated Steatogenys and Hypopygus (Steatogeninae) in that family (Maldonado-Ocampo et al., 2013; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). The monophyly of “Rhamphichthyidae”, excluding Steatogeninae, is supported by four synapomorphies from the dorsolateral head musculature (Chs. 2, 18, 27, 46). Gymnorhamphichthys is the sister group of Iracema + Rhamphichthys (Rhamphichthyini), in agreement with recent hypotheses based on morphological analysis and total evidence (Carvalho, Albert, 2011Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
https://doi.org/10.1590/S1679-6225201100...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
), but contrary to previous morphological studies which supported Iracema as more closely related to Gymnorhamphichthys (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.). The position of the clade composed of Steatogenys + Hypopygus (Steatogeninae) is still a source of generalized controversy between hypotheses based on phenotypic (Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), molecular (e.g., Maldonado-Ocampo et al., 2014Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR. Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
http://dx.doi.org/10.1111/zsc.12035...
) and total evidence (Carvalho, 2013Carvalho TP. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): Diversification in South American Freshwaters. [PhD Thesis]. Lafayette: University of Louisiana; 2013. Available from: https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750
https://www.proquest.com/openview/ffbdff...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
) analyses. In the present study, a monophyletic Steatogeninae is unresolved at a polytomy at the base of Rhamphichthyoidea. No myological characters exist for the entire subfamily, although synapomorphies from musculature were newly found for each of its constituent genera.
As mentioned above, the levator operculi divided in two sections is an exclusive condition in Gymnotiformes, with a reversal in Hypopygus. According to hypotheses of primary homology in the discussion of Ch. 52 above, the portion of the levator operculi present in Hypopygus is homologous to the levator operculi anterior of other gymnotiforms. The single levator operculi in Hypopygus shares all characteristics of the levator operculi anterior in other gymnotiforms (e.g., sites of origin and insertion, and position relative to the R-Avn nerve- cf. Section “General aspects of the dorsolateral head musculature in Gymnotiformes- levator operculi”, above), with no remaining myological component or subsection corresponding to the levator operculi posterior. Thus, a loss of levator operculi posterior is postulated in the genus.
Losses of entire myological components or sections in fishes are unusual. When a muscle, bundle or section is apparently absent, what most often happens is that it is simply not differentiated as a separate element, but its corresponding fibers are still present, undifferentiated from those of another adjacent muscle (e.g., Edgeworth, 1929Edgeworth FH. II. The development of some of the cranial muscles of ganoid fishes. Philos Trans R Soc Lond B Biol Sci. 1929; 217(440–449):39–89. https://doi.org/10.1098/rstb.1929.0002
https://doi.org/10.1098/rstb.1929.0002...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
). However rare, losses of entire myological components do happen in some instances, as is the case of the adductor mandibulae, segmentum mandibularis in several teleosts (e.g., Gymnotiformes- Gymnotidae; Siluriformes- Aspredinidae, Callichthyidae, Loricariidae; Anguiliformes- Anguilla, Ariosoma; Osteoglossiformes- Arapaimidae; LAWP, pers. obs.; Diogo, 2004Diogo R. Morphological evolution, adaptations, homoplasies, constraints, and evolutionary trends: catfishes as a case study on general phylogeny and macroevolution. Enfield: Science Publishers; 2004.; Diogo et al., 2012Diogo R, Matthews LJ, Wood B. A major reason to study muscle anatomy: myology as a tool for evolutionary, developmental, and systematic biology. Biol Syst. 2012; 1(1):1000102. http://dx.doi.org/10.4172/2329-6577.1000102
http://dx.doi.org/10.4172/2329-6577.1000...
; Datovo, Castro, 2012Datovo A, Castro RMC. Anatomy and evolution of the mandibular, hyopalatine, and opercular muscles in characiform fishes (Teleostei: Ostariophysi). Zoology. 2012; 115(2):84–116. https://doi.org/10.1016/j.zool.2011.09.008
https://doi.org/10.1016/j.zool.2011.09.0...
; Datovo, Vari, 2013Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
https://doi.org/10.1371/journal.pone.006...
, 2014Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
https://doi.org/10.1111/zoj.12142...
).
Interestingly, de Santana, Crampton, (2011)de Santana CD, Crampton WGR. Phylogenetic interrelationships, taxonomy, and reductive evolution in the Neotropical electric fish genus Hypopygus (Teleostei, Ostariophysi, Gymnotiformes). Zool J Linn Soc. 2011; 163(4):1096–156. https://doi.org/10.1111/j.1096-3642.2011.00736.x
https://doi.org/10.1111/j.1096-3642.2011...
listed a series of reductive characters for Hypopygus, including partial or total loss of cranial bones, reduction of anal-fin rays, loss of scales, and simplification of laterosensory canal system (reductions reaching their extreme conditions in Hypopygus minissimus de Santana & Crampton, 2011, the smallest know gymnotiform, with ca. 42 mm TL). Those authors recognized Hypopygus as the only miniature taxon within Gymnotiformes. The absence of the levator operculi posterior may be part of that general trend of reduction, with simplification of a myological component. If corroborated by ontogenetic studies, this may be the first case of miniaturization-related reductive modification in myology among Ostariophysi.
Monophyly of Hypopomidae is corroborated in our results, a result also in line with several previous cladistic studies. The origin of the levator arcus palatini being wider than its insertion (Ch. 40) is a new synapomorphy for the family. Our proposed relationships among hypopomid genera, however, disagree with previous hypotheses. Monophyly of Microsternarchini (sensuCox Fernandes et al., 2014Cox Fernandes C, Nogueira A, Alves-Gomes JA.Procerusternarchus pixuna, a new genus and species of electric knifefish (Gymnotiformes: Hypopomidae, Microsternarchini) from the Negro River, South America. Proc Acad Nat Sci Phila. 2014; 163(1):95–118. https://doi.org/10.1635/053.163.0107
https://doi.org/10.1635/053.163.0107...
) is supported, but its position in a polytomy at the base of Hypopomidae and the unresolved relationships among its genera do not support results of other studies, where it was sister group to Hypopomus (Cox Fernandes et al., 2014Cox Fernandes C, Nogueira A, Alves-Gomes JA.Procerusternarchus pixuna, a new genus and species of electric knifefish (Gymnotiformes: Hypopomidae, Microsternarchini) from the Negro River, South America. Proc Acad Nat Sci Phila. 2014; 163(1):95–118. https://doi.org/10.1635/053.163.0107
https://doi.org/10.1635/053.163.0107...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
). The monophyly of Brachyhypopominae (Hypopomus + Brachyhypopomus; sensuAlbert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), usually corroborated in studies of both phenotypic (e.g., Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.) and total evidence (Carvalho, 2013Carvalho TP. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): Diversification in South American Freshwaters. [PhD Thesis]. Lafayette: University of Louisiana; 2013. Available from: https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750
https://www.proquest.com/openview/ffbdff...
) data, was instead refuted herein, with Hypopomus surprisingly supported as sister-group of Akawaio.
Crampton et al. (2016)Crampton WGR, de Santana CD, Waddell JC, Lovejoy NR. A taxonomic revision of the Neotropical electric fish genus Brachyhypopomus (Ostariophysi: Gymnotiformes: Hypopomidae), with descriptions of 15 new species. Neotrop Ichthyol. 2016; 14(4):e150146. https://doi.org/10.1590/1982-0224-20150146
https://doi.org/10.1590/1982-0224-201501...
proposed three potential synapomorphic characters for Brachyhypopomus, however, they also underscored the need of additional analyses to account for ontogenetic variation in the genus. Herein, species of Brachyhypopomus form a monophyletic group, but with all its synapomorphies from morphological complexes other than myology (Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
: chars. 122 and 171). Brachyhypopomus, however, is a highly diverse taxon and examination of additional species is necessary.
Sternopygoidea is monophyletic and sister group to Rhamphichthyoidea, a hypothesis congruent with the vast majority of previous studies (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Albert, Fink, 2007Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
https://doi.org/10.1671/0272-4634(2007)2...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
) and further supported by the attachment of the fibers of the rictalis restricted to the anterior portion of preopercle, not extending beyond the preopercular fossa (Ch. 16).
Within Sternopygoidea, Sternopygidae is supported by three homoplasy-free synapomorphies in dorsolateral head musculature (Chs. 7, 12, 31), including an insertion of the malaris on infraorbitals 1+2 (Ch. 7) which is otherwise unknown within Ostariophysi. Within Sternopygidae, Eigenmanniinae is monophyletic, with Japigny as the sister group to the other genera in the subfamily. Tagliacollo et al., (2016)Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
was the first study to insert Japigny into a published phylogenetic analysis, and concluded that the sole species in the genus was actually a member of Eigenmannia. That hypothesis is not corroborated in our results, which instead support the previous proposals regarding the basalmost position of Japigny within Eigenmaniinae (Vari et al., 2012Vari RP, de Santana CD, Wosiacki WB. South American electric knifefishes of the genus Archolaemus (Ostariophysi, Gymnotiformes): undetected diversity in a clade of rheophiles. Zool J Linn Soc. 2012; 165(3):670–99. https://doi.org/10.1111/j.1096-3642.2012.00827.x
https://doi.org/10.1111/j.1096-3642.2012...
; Dutra, 2015Dutra GM. Sistemática de Eigenmanniinae (Teleostei: Gymnotiformes: Sternopygidae). [PhD Thesis]. Belém: Museu Paraense Emílio Goeldi; 2015.; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
). Archolaemus is sister group to a clade composed of Distocyclus and Eigenmannia + Rhabdolichops, a hypothesis identical to those previously proposed on the basis of phenotypic data (Fink, Fink, 1981Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
https://doi.org/10.1111/j.1096-3642.1981...
; Lundberg, Mago-Leccia, 1986Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
https://www.jstor.org/stable/4064852...
; Albert, Fink, 1996Albert JS, Fink WL. Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus. Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
https://doi.org/10.2307/1446944...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.).
