Abstracts
The troglobitic electric fish Eigenmannia vicentespelaea, endemic to a single cave-system and included in the Brazilian Red List of Threatened Fauna, was studied in relation to population densities and habitat. For comparison, we used the epigean species, E. trilineata. We verify if the population densities recorded for E. vicentespelaea follow the pattern observed for other subterranean fishes; if there are seasonal fluctuations in these densities and which environmental variables explain the densities variation. We estimated abundances and population densities during three consecutive dry seasons concomitant with habitat description and physicochemical variables measurements. For E. vicentespelaea: in six stream reaches in the São Vicente II cave. For E. trilineata: from counts of active fish in rio da Lapa. The mean population density recorded for E. vicentespelaea is considered low (0.17 ind.m-2), similar to those of E. trilineata (0.13 ind.m-2), without significant differences between the years, but with marked fluctuations during dry seasons within each year, suggesting seasonality. Estimated population size of E. vicentespelaea is considered low (270 individuals in average). Depth, water current and substrate, allied to plant debris explain better the variation of densities. Urgent actions for E. vicentespelaea conservation include protection of headsprings in Terra Ronca State Park.
Bambuí karst; Cave habitat; Conservation; Sternopygidae; Troglobitic species
O peixe troglóbio Eigenmannia vicentespelaea, endêmico de um único sistema de cavernas e incluído na Lista Brasileira de Fauna Ameaçada foi estudado em relação às densidades populacionais e hábitat. Para comparação utilizamos a espécie epígea E. trilineata. Verificamos se as densidades populacionais registradas para E. vicentespelaea seguem o padrão observado para outros peixes subterrâneos; se há flutuações sazonais nestas densidades e quais variáveis ambientais explicam as variações nas densidades. Estimamos as abundâncias e densidades populacionais ao longo de três estações secas consecutivas, concomitantes à descrição do habitat e das variáveis físico-químicas. Para E. vicentespelaea: em seis trechos de rio ao longo da caverna São Vicente II. Para E. trilineata: na contagem de peixes ativos no rio da Lapa. A densidade populcional média registrada para E. vicentespelaea é baixa (0,17 ind.m-2), similar à observada para E. trilineata (0,13 ind.m-2), sem diferenças significativas entre os anos, mas com flutuações marcantes ao longo das estações secas dentro de cada ano, sugerindo sazonalidade. O tamanho populacional estimado para E. vicentespelaea é considerado pequeno (270 indivíduos em média). Profundidade, correnteza, substrato, aliadas à concentração de detrito vegetal explicam melhor a variação nas densidades. Ações urgentes para conservação de E. vicentespelaea incluem a proteção das nascentes do Parque Estadual de Terra Ronca.
Introduction
Communities established in subterranean habitats, which are highly fragmented and completely dark in deep (aphotic) zones, tend to contain scarce food (Poulson & White, 1969Poulson, T. L. & W. B. WHITE. 1969. The cave environment. Science, 165: 971-980.). These communities are under strong selective pressures and are naturally isolated, favoring the survival of relict species (Gibert & Deharveng, 2002Gibert, J. & L. Deharveng. 2002. Subterranean ecosystems: a truncated functional biodiversity. BioScience, 52: 473-481.). However, due to their intrinsic fragility and vulnerability to disturbance, this environment is among the most threatened in the world (Sket, 1999Sket, B. 1999. The nature of biodiversity in hypogean waters and how it is endangered. Biodiversity Conservation, 8: 1319-1338.). Therefore, the study of such ecosystems and their components is of great scientific and applied interest for conservation purposes.
Fish are among the most conspicuous components of cave aquatic communities, with more than 170 troglobitic species around the world primarily in China and the Neotropical region (Proudlove, 2010Proudlove, G. S. 2010. Biodiversity and distribution of the subterranean fishes of the world. Pp. 41-64. In: Trajano, E., M. E. Bichuette & B. G. Kapoor (Eds.). Biology of subterranean fishes. Enfield, Science Publishers.). Troglobites are exclusively subterranean organisms, which generally have specializations related to their isolation in the subterranean realm (Wilkens, 2010Wilkens, H. 2010. Genes, modules and the evolution of cave fish. Heredity, 105: 413-422.), the most conspicuous being the reduction or loss of melanic pigmentation and eyes, i.e., the classic troglomorphisms (Holsinger & Culver, 1988Holsinger, J. R. & D. C. Culver. 1988. The invertebrate cave fauna of Virginia and part of eastern Tennessee: zoogeography and ecology. Brimleyana, 14: 1-162.). The subterranean or hypogean environment comprises interconnected subsurface spaces, allowing for dispersion of the species that inhabit these areas (Juberthie, 2000Juberthie, C. 2000. The diversity of the karstic and pseudokarstic hypogean habitats in the world. Pp. 17-39. In: Wilkens, H., D. C. Culver & W. F. Humphreys (Eds.). Ecosystems of the world, subterranean ecosystems. Amsterdan, Elsevier Academic Press.). This environment is characterized by faunistic originality and high endemism (Gibert & Deharveng, 2002Gibert, J. & L. Deharveng. 2002. Subterranean ecosystems: a truncated functional biodiversity. BioScience, 52: 473-481.), due to unique evolutionary events (Culver & Pipan, 2009Culver, D. C. & T. Pipan. 2009. The biology of caves and other subterranean habitats. New York, Oxford University Press.).
Gymnotiformes are an important component of the night-active ichthyofauna in South and Central America, corresponding to a monophyletic group based, among other characters, on the ability of producing and detecting weak electric fields (Mago-Leccia, 1994Mago-Leccia, F. 1994. Electric fishes of the continental waters of America. Classification and catalogue of the electric fishes of the Order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Biblioteca de la Academia de Ciencias Fisicas, Matematicas y Naturales, Caracas, 29: 1-225.; Alves-Gomes et al., 1995Alves-Gomes, J. A., G. Ortí, M. Haygood, A. Meyer & W. Heiligenberg. 1995. Phylogenetic analysis of the South American electric fishes (Order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Molecular Biology and Evolution, 12: 298-318.; Albert & Campos-da-Paz, 1998Albert, J. S. & R. Campos-da-Paz. 1998. Phylogenetic systematics of american knifefishes: a review of the available data. Pp. 409-435. In: Malabarba, L., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Phylogeny and classification of Neotropical fishes. Porto Alegre, Edipucrs.; Albert, 2001Albert, J. S. 2001. Species diversity and phylogenetic systematics of american knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications Museum of Zoology, University of Michigan, 190: 1-127.). Adaptations related to their predominantly nocturnal activity (see Steinbach, 1970Steinbach, A. B. 1970. Diurnal movements and discharge characteristics of eletric gymnotid fishes in the Rio Negro, Brazil. Biological Bulletin, 138: 200-210.; Bennett, 1971aBennett, M. V. L. 1971a. Electric organs. Pp. 349-491. In: Hoar, W. S. & D. J. Randall (Eds.). Fish physiology. New York, Academic Press., 1971bBennett, M. V. L. 1971b. Electroreception. Pp. 493-574. In: Hoar, W. S. & D. J. Randall (Eds.). Fish physiology. New York, Academic Press.; Hopkins, 1974Hopkins, C. D. 1974. Electric communication in fish. American Scientist, 62: 426-437.; Westby, 1988Westby, G. W. M. 1988. The ecology, discharge diversity and predatory behavior of gymnotiform electric fish in coastal streams of French Guiana. Behavioral Ecology and Sociobiology, 22: 341-354.; Kramer, 1990Kramer, B. 1990. Sexual signs in electric fishes. Trends in Ecology and Evolution, 5: 247-250., 1996Kramer, B. 1996. Electric signaling and communication in weakly electric fishes (Gymnotiformes) of South America. Pp. 23-30. In: Val, A. L. & D. T. Randall (Eds.). Physiology and biochemistry of the fishes of the Amazon. Manaus, INPA.), favor the colonization of permanently dark habitats such as those in the subterranean (hypogean) realm. In fact, gymnotiforms are frequently observed in Brazilian caves, with populations regularly found in these habitats, apparently as troglophiles - species with source populations both in hypogean and epigean (surface) habitats, with individuals regularly commuting between them, promoting the introgression of genes selected under epigean regimes into subterranean populations (Trajano, 2012Trajano, E. 2012. Ecological classification of subterranean organisms. Pp. 275-277. In: White, W. B. & D. C. Culver (Eds.). Encyclopedia of caves. Oxford, Elsevier Academic Press.). Among the subterranean gymnotiforms, the sternopygid Eigenmannia vicentespelaea Triques, 1996, from the São Domingos karst area, is distinguished by being the only species known so far to exhibit regression of eyes and melanic pigmentation (classical troglomorphisms, sensu Holsinger & Culver, 1988Holsinger, J. R. & D. C. Culver. 1988. The invertebrate cave fauna of Virginia and part of eastern Tennessee: zoogeography and ecology. Brimleyana, 14: 1-162.), indicative of the troglobitic condition (Bichuette & Trajano, 2006Bichuette, M. E. & E. Trajano. 2006. Morphology and distribution of the cave knifefish Eigenmannia vicentespelaea Triques, 1996 (Gymnotiformes: Sternopygidae) from Central Brazil, with an expanded diagnosis and comments on subterranean evolution. Neotropical Ichthyology, 4: 99-105.).