Eigenmannia is proposed as monophyletic, with a synapomorphic origin of the malaris not exclusively on the hyomandibula (Ch. 3). Eigenmannia was usually considered as paraphyletic in previous phylogenetic studies (Alves-Gomes et al., 1998; Albert et al., 1995; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
; Dutra et al., 2021Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
https://doi.org/10.1111/jzs.12535...
). However, ultraconserved elements and morphological studies support the monophyly of the genus (Alda et al., 2019Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
https://doi.org/10.1093/sysbio/syy085...
). Of course, Eigenmannia is the most diverse genus in its family (Peixoto et al., 2015Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): monophyly and description of seven new species. Zool J Linn Soc. 2015; 175(2):384–414. https://doi.org/10.1111/zoj.12274
https://doi.org/10.1111/zoj.12274...
, 2020Peixoto LAW, Pastana MNL, Ballen GA. New species of glass knifefish genus Eigenmannia (Gymnotiformes: Sternopygidae) with comments on the morphology and function of the enlarged cephalic lateral-line canals of Sternopygidae. J Fish Biol. 2020; 1–12. https://doi.org/10.1111/jfb.14564
https://doi.org/10.1111/jfb.14564...
; Peixoto, Ohara, 2019Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
https://doi.org/10.1371/journal.pone.022...
) and examination of musculature of additional species is critical for a solid assessment of its monophyly.
Two new myological synapomorphies support apteronotid monophyly (Chs. 14, 32), a hypothesis widely supported in the literature (Albert et al., 1994, 1995; Alves-Gomes et al., 1998; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Bernt et al., 2018Bernt MJ, Crampton WGR, Orfinger AB, Albert JS.Melanosternarchus amaru, a new genus and species of electric ghost knifefish (Gymnotiformes: Apteronotidae) from the Amazon Basin. Zootaxa. 2018; 4378(2):451–79. https://doi.org/10.11646/zootaxa.4378.4.1
https://doi.org/10.11646/zootaxa.4378.4....
, 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
). Relationships within Apteronotidae are similar to those presented in Peixoto et al. (2019), with multiple apteronotid subclades unresolved in a polytomy (a situation similar to that proposed in Triques, 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.). Overall, however, there is rampant disagreement with previous studies (Triques, 1993Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130., 2005Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.; Alves-Gomes et al., 1995Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
https://doi.org/10.1093/oxfordjournals.m...
; Albert et al., 1998Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
https://doi.org/10.1111/j.1558-5646.1998...
; Albert, Campos-da-Paz, 1998Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.; Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.; Ivanyisky III, Albert, 2014Ivanyisky III SJ, Albert JS. Systematics and biogeography of Sternarchellini (Gymnotiformes: Apteronotidae): Diversification of electric fishes in large Amazonian rivers. Neotrop Ichthyol. 2014; 12(3):565–84. http://dx.doi.org/10.1590/1982-0224-20130159
http://dx.doi.org/10.1590/1982-0224-2013...
; Tagliacollo et al., 2016Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
https://doi.org/10.1016/j.ympev.2015.11....
; Evans et al., 2017Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
http://dx.doi.org/10.1590/1982-0224-2016...
; Bernt et al., 2018Bernt MJ, Crampton WGR, Orfinger AB, Albert JS.Melanosternarchus amaru, a new genus and species of electric ghost knifefish (Gymnotiformes: Apteronotidae) from the Amazon Basin. Zootaxa. 2018; 4378(2):451–79. https://doi.org/10.11646/zootaxa.4378.4.1
https://doi.org/10.11646/zootaxa.4378.4....
, 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
, 2020Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
; Peixoto et al., 2019Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
). For example, Sternarchorhamphinae (Sternarchorhamphus + Orthosternarchus; sensuAlbert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.), has support from three new myological synapomorphies (Chs. 27, 46, 47), one of them homoplasy-free (Ch. 47). The same holds for ((Porotergus + Sternarchogiton, Tenebrosternarchus) + (Compsaraia + “Apteronotus” gr. bonapartii)), a proposal first advanced in Peixoto et al., (2019)Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022...
which received additional support from one new myological synapomorphy (Ch. 25).
Tenebrosternarchus was proposed by Bernt et al. (2020)Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
, to allocate Sternarchogiton preto de Santana & Crampton, 2007. According to the molecular analysis performed in Bernt et al. (2020)Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
, S. preto was the sister-group of all remaining “Navajini” genera (sensuBernt et al., 2019Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
https://doi.org/10.1016/j.ympev.2019.02....
; Navajini is an invalid name- see Ferraris et al., 2007Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
https://doi.org/10.1590/1982-0224-201600...
), including Sternarchogiton, a situation which would require the proposal of a new genus. Our results contradict those of Bernt et al., (2020)Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
https://doi.org/10.1590/1982-0224-2019-0...
, and support T. preto at a position internested with Sternarchogiton species, with two myological synapomorphies recovered for that clade (Chs. 13, 45).
Character sets from different data sources (e.g., bones, muscles, nuclear and mitochondrial genes) are commonly concatenated in cladistic analyses. This is based on the long-held premise that the most reliable hypothesis of phylogenetic relationship is the one that covers all available data (Cracraft, Mindell, 1989Cracraft J, Mindell DP. The early history of modern birds: a comparison of molecular and morphological evidence. In: Fernholm B, Bremer K, Jornvall J, editors. The hierarchy of life. Amsterdam: Elsevier; 1989. p.389–403.). While this premise is broadly unchallenged, understanding the relationship between evidence and hypotheses requires an understanding of the degree to which different data sources contribute to the support of each branch within a global analysis (Baker, DeSalle, 1997Baker RH, DeSalle R. Multiple sources of character information and the phylogeny of Hawaiian drosophilids. Syst Biol. 1997; 46(4):654–73. https://doi.org/10.1093/sysbio/46.4.654
https://doi.org/10.1093/sysbio/46.4.654...
). Among the procedures to investigate that issue, PBS is one of the most consistent, permitting an estimate of the congruence or incongruence of a specific dataset, quantifying and mapping it on a topology (Baker, DeSalle, 1997Baker RH, DeSalle R. Multiple sources of character information and the phylogeny of Hawaiian drosophilids. Syst Biol. 1997; 46(4):654–73. https://doi.org/10.1093/sysbio/46.4.654
https://doi.org/10.1093/sysbio/46.4.654...
; Koepfli, Wayne, 2003Koepfli KP, Wayne RK. Type I Sts markers are more informative than cytochrome b in phylogenetic reconstruction of the Mustelidae (Mammalia: Carnivora). Syst Biol. 2003; 52(5):571–93. https://doi.org/10.1080/10635150390235368
https://doi.org/10.1080/1063515039023536...
). Negative PBS values (PBS-) for a node indicate that data partitions support relationships not corroborated in the global analyses, while positive PBS values (PBS+) mean complete congruence with that topology (Lambkin, 2004Lambkin CL. Partitioned Bremer support localises significant conflict in bee flies (Diptera: Bombyliidae: Anthracinae). Invertebr Syst. 2004; 18:351–60. https://doi.org/10.1071/IS04004
https://doi.org/10.1071/IS04004...
; Peña et al., 2006Peña C, Wahlberg N, Weingartner E, Kodandaramaiah U, Nylin S, Freitas AVL, Brower AVZ. Higher level phylogeny of Satyrinae butterflies (Lepidoptera: Nymphalidae) based on DNA sequence data. Mol Phylogenet Evol. 2006; 40(1):29–49. https://doi.org/10.1016/j.ympev.2006.02.007
https://doi.org/10.1016/j.ympev.2006.02....
).
The application of PBS on two partitions focal to this study (general morphological characters vs. myological characters) allows an assessment of the influence of dorsolateral head musculature characters within the phylogeny of Gymnotiformes. In the global analysis, the myological data set conflicts in 34 of the 64 nodes of the strict consensus tree (i.e., PBS-), while providing favorable evidence in 29 other nodes (PBS+) and neutral value for a single clade (PBS= 0) (Fig. 36). PBS analysis further reveals that the contribution of myological characters is not uniformly distributed on the tree, instead concentrating on specific portions of the topology.
Strict consensus of MPT’s resulting from parsimony analysis of character matrix in Peixoto et al., (2019)Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
https://doi.org/10.1371/journal.pone.022... , concatenated with dorsolateral head musculature characters of this study [Score: 795; RI: 0.92; CI: 0.37]. PBS values for general morphological characters and myological characters are indicated below each branch. Circle size is arbitrary; gray indicate neutral values of PBS for myologycal characters. Taxon legends as in Fig. 35.
Support from myological characters is disproportionately concentrated in basal portions of the tree, with positive values to almost all more inclusive groups, such as orders (e.g., Characiformes, Siluriphysi, Siluriformes and Gymnotiformes), suborders (Gymnotoidei and Sternopygoidei) and superfamilies (e.g., Rhamphichthyoidea and Sternopygoidea). Nearly all gymnotiform families also received PBS+ from musculature, the only exception being Hypopomidae (PBS: -0.1). Among subfamilies, 63.3% received PBS+, with Steatogeninae being the only one in which myological and general characters conflict. Musculature and general data partitions also disagree regarding the resolution of the Microsternarchini, the only tribe with more than two genera included in the analysis. This is revealed by PBS = -0.1 for the myological partition. Contrastingly, among less inclusive clades, relationships at generic level received PBS+ in 52%, and PBS- in 44% of all nodes. A neutral value (PBS = 0) was found exclusively for the polytomy comprising Tenebrosternarchus and Sternarchogiton, indicating non-existing support from musculature characters. Curiously, general morphology and myological data partitions strongly disagree on interspecific relationships, with musculature characters yielding negative values of PBS for all such minor clades.
Myological characters are thus more effective in reconstructing deeper nodes of the combined tree in the equally weighted parsimony analysis, roughly corresponding to levels of order, suborder, superfamily, family, and subfamily, but with negative or no correlation in the ranks of tribe, intergeneric and interspecific levels (Fig. 37). The reasons for such skewed distribution of support from myology remain a matter of speculation at this time, but it offers interesting lines for future research. It may be related to underlying biomechanical constraints evolved in the early stages of gymnotiform evolution, but with more recent variants subject to less stringent parameters and thus undergoing more labile rates of state change. It also remains to be investigated whether such effects hold for other groups of fishes or if it is instead a phenomenon restricted to gymnotiforms.
Patterns of PBS value distribution of myologycal characters per taxonomic level in the phylogeny based on phenotypic data (Figs. 35, 36). Green bars indicate positive PBS values and red bars indicate negative PBS values. Bar for tribes include only non-monogeneric tribes. Taxon legends: A) Characiformes; B) Siluriformes; C) Siluriphysi; D) Gymnotiformes; E) Sternopygoidei; F) Rhamphichthyoidea; G) Sternopygoidea; H) Gymnotidae; I) Hypopomidae; J) Rhamphichthyidae; K) Sternopygidae; L) Apteronotidae; M) Steatogeninae; N) Eigenmanniinae; O) Sternarchorhynchinae; P) Microsternarchini; Q) Gymnotus; R) Brachyhypopomus; S) Akawaio + Hypopomus; T) Gymnorhamphichthys; U) Iracema + Rhamphichthys; V) Steatogenys; W) Hypopygus; X) Sternopygus; Y) Archolaemus + remaining Eigenmanniinae genera (except Japigny) Z) Distocyclus + remaining Eigenmanniinae genera (except Archolaemus and Japigny); A1) Eigenmannia + Rhabdolichops; B1) Rhabdolichops; C1) R. eastwardi + Rhabdolichops spp.; D1) Eigenmannia spp.; E1) Adontosternarchus; F1) Megadontognathus + Apteronotus; G1) Apteronotus; H1) A. cuchillejo + A. spp.1; I1) ((Porotergus + Tenebrosternarchus, Sternarchogiton) + (Compsaraia + “A.” bonapartii)); J1) (Porotergus + Tenebrosternarchus, Sternarchogiton); K1) (Tenebrosternarchus, Sternarchogiton); L1) (Compsaraia + “A.” bonapartii); M1) Compsaraia; N1) Pariosternarchus + Sternarchella; O1) Sternarchella; P1) Orthosternarchus + Sternarchorhamphus; Q1) Platyurosternarchus + Sternarchorhynchus; R1) Platyurosternarchus; S1) Sternarchorhynchus; T1) G. curupira + Gymnotus subgroups; U1) Gymnotus spp.2, 3, 4, 5; V1) Gymnotus spp.3; W1) Gymnotus spp.4; X1) Gymnotus spp.5; Y1) Gymnotus spp.6; Z1) Gymnotus spp.7; A2) B. beebei + Brachyhypopomus subgroups; B2) B. draco + Brachyhypopomus spp.2; C2) Brachyhypopomus spp.3; D2) Hypopygus spp.; E2) Rhabdolichops spp.; F2) Adontosternarchus spp.; G2) Apteronotus spp.1; H2) Apteronotus spp.2 + Apteronotus spp.3; I2) Apteronotus spp.2; J2) Apteronotus spp.3; K2) “A.” bonapartii; L2) Sternarchorhynchus spp.
Evidence presented here suggests the relevance of musculature as a source of reliable phylogenetic signal, a view foreshadowed by Borden, (1999)Borden WC. Comparative myology of the unicornfishes, Naso (Acanthuridae, Percomorpha), with implications for phylogenetic analysis. J Morphol. 1999; 239(2):191–224. https://doi.org/10.1002/(SICI)1097-4687(199902)239:2%3C191::AID-JMOR6%3E3.0.CO;2-2
https://doi.org/10.1002/(SICI)1097-4687(...
. That author found little intraspecific variability in the muscles of Naso species (Acanthuridae; Perciformes) when compared to other anatomical complexes (e.g., osteology) and, in combination with data from other studies, concluded that myological characters are reliable indicators of relationships at deep levels of phylogeny. Therefore, in spite of the difficulties related to the acquisition of material, myological preparation and dissection (Datovo, Bockmann, 2010Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol. 2010; 8(2):193–246. http://dx.doi.org/10.1590/S1679-62252010000200001
http://dx.doi.org/10.1590/S1679-62252010...
), the information thus gained is worth the effort, especially in deeper components of the relationships in fishes.
Some studies (Diogo, 2004Diogo R. Morphological evolution, adaptations, homoplasies, constraints, and evolutionary trends: catfishes as a case study on general phylogeny and macroevolution. Enfield: Science Publishers; 2004.; Diogo et al., 2012Diogo R, Matthews LJ, Wood B. A major reason to study muscle anatomy: myology as a tool for evolutionary, developmental, and systematic biology. Biol Syst. 2012; 1(1):1000102. http://dx.doi.org/10.4172/2329-6577.1000102
http://dx.doi.org/10.4172/2329-6577.1000...
) attempted to empirically investigate the influence and relevance of myological characters in cladistic analyses by comparing the mean consistency and retention indices of myological and osteological characters, concluding that the former have, on average, higher values than those of the latter. Such results show that some data sets are more homoplastic than others (consistency index) and express the degree to which global character change is informative in the favored phylogeny (retention index). Those metrics, however, do not allow an estimate of the contribution of each data source at different taxonomic levels across the phylogeny. In this paper, we have shown that PBS reveals the location and degree of contribution of each data set to the topology resulting from concatenated analyses (Baker, DeSalle, 1997Baker RH, DeSalle R. Multiple sources of character information and the phylogeny of Hawaiian drosophilids. Syst Biol. 1997; 46(4):654–73. https://doi.org/10.1093/sysbio/46.4.654
https://doi.org/10.1093/sysbio/46.4.654...
; Lambkin et al., 2002Lambkin CL, Lee MSY, Winterton SL, Yeates DK. Partitioned Bremer support and multiple trees. Cladistics. 2002; 18(4):436–44. https://doi.org/10.1111/j.1096-0031.2002.tb00159.x
https://doi.org/10.1111/j.1096-0031.2002...
) and is a valid tool to detect the influence (or congruence) of characters from different sources at different levels of the phylogeny.
ANALYSIS 2- Summary of myological synapomorphies
In this section we list all myological synapomorphies currently known for the clades found in this study. They are listed per clade, following the numbering in preceding character description sections and with information on their specific cases of homoplastic changes when pertinent.
Clade Siluriformes + Gymnotiformes
- Origin of stegalis including sphenotic (Ch. 18, State 1; reversed in Eigenmanniinae, Porotergus, Pariosternarchus and Clade Gymnorhamphichthys + Iracema + Rhamphichthys).
- Origin of levator arcus palatini including frontal (Ch. 38, State 1; reversed to State 0 in Electrophorus and Clade Archolaemus + Distocyclus + Eigenmannia + Rhabdolichops).
- Anterolateral fibers of levator arcus palatini oriented obliquely relative to the horizontal arm of preopercle, forming a 45° angle relative to longitudinal axis of head (Ch. 46, State 1; reversed in Electrophorus, Eigenmanniinae, Sternarchorhynchinae and Clade Gymnorhamphichthys + Iracema + Rhamphichthys).
- Levator arcus palatini overlapping pterosphenoid almost entirely, except for its anterior region (Ch. 47, State 1; reversed in Electrophorus).
Gymnotiformes
- Origin of stegalis including parasphenoid (Ch. 19, State 1).
- Stegalis positioned lateral to entire adductor arcus palatini, totally overlapping it (Ch. 23, State 1).
- Orientation of anterolateral fibers of levator arcus palatini at insertion lateral relative to adductor mandibulae, pars malaris: (Ch. 42, State 1).
- Posterolateral fibers of levator arcus palatini at insertion lateral relative to adductor mandibulae, pars malaris (Ch. 43, State 1).
- Levator operculi posterior present (Ch. 52, State 1; reversed to State 0 in Hypopygus).
- Insertion of levator operculi on a dorsal crest on lateral surface of opercle (Ch. 53, State 1).
- R-Avn nerve lateral relative to levator operculi anterior or its anterior fibers (Ch. 54, State 1; reversed to State 0 in Eigenmanniinae).
Gymnotidae
- Origin of stegalis including frontal (Ch. 20, State 1).
- Segmentum mandibularis absent (Ch. 27, State 1; convergent in Archolaemus, Sternarchorhynchinae and in Clade Iracema + Gymnorhamphichthys + Rhamphichthys).
- Origin of adductor hyomandibulae including sphenotic (Ch. 55, State 1).
Gymnotus
- Origin of levator arcus palatini twice that of insertion (Ch. 40, State 3).
Electrophorus
- Origin of levator arcus palatini not including frontal (Ch. 38, state 0; reversal of State 1 in Clade Gymnotiformes + Siluriformes; also reversed in Clade Archolaemus + Distocyclus + Eigenmannia + Rhabdolichops).
- Levator arcus palatini subdivided in two sections (Ch. 41, State 1).
- Anteromesial fibers of levator arcus palatini at insertion lateral to adductor mandibulae, pars malaris (Ch. 44, State 1; convergent in Clade Orthosternarchus + Sternarchorhamphus).
- Anterolateral fibers of levator arcus palatini approximately straight relative to the horizontal arm of preopercle, forming angle of ca. 90° relative to the longitudinal axis of head (Ch. 46, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; reversed also in Eigenmanniinae, Sternarchorhynchinae and Clade Gymnorhamphichthys + Iracema + Rhamphichthys).
- Levator arcus palatini overlapping pterosphenoid only at posterior margin (Ch. 47, State 0, reversal of State 1 in Clade Siluriformes + Gymnotiformes).
- Dilatator operculi divided into two sections (Ch. 49, State 1).
Sternopygoidei
- Origin of stegalis including pterosphenoid (Ch. 21, State 1; reversed to State 0 in Gymnorhamphichthys, Porotergus, Pariosternarchus, Parapteronotus and Clade Distocyclus + Eigenmannia + Rhabdolichops).