Ecological studies on gymnotiforms are scarce (Lissmann, 1961Lissmann, H. W. 1961. Ecological studies on gymnotids. Pp. 215-226. In: Chagas, C. & A. P. Carvalho (Eds.). Bioelectrogenesis. Amsterdan, Elsevier.; Steinbach, 1970Steinbach, A. B. 1970. Diurnal movements and discharge characteristics of eletric gymnotid fishes in the Rio Negro, Brazil. Biological Bulletin, 138: 200-210.; Hagedorn, 1988Hagedorn, M. 1988. Ecology and behavior of a pulse-type electric fish, Hypopomus occidentalis (Gymnotiformes, Hypopomidae), in a fresh-water stream in Panama. Copeia, 1988: 324-335.; Westby, 1988Westby, G. W. M. 1988. The ecology, discharge diversity and predatory behavior of gymnotiform electric fish in coastal streams of French Guiana. Behavioral Ecology and Sociobiology, 22: 341-354.; Alves-Gomes, 1997; Crampton, 1998Crampton, W. G. R. 1998. Electric signal design and habitat preferences in a species rich assemblage of gymnotiform fishes from the Upper Amazon basin. Anais da Academia Brasileira de Ciências, 70: 805-847.), and few data on basic aspects of their natural history have been available up to now, most of which consider aspects of their reproduction and feeding biology (Giora et al., 2005Giora, J., C. B. Fialho & A. P. Dufech. 2005. Feeding habit of Eigenmannia trilineata Lopez & Castello, 1966 (Teleostei: Sternopygidae) of Parque Estadual de Itapuã, RS, Brazil. Neotropical Ichthyology, 3: 291-298.; Giora & Fialho, 2009Giora, J. & C. B. Fialho. 2009. Reproductive biology of weakly electric fish Eigenmannia trilineata López and Castello, 1966 (Teleostei, Sternopygidae). Brazilian Archives of Biology and Technology, 52: 617-628.; Schaan et al., 2009Schaan, A. B.; J. Giora & C. B. Fialho. 2009. Reproductive biology of the Neotropical electric fish Brachyhypoppomus draco (Teleostei: Hypoppomidae) from southern Brazil. Neotropical Ichthyology, 7: 737-744.; Giora et al., 2011Giora, J., H. M. Tarasconi & C. B. Fialho. 2011. Reproduction and feeding habits of the highly seasonal Brachyhypopomus bombilla (Gymnotiformes: Hypoppomidae) from southern Brazil, with evidence for a dormancy period. Environmental Biology of Fishes, 94: 649-662.). Further, relatively few troglobitic fishes have been studied in detail, focusing on population traits such as population sizes and densities or individual movements and growth (as a life history component) (see Trajano, 2001Trajano, E. 2001. Ecology of subterranean fishes: an overview. Pp. 133-160. In: Romero, A. (Ed.). The Biology of hypogean fishes. Dordrecht, Kluwer Academic Publishers.; Trajano & Bichuette, 2010Trajano E. & M. E. Bichuette. 2010. Subterranean fishes of Brazil. Pp. 331-355. In: Trajano, E., M. E. Bichuette & B. G. Kapoor (Eds.). Biology of subterranean fishes. Enfield, Science Publishers.). Such knowledge is not only scientifically relevant but also fundamental for the establishment of efficient conservation policies. A classification of cave fishes based on population densities was proposed by Trajano (2001)Trajano, E. 2001. Ecology of subterranean fishes: an overview. Pp. 133-160. In: Romero, A. (Ed.). The Biology of hypogean fishes. Dordrecht, Kluwer Academic Publishers.: (1) species with low population densities (<0.1 ind.m-2); (2) species with intermediate population densities (0.1 - 1.0 ind.m-2); and (3) species with high population densities (>1.0 ind.m-2). In Brazil, most troglobitic populations are in the first category and there is no record for the last one, except for some special cases such as local concentration of fishes in pools or small subterranean stream reaches at the end of the dry season (Trajano & Bichuette, 2010Trajano E. & M. E. Bichuette. 2010. Subterranean fishes of Brazil. Pp. 331-355. In: Trajano, E., M. E. Bichuette & B. G. Kapoor (Eds.). Biology of subterranean fishes. Enfield, Science Publishers.).