- Insertion of adductor arcus palatini on dorsolateral and median portion of endopterygoid, reaching middle of bone (Ch. 37, State 1; reversed to State 0 in Gymnorhamphichthys).
Rhamphichthyoidea
- Malaris inserting on antorbital (Ch. 6, State 1).
Hypopomidae
- Origin of levator arcus palatini wider than its insertion, up to 150% width insertion (Ch. 40, State 1; modified into State 2 in Sternopygus and in Rhabdolichops).
Clade Gymnorhamphichthys + Iracema + Rhamphichthys
- Rictalis and stegalis partially continuous, forming ricto-stegalis (Ch. 2, State 1)
- Origin of stegalis not including sphenotic (Ch. 18, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; also reversed in Eigenmanniinae, Porotergus and Pariosternarchus).
- Segmentum mandibularis absent (Ch. 27, State 1; convergent in Archolaemus and Sternarchorhynchinae).
- Anterolateral fibers of levator arcus palatini oriented approximately straight relative to the horizontal arm of preopercle, forming an angle of ca. 90° relative to the longitudinal axis of head (Ch. 46, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; also reversed in Electrophorus, Eigenmanniinae and Sternarchorhynchinae).
Gymnorhamphichthys
- Origin of stegalis not including pterosphenoid (Ch. 21, State 0; reversal of State 1 in Sternopygoidei; also reversed in Porotergus, Pariosternarchus, Parapteronotus and Clade Distocyclus + Eigenmannia + Rhabdolichops).
- Insertion of adductor arcus palatini limited to dorsolateral portion of endopterygoid, not extending beyond middle of bone (Ch. 37, State 0; reversal of State 1 in Sternopygoidei).
Steatogeninae
No myological synapomorphies found.
Hypopygus
- Levator operculi posterior absent (Ch. 52, State 0; reversal of State 1, in Clade Siluriformes + Gymnotiformes).
Steatogenys
- Insertion of segmentum mandibularis restricted to anguloarticular (Ch. 29, State 2).
- Dilatator operculi lateral to levator arcus palatini, covering anterior half of latter (Ch. 45, State 0).
- Origin of levator operculi including hyomandibula (Ch. 51, State 1; convergent in Eigenmanniinae).
Sternopygoidea
- Attachment of ventrolateral fibers of rictalis restricted to anterior portion of preopercle, not extending beyond preopercular fossa (Ch. 16, State 1).
Sternopygidae
- Malaris inserting on infraorbital 1+2 (Ch. 7, State 1).
- Connection of malaris fibrous (Ch. 12, State 1).
- Transverse ligament well differentiated (Ch. 31, State 1).
Sternopyginae
- Origin of levator arcus palatini including pterosphenoid (Ch. 39, State 1; convergent in Rhabdolichops and Adontosternarchus).
- Origin and insertion of levator arcus palatini equally wide (Ch. 40, State 2; convergent in Rhabdolichops).
Eigenmanniinae
- Origin of stegalis not including sphenotic (Ch. 18, State 0; reversal of State 1, in Clade Siluriformes + Gymnotiformes; also reversed in Clade Gymnorhamphichthys + Iracema + Rhamphichthys, Eigenmanniinae, Porotergus and Pariosternarchus).
- Levator arcus palatini overlapping posterior third of pterosphenoid (Ch. 47, State 3)
- Origin of levator operculi including hyomandibula (Ch. 51, State 1; convergent in Steatogenys).
- R-Avn nerve entirely mesial to levator operculi (Ch. 54, State 0; reversal of State 1 in Gymnotiformes).
Clade Archolaemus + Distocyclus + Eigenmannia + Rhabdolichops
- Origin of levator arcus palatini not including frontal (Ch. 38, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; also reversed in Electrophorus).
- Anterolateral fibers of levator arcus palatini oriented approximately straight relative to the horizontal arm of preopercle, forming ca. 90° angle relative to longitudinal axis of head (Ch. 46, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; also reversed in Electrophorus, “Rhamphichthyidae” and Sternarchorhynchinae).
Archolaemus blax
- Malaris totally overlapping stegalis (Ch. 24, State 1).
- Segmentum mandibularis absent (Ch. 27, State 1; convergent in Sternarchorhynchinae).
- Origin of adductor operculi not including prootic (Ch. 56, State 1).
Clade Distocyclus + Eigenmannia + Rhabdolichops
- Origin of stegalis not including pterosphenoid (Ch. 21, State 0; reversal of State 1 in Sternopygoidei; also reversed in Gymnorhamphichthys, Pariosternarchus, Porotergus and Parapteronotus).
Eigenmannia
No myological synapomorphies found.
Rhabdolichops
- Lateral fibers of rictalis inserting on posterior margin of anguloarticular (Ch. 17, State 1; convergent in Adontosternarchus and Parapteronotus).
- Segmentum mandibularis contacting entire dorsal margin of Meckel´s cartilage (Ch. 28, State 1; convergent in Parapteronotus).
- Origin of levator arcus palatini including pterosphenoid (Ch. 39, State 1; convergent in Sternopygus and Adontosternarchus).
- Origin and insertion of levator arcus palatini equally wide (Ch. 40, State 2; convergent in Sternopyginae).
Apteronotidae
- Malaris entirely differentiated into a lateral layer of buccopalatal membrane (Ch. 14, State 1).
- Length of endomaxilar ligament equal to that of fibrous portion of malaris (Ch. 32, state 1; reversed to State 0 in Clade Compsaraia + “Apteronotus” gr. bonapartii and Pariosternarchus).
Adontosternarchus
- Insertion of ventrolateral fibers of malaris inserting on posterior margin of dentary and anguloarticular (Ch. 9, State 1).
- Lateral fibers of rictalis inserting on posterior margin of anguloarticular (Ch. 17, State 1; convergent in Rhabdolichops and Parapteronotus).
- Stegalis positioned laterally to middle and posterior portion of adductor arcus palatini (Ch. 23, State 2; convergent in Clade Orthosternarchus tamandua + Sternarchorhamphus muelleri).
- Origin of levator arcus palatini including pterosphenoid (Ch. 39, State 1; convergent in Rhabdolichops and Sternopyginae).
Clade Apteronotus + Compsaraia + “ Apteronotus ” gr. bonapartii + Porotergus gimbeli + Tenebrosternarchus + Sternarchogiton
- Malaris positioned laterally to dorsal portion of rictalis and to ventromedial portion of stegalis, overlapping both almost entirely (Ch. 25, State 1; convergent in Pariosternarchus).
Clade Compsaraia + “ Apteronotus ” gr. bonapartii
- Length of endomaxillary ligament shorter than 2/3 length of fibrous portion of malaris (Ch. 32, State 0; reversal from State 1 in Apteronotidae; also reversed in Pariosternarchus).
“ Apteronotus ” gr. bonapartii
- Malaris entirely differentiated into promalaris and retromalaris, from origin to insertion (Ch. 13, State 3).
- Dorsal portion of malaris (promalaris) positioned dorsolaterally to dorsal portion of rictalis and stegalis, with its ventral region (retromalaris) ventrolateral to dorsal portion of rictalis and stegalis (Ch. 25, State 3).
Porotergus gimbeli
- Origin of stegalis not including sphenotic (Ch. 18, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; also reversed in Eigenmanniinae, Pariosternarchus and in Clade Gymnorhamphichthys + Iracema + Rhamphichthys).
- Origin of stegalis not including pterosphenoid (Ch. 21, State 0; reversal of State 1 in Sternopygoidei; also reversed in Gymnorhamphichthys, Pariosternarchus, Parapteronotus and Clade Distocyclus + Eigenmannia + Rhabdolichops).
Clade Tenebrosternarchus + Sternarchogiton
- Malaris with median and dorsoposterior regions of promalaris differentiated from its ventromedial region and from retromalaris (Ch. 13, State 2).
- Dilatator operculi positioned laterally to levator arcus palatini, covering less than anterior half of latter (Ch. 45, State 1; convergent in Orthosternarchus).
Sternarchellini
No myological synapomorphies found.
Pariosternarchus amazonensis
- Origin of stegalis not including sphenotic (Ch. 18, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; also reversed in Eigenmanniinae, Porotergus and in Clade Gymnorhamphichthys + Iracema + Rhamphichthys).
- Origin of stegalis not including pterosphenoid (Ch. 21, State 0; reversal of State 1 in Sternopygoidei; also reversed in Porotergus, Gymnorhamphichthys, Parapteronotus and Clade Distocyclus + Eigenmannia + Rhabdolichops).
- Malaris positioned laterally to dorsal portion of rictalis and to ventromedial portion of stegalis, overlapping both almost entirely (Ch. 25, State 1; convergent in Clade Apteronotus + Compsaraia + “Apteronotus” gr. bonapartii + Porotergus gimbeli + Tenebrosternarchus + Sternarchogiton).
- Length of endomaxillary ligament shorter than 2/3 length of fibrous portion of malaris (Ch. 32, State 0; reversal from State 1 in Apteronotidae; also reversed in Clade Compsaraia + “Apteronotus” gr. bonapartii).
Sternarchella
- Mesial fibers of malaris inserting on posterior margin of anguloarticular (Ch. 10, State 1).
- Malaris inserting on mesethmoid and premaxilla (Ch. 11, State 1).
- Endomaxillary ligament dorsolateral to autopalatine (Ch. 33, State 1).
- Origin of dorsal fibers of dilatator operculi on sphenotic and pterotic overlapping the bones completely and extending to suture with parietal (Ch. 50, State 1).
Sternarchorhynchinae
- Segmentum mandibularis absent (Ch. 27, State 1; convergent in Gymnotidae,
Archolaemus, and Clade Iracema + Gymnorhamphichthys + Rhamphichthys).
- Anterolateral fibers of levator arcus palatini approximately straight relative to the horizontal arm of preopercle, forming angle of ca. 90° relative to the longitudinal axis of head (Ch. 46, State 0; reversal of State 1 in Clade Siluriformes + Gymnotiformes; reversed also in Electrophorus, Eigenmanniinae and Clade Gymnorhamphichthys + Iracema + Rhamphichthys).
- Levator arcus palatini overlapping half of pterosphenoid (Ch. 47, State 2).
Clade Orthosternarchus tamandua + Sternarchorhamphus muelleri
- Stegalis positioned laterally to middle and posterior portion of adductor arcus palatini (Ch. 23, State 2; convergent in Adontosternarchus).
- Anteromesial fibers of levator arcus palatini at insertion lateral to adductor mandibulae, pars malaris (Ch. 44, State 1; convergent in Electrophorus).
Orthosternarchus tamandua
- Intermuscular bones present in adductor mandibulae, segmentum facialis (Ch. 26, State 1).
- Dilatator operculi positioned laterally to levator arcus palatini, covering less than anterior half of latter (Ch. 45, State 1; convergent in Clade Tenebrosternarchus + Sternarchogiton).
Sternarchorhynchus
- Stegalis inserting on posteromesial margin of dentary (Ch. 33, State 1).
Parapteronotus hasemani
- Lateral fibers of rictalis inserting on posterior margin of anguloarticular (Ch. 17, State 1; convergent in Adontosternarchus and Rhabdolichops).
- Origin of stegalis not including pterosphenoid (Ch. 21, State 0; reversal of State 1 in Sternopygoidei; also reversed in Porotergus, Gymnorhamphichthys, Pariosternarchus and Clade Distocyclus + Eigenmannia + Rhabdolichops).
- Segmentum mandibularis contacting entire dorsal margin of Meckel´s cartilage (Ch. 28, State 1; convergent in Rhabdolichops).
ACKNOWLEDGEMENTS
Authors are grateful to Cláudio de Oliveira (LBP); Lúcia Rapp Py-Daniel and Renildo R. de Oliveira (INPA); Carlos Lucena (MCP); John Sparks and Barbara Brown (AMNH); Mark Sabaj and Mariangeles Arce (ANSP); David Catania (California Academy of Sciences); Patrice Pruvost, Aurélie Laurent, Zora Gabsi and Lina-María Duque-Vélez (MNHN); Caleb McMahan (FMNH); Wolmar Wosiacki, Izaura Maschio, and Angelo Dourado (MPEG); and Lynne Parenti, Richard Vari, David Johnson, Jeffrey Clayton, Kris Murphy and Sandra Rareron (USNM) for the loan of specimens and assistance during visits to their institutions. This study benefitted from suggestions of Carlos de Santana, Marcelo Britto, Ricardo Campos-da-Paz and Aléssio Datovo, as part of their participation in the Ph.D Committee of the first author in the Graduate Program of the Museu de Zoologia, Universidade de São Paulo. Additional suggestions by Rodrigo Caires and Guilherme Dutra are also gratefully acknowledged. The authors are indebt to two anonymous reviewers for carefully reviewing this paper. This study was funded by FAPESP (#2013/09926–3; #2015/24709–4; #2018/05084–1 to LAWP) and CNPq (#310688/2019–1 to MdP). Financial support was also provided by the Diversity and Evolution of Gymnotiformes Project (FAPESP/Smithsonian Institution #2016/19075–9).
REFERENCES
- Aguilera O. La musculature estriada en los peces Gymnotiformes (Teleostei-Ostariophysi): Musculatura facial. Acta Biol Venez. 1986; 12(2):13–23.
- Aguilera O, Machado-Allison A. La musculatura em los peces Gymnotiformes (Teleostei-Ostariophysi): Arcos Branquiales. Acta Biol Venez. 1993; 14:21–32.
- Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Pub - Mus Zool, Univ Mich. 2001; 190; 1–127.
- Albert JS, Campos-da-Paz R. Phylogenetic systematics of Gymnotiformes with diagnoses of 58 clades: a review of available data. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.419–60.
- Albert JS, Crampton WGR. Diversity and phylogeny of neotropical electric fishes (Gymnotiformes). In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005a. p.360–409. https://doi.org/10.1007/0-387-28275-0_13
» https://doi.org/10.1007/0-387-28275-0_13 - Albert JS, Crampton WGR. Electroreception and electrogenesis. In: Evans DH, Claiborne JB, editors. The Physiology of fishes. Boca Raton: CRC Press; 2005b. p.431–72.
- Albert JS, Crampton WGR. A new species of electric knifefish, genus Compsaraia (Gymnotiformes: Apteronotidae) from the Amazon River, with extreme sexual dimorphism in snout and jaw length. Syst Biodivers. 2009; 7(1):81–92. https://doi.org/10.1017/S1477200008002934
» https://doi.org/10.1017/S1477200008002934 - Albert JS, Crampton WGR, Thorsen DH, Lovejoy NR. Phylogenetic systematics and historical biogeography of the Neotropical electric fish Gymnotus (Teleostei: Gymnotidae). Syst Biodivers. 2005; 2(4):375–417. https://doi.org/10.1017/S1477200004001574
» https://doi.org/10.1017/S1477200004001574 - Albert JS, Fink WL Sternopygus xingu, a new species of electric fish from Brazil (Teleostei: Gymnotoidei), with comments on the phylogenetic position of Sternopygus Copeia. 1996; 1996(1):85–102. https://doi.org/10.2307/1446944
» https://doi.org/10.2307/1446944 - Albert JS, Fink WL. Phylogenetic relationships of fossil Neotropical electric fishes (Osteichthyes: Gymnotiformes) from the Upper Miocene of Bolivia. J Vertebr Paleontol. 2007; 27(1):17–25. https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2
» https://doi.org/10.1671/0272-4634(2007)27[17:PROFNE]2.0.CO;2 - Albert JS, Lannoo MJ, Yuri T. Testing hypotheses of neural evolution in gymnotiform electric fishes using phylogenetic character data. Evolution. 1998; 52(6):1760–80. https://doi.org/10.1111/j.1558-5646.1998.tb02255.x
» https://doi.org/10.1111/j.1558-5646.1998.tb02255.x - Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical Fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. https://doi.org/10.1093/sysbio/syy085
» https://doi.org/10.1093/sysbio/syy085 - Alves-Gomes JA. The phylogenetic position of the South American electric fish genus Sternopygus and Archolaemus (Ostariophysi: Gymnotiformes) according to 12S e 16S Mitochondrial DNA Sequences. In: Malabarba LR, Reis ER, Vari R, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of neotropical fishes. Porto Alegre: Edipucrs; 1998. p.447–59.
- Alves-Gomes J, Ortí G, Haygood M, Heiligenberg W, Meyer A. Phylogenetic analysis of the South American electric fishes (order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Mol Biol Evol. 1995; 12(2):298–318. https://doi.org/10.1093/oxfordjournals.molbev.a040204
» https://doi.org/10.1093/oxfordjournals.molbev.a040204 - Baker RH, DeSalle R. Multiple sources of character information and the phylogeny of Hawaiian drosophilids. Syst Biol. 1997; 46(4):654–73. https://doi.org/10.1093/sysbio/46.4.654
» https://doi.org/10.1093/sysbio/46.4.654 - Bernt MJ, Crampton WGR, Orfinger AB, Albert JS.Melanosternarchus amaru, a new genus and species of electric ghost knifefish (Gymnotiformes: Apteronotidae) from the Amazon Basin. Zootaxa. 2018; 4378(2):451–79. https://doi.org/10.11646/zootaxa.4378.4.1
» https://doi.org/10.11646/zootaxa.4378.4.1 - Bernt MJ, Fronk AH, Evans KM, Albert JS. A redescription of deep-channel ghost knifefish, Sternarchogiton preto (Gymnotiformes: Apteronotidae), with assignment to a new genus. Neotrop Ichthyol. 2020; 18(1):e190126. https://doi.org/10.1590/1982-0224-2019-0126
» https://doi.org/10.1590/1982-0224-2019-0126 - Bernt MJ, Tagliacollo VA, Albert JS. Molecular Phylogeny of the ghost knifefishes (Gymnotiformes: Apteronotidae). Mol Phylogenet Evol. 2019; 135:297–307. https://doi.org/10.1016/j.ympev.2019.02.019
» https://doi.org/10.1016/j.ympev.2019.02.019 - Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol. 2017; 17(162):1–40. https://doi.org/10.1186/s12862-017-0958-3
» https://doi.org/10.1186/s12862-017-0958-3 - Borden WC. Comparative myology of the unicornfishes, Naso (Acanthuridae, Percomorpha), with implications for phylogenetic analysis. J Morphol. 1999; 239(2):191–224. https://doi.org/10.1002/(SICI)1097-4687(199902)239:2%3C191::AID-JMOR6%3E3.0.CO;2-2
» https://doi.org/10.1002/(SICI)1097-4687(199902)239:2%3C191::AID-JMOR6%3E3.0.CO;2-2 - Bremer K. Branch support and tree stability. Cladistics. 1994; 10(3):295–304. https://doi.org/10.1111/j.1096-0031.1994.tb00179.x
» https://doi.org/10.1111/j.1096-0031.1994.tb00179.x - Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory nerves in gymnotiform fish. J Comp Neurol. 1982; 211(2):139–53. https://doi.org/10.1002/cne.902110204
» https://doi.org/10.1002/cne.902110204 - Carvalho TP. Systematics and evolution of the toothless knifefishes Rhamphichthyoidea Mago-Leccia (Actinopterygii: Gymnotiformes): Diversification in South American Freshwaters. [PhD Thesis]. Lafayette: University of Louisiana; 2013. Available from: https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750
» https://www.proquest.com/openview/ffbdff290b7617a64774f88ff98dd0b5/1?pq-origsite=gscholar&cbl=18750 - Carvalho TP, Albert JS. Redescription and phylogenetic position of the enigmatic Neotropical electric fish Iracema caiana Triques (Gymnotiformes: Rhamphichthyidae) using x-ray computed tomography. Neotrop Ichthyol. 2011; 9(3):457–69. https://doi.org/10.1590/S1679-62252011000300001
» https://doi.org/10.1590/S1679-62252011000300001 - Chardon M, de la Hoz E. Notes sur le squelette, les muscles, les tendons et le cerveau des Gymnotoidei. Ann Sci Nat, Zool Biol Anim. 1973; 15(1):1–10.