In this study we established three central questions relative to population parameters of E. vicentespelaea: 1) Do population densities of E. vicentespelaea follow the pattern observed for other subterranean fishes, and are they comparable with those recorded for a related epigean species, E. trilineata? 2) Are there seasonal fluctuations in the population densities of E. vicentespelaea? 3) Which environmental variables explain the variation in population densities of E. vicentespelaea? To answer these questions we investigated, during three consecutive dry seasons, aspects of population biology (population densities) and habitat characteristics of E. vicentespelaea. For comparison, we chose its epigean relative, E. trilineata, which occurs in the same karstic area, but in another watershed.
Materials and Methods
Studied species. Eigenmannia vicentespelaea was described in 1996 based on two specimens collected by speleologists in 1978 at São Vicente II cave, Paranã basin. It has reduced eyes compared to epigean Eigenmannia species, such as E. trilineata, found in the same area, but not in the São Vicente watershed. Our morphological study (Bichuette & Trajano, 2006Bichuette, M. E. & E. Trajano. 2006. Morphology and distribution of the cave knifefish Eigenmannia vicentespelaea Triques, 1996 (Gymnotiformes: Sternopygidae) from Central Brazil, with an expanded diagnosis and comments on subterranean evolution. Neotropical Ichthyology, 4: 99-105.) showed prominent variability of pigmentation and eye condition (from pigmented individuals with eyes to depigmented individuals with no externally visible eyes) and a maximum length of 164.5 mm. Because such variation is not observed in epigean Eigenmannia species, it supports the troglobitic status for E. vicentespelaea, indicating isolation determined by morphological differentiation, consistent with geographic isolation in the São Vicente cave system. The epigean population herein chosen for comparison was that occurring at rio da Lapa, part of Paranã basin, identified as E. trilineata. Eigenmannia trilineata has a wide distribution, from the rio Paraná to the rio Paraguay basins, with a maximum length of 250 mm (Albert, 2003Albert, J. S. 2003. Family Sternopygidae. Pp. 487-491. In: Reis R. E., S. O. Kullander & C. J. Ferraris Jr (Eds.). Checklist of the Freshwater Fishes of South and Central America. Porto Alegre, Edipucrs.). Identification of both species follows the original descriptions and thus their diagnosis, as well as additional morphological studies (Lopez & Castello, 1966Lopez, R. B. & H. P. Castello. 1966. Eigenmannia trilineata (Teleostomi, Sternopygidae), nueva especie hallada en el Río de La Plata. Comunicaciones del Museo Argentino de Ciencias Naturales Bernardino Rivadavia, 4: 7-12.; Triques, 1996Triques, M. L. 1996. Eigenmannia vicentespelea, a new species of cave dwelling electrogenic neotropical fish (Ostariophisi: Gymnotiformes: Sternopygidae). Revue Française d'Aquariologie, 23: 1-4.; Bichuette & Trajano, 2006Bichuette, M. E. & E. Trajano. 2006. Morphology and distribution of the cave knifefish Eigenmannia vicentespelaea Triques, 1996 (Gymnotiformes: Sternopygidae) from Central Brazil, with an expanded diagnosis and comments on subterranean evolution. Neotropical Ichthyology, 4: 99-105.).
Study area. The São Domingos karst area, ca. 500 km2, is part of the Bambui geological group (Auler & Farrant, 1996Auler, A. & A. R. Farrant. 1996. A brief introduction to karst and caves in Brazil. Proceedings of the University of Bristol Spelaeological Society, 20: 187-200.) and the upper rio Tocantins basin. It is situated in the Cerrado domain (Savannah-like vegetation), characterized by a tropical semi-humid climate, with 5-6 dry months from April/May to October (Nimer, 1989Nimer, E. 1989. Climatologia do Brasil. Rio de Janeiro, Supren.). The study area is located within the Terra Ronca State Park (46o10'-46o30'S, 13o30'-13o50'W), São Domingos municipality, in the Goiás State, Brazil (Fig. 1) and is considered a global high diversity area of subterranean ichthyofauna, with several stable cave populations, including seven troglobitic nominal species and many troglophilic populations (Fernandez & Bichuette, 2002; Bichuette & Trajano, 2003Bichuette, M. E. & E. Trajano. 2003. Epigean and subterranean ichthyofauna from the São Domingos karst area, Upper Tocantins River basin, Central Brazil. Journal of Fish Biology, 63: 1100-1121., 2004; Trajano et al., 2004Trajano, E., R. E. Reis & M. E. Bichuette. 2004. Pimelodella spelaea: A new cave catfish from Central Brazil, with data on ecology and evolutionary considerations (Siluriformes: Heptapteridae). Copeia, 2004: 315-325.).
Map of São Domingos karst area showing the localities of Eigenmannia vicentespelaea and E. trilineata in the rio Paranã basin, upper rio Tocantins, with neighboring states. Inset: map of South America.
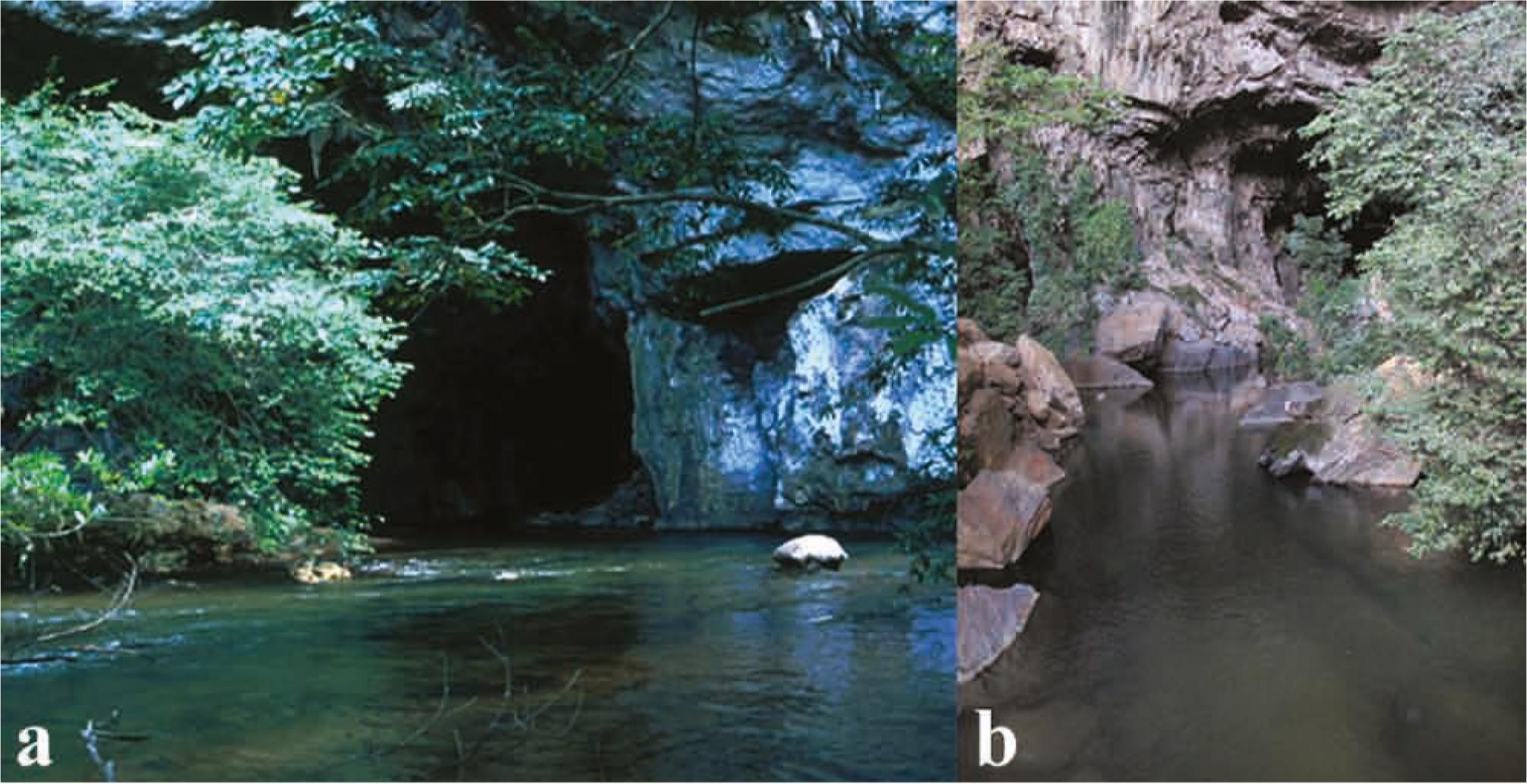
Populations of E. vicentespelaea have been found in two caves from a single integrated cave system (with conduits extending continuously between the input and output points of karst rock, Ford & Williams, 2007): São Vicente I cave, upstream, and São Vicente II cave, downstream, forming the São Vicente cave system and separated by a 500 m long