- Chardon M, de la Hoz E. Towards an improved classification of the gymnotiform fishes by the use of the splanchnocranium characters. Acta Biol Jugoslav, Beograd. 1974; 6:15–25.
- Chardon M, de la Hoz E. Remarques anatomiques et fonctionnelles à propos du suspensorium et de la série operculaire chez Sternopygus macrurus (Bloch & Schneider) et Egenmannia virescens (Val) (Teleostei, Gymnotoidei). Ann Soc R Zool Belg. 1977; 106(2–4):177–91.
- Chen WJ, Lavoué S, Mayden RL. Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution. 2013; 67(8):2218–39. https://doi.org/10.1111/evo.12104
» https://doi.org/10.1111/evo.12104 - Correa SB, Crampton WGR, Albert JS. Three new species of the neotropical electric fish Rhabdolichops (Gymnotiformes: Sternopygidae) from the central Amazon, with a new diagnosis of the genus. Copeia. 2006; 2006(1):27–42. https://doi.org/10.1643/0045-8511(2006)006[0027:TNSOTN]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2006)006[0027:TNSOTN]2.0.CO;2 - Cox Fernandes C, Lundberg JG, Riginos C. Largest of all electric-fish snouts: hypermorphic facial growth in male Apteronotus hasemani and the identity of Apteronotus anas (Gymnotiformes: Apteronotidae). Copeia. 2002; 2002(1):52–61. https://doi.org/10.1643/0045-8511(2002)002[0052:LOAEFS]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2002)002[0052:LOAEFS]2.0.CO;2 - Cox Fernandes C, Nogueira A, Alves-Gomes JA.Procerusternarchus pixuna, a new genus and species of electric knifefish (Gymnotiformes: Hypopomidae, Microsternarchini) from the Negro River, South America. Proc Acad Nat Sci Phila. 2014; 163(1):95–118. https://doi.org/10.1635/053.163.0107
» https://doi.org/10.1635/053.163.0107 - Cracraft J, Mindell DP. The early history of modern birds: a comparison of molecular and morphological evidence. In: Fernholm B, Bremer K, Jornvall J, editors. The hierarchy of life. Amsterdam: Elsevier; 1989. p.389–403.
- Crampton WGR. Electric signal design and habitat preferences in a species rich assemblage of gymnotiform fishes from the upper Amazon basin. An Acad Bras Cienc. 1998; 70:805–47.
- Crampton WGR. Electroreception, electrogenesis and signal evolution. J Fish Biol. 2019; 95(1):92–134. https://doi.org/10.1111/jfb.13922
» https://doi.org/10.1111/jfb.13922 - Crampton WGR, Albert JS. Evolution of electric signal diversity in gymnotiform fishes. In: Ladich F, Collin SP, Moller P, Kapoor BG, editors. Communication in fishes. Enfield: Science Publishers; 2006. p.647–731.
- Crampton WGR, Rodríguez-Cattáneo A, Lovejoy NR, Caputi AA. Proximate and ultimate causes of signal diversity in the electric fish Gymnotus J Exp Biol. 2013; 216(13):2523–41. https://doi.org/10.1242/jeb.083261
» https://doi.org/10.1242/jeb.083261 - Crampton WGR, de Santana CD, Waddell JC, Lovejoy NR. A taxonomic revision of the Neotropical electric fish genus Brachyhypopomus (Ostariophysi: Gymnotiformes: Hypopomidae), with descriptions of 15 new species. Neotrop Ichthyol. 2016; 14(4):e150146. https://doi.org/10.1590/1982-0224-20150146
» https://doi.org/10.1590/1982-0224-20150146 - Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol. 2010; 8(2):193–246. http://dx.doi.org/10.1590/S1679-62252010000200001
» http://dx.doi.org/10.1590/S1679-62252010000200001 - Datovo A, Castro RMC. Anatomy and evolution of the mandibular, hyopalatine, and opercular muscles in characiform fishes (Teleostei: Ostariophysi). Zoology. 2012; 115(2):84–116. https://doi.org/10.1016/j.zool.2011.09.008
» https://doi.org/10.1016/j.zool.2011.09.008 - Datovo A, Rizzato PP. Evolution of the facial musculature in basal ray-finned fishes. Front Zool. 2018; 15(40):1–29. https://doi.org/10.1186/s12983-018-0285-6
» https://doi.org/10.1186/s12983-018-0285-6 - Datovo A, Vari RP. The jaw adductor muscle complex in teleostean fishes: evolution, homologies and revised nomenclature (Osteichthyes: Actinopterygii). PLoS ONE. 2013; 8(4):e60846. https://doi.org/10.1371/journal.pone.0060846
» https://doi.org/10.1371/journal.pone.0060846 - Datovo A, Vari RP. The adductor mandibulae muscle complex in lower teleostean fishes (Osteichthyes: Actinopterygii): comparative anatomy, synonymy, and phylogenetic implications. Zool J Linn Soc. 2014; 171(3):554–622. https://doi.org/10.1111/zoj.12142
» https://doi.org/10.1111/zoj.12142 - Dagosta FC, de Pinna MCC. The fishes of the Amazon: Distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1–163. Available from: http://digitallibrary.amnh.org/handle/2246/6940
» http://digitallibrary.amnh.org/handle/2246/6940 - Dietz PA. Beiträge zur Kenntnis der Kiefer - und Kiemenbogenmuskulatur der Teleostier. I, Die Kiefer und Kiemenbogenmuskeln der Acanthopterygier. Mitt Zool Sta Neapel. 1914; 22:99–162.
- Diogo R. Morphological evolution, adaptations, homoplasies, constraints, and evolutionary trends: catfishes as a case study on general phylogeny and macroevolution. Enfield: Science Publishers; 2004.
- Diogo R, Chardon M. Homologies among different adductor mandibulae sections of teleostean fishes, with special regard to catfishes (Teleostei: Siluriformes). J Morphol. 2000; 243(2):193–208. https://doi.org/10.1002/(SICI)1097-4687(200002)243:2<193::AID-JMOR8>3.0.CO;2-2
» https://doi.org/10.1002/(SICI)1097-4687(200002)243:2<193::AID-JMOR8>3.0.CO;2-2 - Diogo R, Doadrio I, Vandewalle P. Teleostean phylogeny based on osteological and myological characters. Int J Morphol. 2008; 26(3):463–522. http://dx.doi.org/10.4067/S0717-95022008000300001
» http://dx.doi.org/10.4067/S0717-95022008000300001 - Diogo R, Matthews LJ, Wood B. A major reason to study muscle anatomy: myology as a tool for evolutionary, developmental, and systematic biology. Biol Syst. 2012; 1(1):1000102. http://dx.doi.org/10.4172/2329-6577.1000102
» http://dx.doi.org/10.4172/2329-6577.1000102 - Diogo R, Vandewalle P. Review of superficial cranial musculature of catfishes, with comments on plesiomorphic states. In: Arratia G, Kapoor B, Chardon M, Diogo R, editors. Catfishes. Enfield: Science Publishers; 2003. p.47–70.
- Dutra GM. Sistemática de Eigenmanniinae (Teleostei: Gymnotiformes: Sternopygidae). [PhD Thesis]. Belém: Museu Paraense Emílio Goeldi; 2015.
- Dutra GM, Jerep FC, Vari RP, de Santana CD. The pseudotympanum in the Gymnotiformes (Teleostei, Ostariophysi, Otophysi): homology and evolution of a previously unexplored system in Neotropical electric fishes. Zool J Linn Soc. 2015; 174(1):114–29. https://doi.org/10.1111/zoj.12221
» https://doi.org/10.1111/zoj.12221 - Dutra GM, Peixoto LAWP, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021. https://doi.org/10.1111/jzs.12535
» https://doi.org/10.1111/jzs.12535 - Edgeworth FH. II. The development of some of the cranial muscles of ganoid fishes. Philos Trans R Soc Lond B Biol Sci. 1929; 217(440–449):39–89. https://doi.org/10.1098/rstb.1929.0002
» https://doi.org/10.1098/rstb.1929.0002 - Edgeworth FH. The cranial muscles of the vertebrates. Cambridge: Cambridge University Press; 1935.
- Elbassiouny AA, Schott RK, Waddell JC, Kolmann MA, Lehmberg ES, Nynatten AV, Crampton WGR, Chang BSW, Lovejoy NR. Mitochondrial genomes of the South American electric knifefishes (Order Gymnotiformes). Mitochondrial DNA B Resour. 2016; 1(1):401–03. https://doi.org/10.1080/23802359.2016.1174090
» https://doi.org/10.1080/23802359.2016.1174090 - Evans KM, Crampton WGR, Albert JS. Taxonomic revision of the deep channel electric fish genus Sternarchella (Teleostei: Gymnotiformes: Apteronotidae), with descriptions of two new species. Neotrop Ichthyol. 2017; 15(2):e160168. http://dx.doi.org/10.1590/1982-0224-20160168
» http://dx.doi.org/10.1590/1982-0224-20160168 - Evans KM, Bernt MJ, Kolmann MA, Ford KL, Albert JS. Why the long face? Static allometry in the sexually dimorphic phenotypes of Neotropical electric fishes. Zool J Linn Soc. 2019a; 186(3):633–49. https://doi.org/10.1093/zoolinnean/zly076
» https://doi.org/10.1093/zoolinnean/zly076 - Evans KM, Vidal-García M, Tagliacollo VA, Taylor SJ, Fenolio DB. Bony patchwork: mosaic patterns of evolution in the skull of electric fishes (Apteronotidae: Gymnotiformes). Integr Comp Biol. 2019b; 59(2):420–31. https://doi.org/10.1093/icb/icz026
» https://doi.org/10.1093/icb/icz026 - Farris J. A successive approximations approach to character weighting. Syst Zool. 1969; 18(4):374–85. https://doi.org/10.2307/2412182
» https://doi.org/10.2307/2412182 - Farris J. The retention index and the rescaled consistency index. Cladistics. 1989; 5(4):417–19. https://doi.org/10.1111/j.1096-0031.1989.tb00573.x
» https://doi.org/10.1111/j.1096-0031.1989.tb00573.x - Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985; 39(4):783–91.