epigean stream reach (Fig. 2a). We studied E. vicentespelaea from São Vicente II cave (SVII cave) (13º 5'S, 46º 4'W), with 4,703 m of mapped passageways; the river conduit is approximately 700 m long and 3 m in width, totaling an area of approximately 2,100m2. In this cave, E. vicentespelaea occurs in the twilight zone (regions with indirect light incidence) and the aphotic zone (regions of permanent darkness).
a) Sinkhole of the São Vicente cave system (entrance of the São Vicente I cave), type-locality of Eigenmannia vicentespelaea. Photography: A. Gambarini; (b) rio da Lapa showing the place of E. trilineata occurrence, a typical slow to moderate water current habitat. Photography: M. E. Bichuette.
Eigenmannia trilineata has been recorded in the epigean reach of rio da Lapa (RL, Fig. 2b) (13º44'S 46º21'W, Fig. 2), upstream of the sinkhole going to the Terra Ronca cave, and in some subterranean stream reaches of the São Mateus III and Angélica caves (Bichuette & Trajano, 2003Bichuette, M. E. & E. Trajano. 2003. Epigean and subterranean ichthyofauna from the São Domingos karst area, Upper Tocantins River basin, Central Brazil. Journal of Fish Biology, 63: 1100-1121.), which belong to different watersheds. Eigenmannia trilineata has not been found in the São Vicente cave system and is physically isolated from E. vicentespelaea. The above-mentioned streams run westward and parallel to each other, both into the rio Paranã, one of the main tributaries of the upper rio Tocantins.
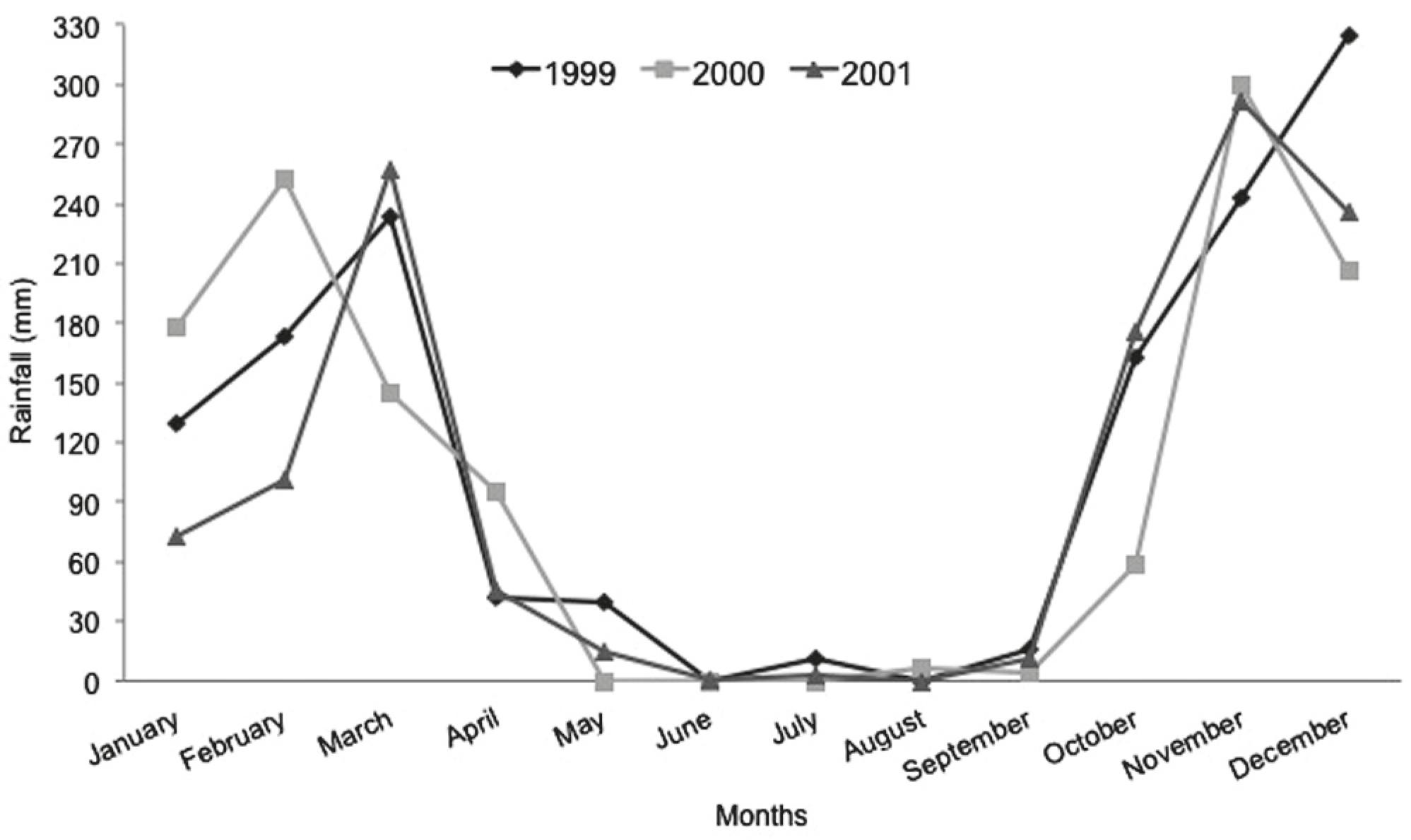
Density estimation. We studied the population of E. vicentespelaea through visual censuses during seven field trips over three annual cycles (1999, 2000 and 2001), always in the dry season. It is not safe to carry out fieldwork in the rainy season due to the occurrence of drastic flash floods in the cave systems of Goiás, which make most of the habitat inaccessible. We defined three periods in the dry season: beginning (May - two samples; 2000 and 2001), middle (July and August - three samples; 1999, 2000 and 2001) and end (September - two samples; 1999 and 2000). For an accurate determination of the beginning, middle and end of each dry season, we used precipitation data for 1999, 2000 and 2001 from a meteorological station of INMET (Instituto Nacional de Meteorologia) installed in the study area. The rainfall values recorded for these years is presented in Fig. 3, indicating two marked seasons in the São Domingos karst area: a dry season between May and September and a rainy season between October and March/April, consistent with the historical data for the region.
Monthly rainfall recorded in the years of 1999, 2000 and 2001. Source: INMET, Posse municipality, Goiás State, central Brazil.
In order to estimate the monthly population densities in the SVII cave, we counted the fishes along six stream reaches of different lengths and areas (totaling ca. 172 m2), detailed in Table 1. The counts were conducted via underwater observations by one observer (M.E. Bichuette) on reaches 2, 3, 4 and 5 or observations from the surface (reaches 1 and 6 - due to shallow water), using underwater lamps and crossing each reach only once, always from downstream to upstream. The observer did not use any anchorage apparatus (e.g., a rope). The water in all reaches was clear, which enabled accurate observations. We standardized the time of observations according to the area of each stream reach (Table 1). We estimated the densities (absolute and mean) per reach and also per sampling event (month and year/ stage of dry season). Using the largest population estimated, we used the river area at the SVII cave to estimate total population (average population density x 2,100 m2).
Environmental characteristics of stream reaches from the São Vicente II cave (SVII) and epigean rio da Lapa. Plant debris: low (< 1/2 of the stretch bottom), medium (1/2 of the stretch bottom) or high (> 2/3 of the stretch bottom).