- Ferraris CJ Jr., de Santana CD, Vari VP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
» https://doi.org/10.1590/1982-0224-20160067 - Fink S, Fink WL. Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc. 1981; 72(4):297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
» https://doi.org/10.1111/j.1096-3642.1981.tb01575.x - Fink S, Fink WL. Interrelationships of Ostariophysan fishes (Teleostei). In: Stiassny ML, Parenti LR, Johnson D, editors. Interrelationships of fishes. San Diego: Academic Press; 1996. p.209–49.
- Freihofer WC. Patterns of the ramus lateralis accessorius and their systematic significance in teleostean fishes. Stanf Ichthyol Bull; 1963(8):81–189.
- Freihofer WC. Cranial nerves of a percoid fish, Polycentrus schomburgkii (family Nandidae), a contribution to the morphology and classification of the order Perciformes. Occas Pap Calif Acad Sci. 1978; 128:1–178.
- Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2020. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Gayet M, Meunier FJ, Kirschbaum F Ellisella kirschbaumi Gayet & Meunier, 1991, gymnotiforme fossile de Bolivie et ses relations phylogénétiques au sein des formes actuelles. Cybium. 1994; 18(3):273–306.
- Geerinckx T, Huysentruyt F, Adriaens D. Ontogeny of the jaw and maxillary barbel musculature in the armoured catfish families Loricariidae and Callichthyidae (Loricarioidea, Siluriformes), with a discussion on muscle homologies. Zool J Linn Soc. 2009; 155(1):76–96. https://doi.org/10.1111/j.1096-3642.2008.00434.x
» https://doi.org/10.1111/j.1096-3642.2008.00434.x - Giribet G. Efficient tree searches with available algorithms. Evol Bioinform Online. 2007; 3:341–56. https://doi.org/10.1177/117693430700300014
» https://doi.org/10.1177/117693430700300014 - Goloboff PA, Catalano SA. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016; 32(3):221–38. https://doi.org/10.1111/cla.12160
» https://doi.org/10.1111/cla.12160 - Goloboff PA, Farris JS. Methods for quick consensus estimation. Cladistics. 2001; 17(1):26–34. https://doi.org/10.1111/j.1096-0031.2001.tb00102.x
» https://doi.org/10.1111/j.1096-0031.2001.tb00102.x - Goloboff PA, Farris JS, Källersjö M, Oxelman B, Ramírez MJ, Szumik CA. Improvements to resampling measures of group support. Cladistics. 2003; 19:324–32. https://doi.org/10.1111/j.1096-0031.2003.tb00376.x
» https://doi.org/10.1111/j.1096-0031.2003.tb00376.x - Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008; 24(5):774–86. https://doi.org/10.1111/j.1096-0031.2008.00217.x
» https://doi.org/10.1111/j.1096-0031.2008.00217.x - Gosline WA. Jaw muscle configuration in some higher teleostean fishes. Copeia. 1986; 1986(3):705–13. https://doi.org/10.2307/1444953
» https://doi.org/10.2307/1444953 - Hadley A. CombineZP: GNU public license software [Internet]; 2009. Available from: https://combinezp.software.informer.com/
» https://combinezp.software.informer.com/ - Haines RW. The posterior end of Meckel’s cartilage and related ossifications in bony fishes. J Cell Sci. 1937; 80(317):1–38. https://doi.org/10.1242/jcs.s2-80.317.1
» https://doi.org/10.1242/jcs.s2-80.317.1 - Hilton EJ, Cox Fernandes C. Sexual dimorphism in Apteronotus bonapartii (Gymnotiformes: Apteronotidae). Copeia. 2006; 2006(4):826–33. http://dx.doi.org/10.1643/0045-8511(2006)6[826:SDIABG]2.0.CO;2
» http://dx.doi.org/10.1643/0045-8511(2006)6[826:SDIABG]2.0.CO;2 - Hilton EJ, Cox Fernandes C, Sullivan JP, Lundberg JG, Campos-da-Paz R. Redescription of Orthosternarchus tamandua (Boulenger, 1898) (Gymnotiformes, Apteronotidae), with reviews of its ecology, electric organ discharges, external morphology, osteology, and phylogenetic affinities. Proc Acad Nat Sci Phila. 2007; 156(1):1–25. https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2
» https://doi.org/10.1635/0097-3157(2007)156[1:ROOTBG]2.0.CO;2 - Ho D. Notepad++. 7.5.3 [Internet]; 2019. Available from: http://notepad-plus-plus.org
» http://notepad-plus-plus.org - Hopkins CD. Design features for electric communication. J Exp Biol. 1999; 202(10):1217–28. https://doi.org/10.1242/jeb.202.10.1217
» https://doi.org/10.1242/jeb.202.10.1217 - Howes JG. Cranial muscles of loricarioid catfishes, their homologies and value as taxonomic characters (Teleostei: Siluroidei). Bull Br Mus Nat Hist Zool. 1983; 45:309–45. https://doi.org/10.5962/bhl.part.28003
» https://doi.org/10.5962/bhl.part.28003 - de la Hoz E, Chardon M. Skeleton, muscles, ligaments and swim-bladder of a gymnotid fish, Sternopygus macrurus Bloch & Schneider (Ostariophysi: Gymnotoidei). Bull Soc R Sci Liège. 1984; 53:9–53.
- Huysentruyt F, Moerkerke B, Devaere S, Adriaens D. Early development and allometric growth in the armoured catfish Corydoras aeneus (Gill, 1858). Hydrobiologia. 2009; 627:45–54. https://doi.org/10.1007/s10750-009-9714-z
» https://doi.org/10.1007/s10750-009-9714-z - Ivanyisky III SJ, Albert JS. Systematics and biogeography of Sternarchellini (Gymnotiformes: Apteronotidae): Diversification of electric fishes in large Amazonian rivers. Neotrop Ichthyol. 2014; 12(3):565–84. http://dx.doi.org/10.1590/1982-0224-20130159
» http://dx.doi.org/10.1590/1982-0224-20130159 - Keeffe R, Hilton EJ, De Souza MJFT, Cox Fernandes C. Cranial morphology and osteology of the sexually dimorphic electric fish, Compsaraia samueli Albert & Crampton (Apteronotidae, Gymnotiformes), with comparisons to C. compsa (Mago-Leccia). Zootaxa. 2019; 4555(1):101–12. https://doi.org/10.11646/zootaxa.4555.1.8
» https://doi.org/10.11646/zootaxa.4555.1.8 - Koepfli KP, Wayne RK. Type I Sts markers are more informative than cytochrome b in phylogenetic reconstruction of the Mustelidae (Mammalia: Carnivora). Syst Biol. 2003; 52(5):571–93. https://doi.org/10.1080/10635150390235368
» https://doi.org/10.1080/10635150390235368 - Lambkin CL. Partitioned Bremer support localises significant conflict in bee flies (Diptera: Bombyliidae: Anthracinae). Invertebr Syst. 2004; 18:351–60. https://doi.org/10.1071/IS04004
» https://doi.org/10.1071/IS04004 - Lambkin CL, Lee MSY, Winterton SL, Yeates DK. Partitioned Bremer support and multiple trees. Cladistics. 2002; 18(4):436–44. https://doi.org/10.1111/j.1096-0031.2002.tb00159.x
» https://doi.org/10.1111/j.1096-0031.2002.tb00159.x - Lannoo MJ, Maler L, Tinner B. Ganglion cell arrangement and axonal trajectories in the anterior lateral line nerve of the weakly electric fish Apteronotus leptorhynchus (Gymnotiformes). J Comp Neurol. 1989; 280(3):331–42. https://doi.org/10.1002/cne.902800302
» https://doi.org/10.1002/cne.902800302 - Lavoué S, Miya M, Inoue JG, Saitoh K, Ishiguro NB, Nishida M. Molecular systematics of the gonorynchiform fishes (Teleostei) based on whole mitogenome sequences: implications for higher-level relationships within the Otocephala. Mol Phylogenet Evol. 2011; 37(1):165–77. https://doi.org/10.1016/j.ympev.2005.03.024
» https://doi.org/10.1016/j.ympev.2005.03.024 - Lundberg JG, Cox Fernandes C. A new species of South American ghost knifefish (Apteronotidae: Adontosternarchus) from the Amazon Basin. Proc Acad Nat Sci Phila. 2007; 156(1):27–37. https://doi.org/10.1635/0097-3157(2007)156[27:ANSOSA]2.0.CO;2
» https://doi.org/10.1635/0097-3157(2007)156[27:ANSOSA]2.0.CO;2 - Lundberg JG, Mago-Leccia F. A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proc Acad Nat Sci Phila. 1986; 138(1):53–85. https://www.jstor.org/stable/4064852
» https://www.jstor.org/stable/4064852 - Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cien Venez. 1978; 29:1–51.
- Mago-Leccia F. Electric fishes of the continental water of America: classification and catalogue of the electric fishes of the order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Caracas: Biblioteca de la Academia de Ciencias, Fisicas, Matematicas y Naturales; 1994.
- Mago-Leccia F, Zaret TM. The taxonomic status of Rhabdolichops troscheli (Kaup, 1856), and speculations on gymnotiform evolution. Environ Biol Fishes. 1978; 3:379–84. https://doi.org/10.1007/BF00000530
» https://doi.org/10.1007/BF00000530 - Maldonado-Ocampo JA, López-Fernández H, Taphorn DC, Bernard CR, Crampton WGR, Lovejoy NR Akawaio penak, a new genus and species of Neotropical electric fish (Gymnotiformes, Hypopomidae) endemic to the upper Mazaruni River in the Guiana Shield. Zool Scr. 2014; 43(1):24–33. http://dx.doi.org/10.1111/zsc.12035
» http://dx.doi.org/10.1111/zsc.12035 - Miyake T, McEachran JD, Hall BK. Edgeworth’s legacy of cranial muscle development with an analysis of muscles in the ventral gill arch region of batoid fishes (Chondrichthyes: Batoidea). J Morphol. 1992; 212(3):213–56. https://doi.org/10.1002/jmor.1052120304
» https://doi.org/10.1002/jmor.1052120304 - Moller P. Electric fishes: history and behavior. London: Chapman & Hall; 1995.
- Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M. Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol. 2011; 11(177):1–25. https://doi.org/10.1186/1471-2148-11-177
» https://doi.org/10.1186/1471-2148-11-177 - Ortí G, Meyer A. Molecular evolution of ependymin and the phylogenetic resolution of early divergences among euteleost fishes. Mol Biol Evol. 1996; 13(4):556–73. https://doi.org/10.1093/oxfordjournals.molbev.a025616
» https://doi.org/10.1093/oxfordjournals.molbev.a025616 - Ortí G, Meyer A. The radiation of characiform fishes and the limits of resolution of mitochondrial ribosomal DNA sequences. Syst Biol. 1997; 46(1):75–100. https://doi.org/10.1093/sysbio/46.1.75
» https://doi.org/10.1093/sysbio/46.1.75 - Osse JWM. Functional morphology of the head of the perch (Perca fluviatilis L.): an electromyographic study. Neth J Zool. 1969; 19(3):289–392. https://doi.org/10.1163/002829669X00134
» https://doi.org/10.1163/002829669X00134 - Pastana MNL, Bockmann FA, Datovo A. The cephalic lateral-line system of Characiformes (Teleostei: Ostariophysi): anatomy and phylogenetic implications. Zool J Linn Soc. 2020; 189(1):1–46. https://doi.org/10.1093/zoolinnean/zlz105
» https://doi.org/10.1093/zoolinnean/zlz105 - Pastana MNL, Johnson GD, Datovo A. Comprehensive phenotypic phylogenetic analysis supports the monophyly of stromateiform fishes (Teleostei: Percomorphacea). Zool J Linn Soc. 2021; zlab058. https://doi.org/10.1093/zoolinnean/zlab058
» https://doi.org/10.1093/zoolinnean/zlab058 - Patterson C. Morphological characters and homology. In: Joysey KA, Friday AE, editors. Problems of phylogenetic reconstruction. London: Academic Press; 1982. p.21–74.
- Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): monophyly and description of seven new species. Zool J Linn Soc. 2015; 175(2):384–414. https://doi.org/10.1111/zoj.12274
» https://doi.org/10.1111/zoj.12274 - Peixoto LAW, Datovo A, Campos-da-Paz R, de Santana CD, Menezes NA. Anatomical, taxonomic, and phylogenetic reappraisal of a poorly know ghost knifefish, Tembeassu marauna (Ostariophysi: Gymnotiformes), using X-ray microcomputed tomography. PLoS ONE. 2019; 14(11):e0225342. https://doi.org/10.1371/journal.pone.0225342
» https://doi.org/10.1371/journal.pone.0225342 - Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
» https://doi.org/10.1371/journal.pone.0220287 - Peixoto LAW, Pastana MNL, Ballen GA. New species of glass knifefish genus Eigenmannia (Gymnotiformes: Sternopygidae) with comments on the morphology and function of the enlarged cephalic lateral-line canals of Sternopygidae. J Fish Biol. 2020; 1–12. https://doi.org/10.1111/jfb.14564
» https://doi.org/10.1111/jfb.14564 - Peña C, Wahlberg N, Weingartner E, Kodandaramaiah U, Nylin S, Freitas AVL, Brower AVZ. Higher level phylogeny of Satyrinae butterflies (Lepidoptera: Nymphalidae) based on DNA sequence data. Mol Phylogenet Evol. 2006; 40(1):29–49. https://doi.org/10.1016/j.ympev.2006.02.007
» https://doi.org/10.1016/j.ympev.2006.02.007 - de Pinna MCC. Concepts and tests of homology in the cladistic paradigm. Cladistics. 1991; 7(4):367–94. https://doi.org/10.1111/j.1096-0031.1991.tb00045.x
» https://doi.org/10.1111/j.1096-0031.1991.tb00045.x - Rapp Py-Daniel LH, Cox Fernandes C. Dimorfismo sexual em Siluriformes e Gymnotiformes (Ostariophysi) da Amazônia. Acta Amaz. 2005; 35(1):97–110. https://doi.org/10.1590/S0044-59672005000100015
» https://doi.org/10.1590/S0044-59672005000100015 - Ridewood WG. On the cranial osteology of the clupeoid fishes. J Zoo. 1904; 74:448–93.
- Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
» https://doi.org/10.1643/ASIHCODONS2020 - Saitoh K, Miya M, Inoué JG, Ishiguro NB, Nishida M. Mitochondrial genomics of ostariophysan fishes: perspectives on phylogeny and biogeography. J Mol Evol. 2003; 56:64–472. https://doi.org/10.1007/s00239-002-2417-y
» https://doi.org/10.1007/s00239-002-2417-y - de Santana CD, Crampton WGR. Phylogenetic interrelationships, taxonomy, and reductive evolution in the Neotropical electric fish genus Hypopygus (Teleostei, Ostariophysi, Gymnotiformes). Zool J Linn Soc. 2011; 163(4):1096–156. https://doi.org/10.1111/j.1096-3642.2011.00736.x
» https://doi.org/10.1111/j.1096-3642.2011.00736.x - de Santana CD, Vari RP. Electric fishes of the genus Sternarchorhynchus (Teleostei, Ostariophysi, Gymnotiformes); phylogenetic and revisionary studies. Zool J Linn Soc. 2010; 159(1):223–371. https://doi.org/10.1111/j.1096-3642.2009.00588.x
» https://doi.org/10.1111/j.1096-3642.2009.00588.x - de Santana CD, Vari RP. New species of Adontosternarchus (Gymnotiformes, Apteronotidae) from the Rio Purus Basin, Brazil. Copeia. 2012; 2012(3):535–40. https://doi.org/10.1643/CI-11-135
» https://doi.org/10.1643/CI-11-135 - de Santana CD, Vari RP, Wosiacki WB. The untold story of the caudal skeleton in the electric eel (Ostariophysi: Gymnotiformes: Electrophorus). PLoS ONE. 2013; 8(7):e68719. https://doi.org/10.1371/journal.pone.0068719
» https://doi.org/10.1371/journal.pone.0068719 - Starks EC. The sesamoid articular: a bone in the mandible of fishes. Stanford: Stanford University Press; 1916.
- Tagliacollo VA, Bernt MJ, Craig JM, Oliveira C, Albert JS. Model-based total evidence phylogeny of Neotropical electric knifefishes (Teleostei, Gymnotiformes). Mol Phylogenet Evol. 2016; 95:20–33. https://doi.org/10.1016/j.ympev.2015.11.007
» https://doi.org/10.1016/j.ympev.2015.11.007 - Takahasi N. On the homology of the cranial muscles of the cypriniform fishes. J Morphol. 1925; 40(1):1–109. https://doi.org/10.1002/jmor.1050400102
» https://doi.org/10.1002/jmor.1050400102 - Triques ML. Filogenia dos gêneros de Gymnotiformes (Actinopterygii, Ostariophysi), com base em caracteres esqueléticos. Comun Mus Ciênc PUCRS, Sér Zool. 1993; 6:85–130.
- Triques ML. Análise cladística dos caracteres de anatomia externa e esquelética de Apteronotidae (Teleostei: Gymnotiformes). Lundiana. 2005; 6(2):121–49.
- Triques ML Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool. 2011; 61(3):299–306.
- Vari RP, de Santana CD, Wosiacki WB. South American electric knifefishes of the genus Archolaemus (Ostariophysi, Gymnotiformes): undetected diversity in a clade of rheophiles. Zool J Linn Soc. 2012; 165(3):670–99. https://doi.org/10.1111/j.1096-3642.2012.00827.x
» https://doi.org/10.1111/j.1096-3642.2012.00827.x - Vischer HA, Lannoo MJ, Heiligenberg W. Development of the electrosensory nervous system in Eigenmannia (Gymnotiformes): I. The peripheral nervous system. J Comp Neurol. 1989; 290:16–40. https://doi.org/10.1002/cne.902900103
» https://doi.org/10.1002/cne.902900103 - Weitzman SH. The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichth Bull. 1962; 8:1–77.
- Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Acad Nat Sci Phila. 1974a; 125(12):225–317. Available from: https://www.jstor.org/stable/4064691
» https://www.jstor.org/stable/4064691 - Winterbottom R. The familial phylogeny of the Tetraodontiformes (Acanthopterygii: Pisces) as evidenced by their comparative myology. Smithson Contrib Zool. 1974b; 155:1–201. https://doi.org/10.5479/si.00810282.155
» https://doi.org/10.5479/si.00810282.155 - Wu KY, Shen SC. Review of the teleostean adductor mandibulae and its significance to the systematic positions of the Polymixiiformes, Lampridiformes, and Triacanthoidei. Zool Stud. 2004; 43(4):712–36.
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Peixoto LAW, de Pinna M. Patterns of diversification and phylogenetic structure in the dorsolateral head musculature of Neotropical electric eels (Ostariophysi: Gymnotiformes), with a myological synonymy. Neotrop Ichthyol. 2022; 20(1):e210009. https://doi.org/10.1590/1982-0224-2021-0009
Edited-by
Publication Dates
-
Publication in this collection
01 Apr 2022 -
Date of issue
2022
History
-
Received
10 Jan 2021 -
Accepted
26 Oct 2021