The sampled area for E. trilineata in RL, located 100 m upstream from the Terra Ronca cave entrance (sinkhole), was ca. 100 m2 (40 m long; 2.5 wide) (Table 1). A rope was anchored just upstream the transect to hold the observer in position. We counted the active E. trilineata in the area and estimated the population densities. We conducted the sampling events for four hours in April 2001 and four hours in August 2001, with two observations per hour. The observer (M.E. Bichuette) covered all transect, from downstream to upstream, in 15 minutes, totaling 16 counts.
Environmental variables and habitat characteristics. We used a Horiba apparatus model U-10 multimeter to measure pH, conductivity (µS.cm-1) and temperature (oC) in the middle of each reach, and correlated these to population densities. For the SVII cave, we made 42 measurements (seven samples per each of the six sectors), and for RL we made 16 measurements (eight on May 2001 and eight on August 2001, hourly).
We also recorded other habitat characteristics for detection of seasonal fluctuations and possible fish preferences for microhabitats: average depth (from 0.3 to 0.7 m), water current (slow, moderate or fast; visually determined), amount of plant debris (low: less than 1/2 of the reach bottom area; moderate: 1/2 of the reach bottom area; high: more than 2/3 of the reach bottom area) and substrate type (silt, sand, gravel, large rocks; determined visually in accordance with the percentage of substrate type covering the streambed) (see Table 1 for details).
Data Analysis. Box-plot diagrams were made to show variation in population densities between the three dry seasons. Using these data, we conducted an analysis of variance (two-way ANOVA) to test if there was a significant effect of year or dry season period on population (factors: seasons- beginning, middle and end of dry season; years, 1999, 2000 and 2001). All tests were performed at p < 0.05 (Zar, 1996Zar, J. H. 1996. Biostatistical analysis. New Jersey, Prentice-Hall.).
In order to find out which independent variables (environmental characteristics) best explain variation in population densities, we performed a Principal Component Analysis (PCA) (Gotelli & Ellison, 2011Gotelli, N. J. & A. M. Ellison. 2011. Princípios de estatística em ecologia. Porto Alegre, Artmed.). In this analysis, we used continuous (depth, pH, conductivity and temperature) and categorical variables (water current, amount of plant debris and substrate type). We ranked the categorical variables as follows: water current (slow - 1; moderate - 2; slow to moderate - 3; fast - 4); amount of plant debris (low - 1; medium - 2; high - 3) and substrate type (silt and sand -1; silt, sand and gravels - 2; silt, sand and large rocks - 3; silt and large blocks - 4), and we normalized the continuous variables, when necessary. For statistical analyses, we used R software (R Core Team 2013, version 3.0.1.) and Past software (version 2.16; Hammer et al., 2001Hammer, Ø., D. A. T. Harper & P. D. Ryan. 2001. PAST. Paleontological statistics software package for education and data analysis. Paleontologia Eletronica, 4: 9 pp.).
Results
Population density and habitat distribution. Descriptive statistics are presented in Table 2. We estimated the abundances considering the areas sampled (172 m2) and the total area of São Vicente II cave river (2,100 m2) (Table 2). Grouping the dry seasons and years (1999-2001), we verify that the mean population densities relative to the dry season varied from 0.05 to 0.17 ind.m-2 (Table 2). Considering each dry season per year, we note a clear decrease in population densities in 1999 and 2000 and the opposite in 2001 (Fig. 4a), with no significant differences between years (p = 0.05349, Table 3). However, significant differences were recorded within dry seasons annually (p = 0.00619, Table 3; Fig. 4b), specifically in 1999 (from the middle to the end of the dry season, p = 0.001) and 2000 (also from the middle to the end of the dry season, p = 0.001, Table 3); for 2001, the values were not significant (p = 0.15725).
Density data (ind.m-2) for Eigenmannia vicentespelaea in six reaches from São Vicente II cave (SVII). Abundance per month*: absolute values per month. Abundance per month**: mean density x total area (2,100 m2).
values from ANOVA two-way analysis for population data of Eigenmannia vicentespelaea. BDS-EDS, interaction between beginning of dry season and end of dry season; df, degrees of freedom; Sum SQ, sum of squares; Mean SQ, mean of squares; p, level of significance; *close to significant values; **significant values.
Box-plots showing the means and standard deviations (Sd) of population densities data for Eigenmannia vicentespelaea along the years 1999, 2000 and 2001 (a) and between the dry seasons, independent of the years (b) BDS, beginning of dry season; MDS, middle of dry season; EDS, end of dry season.
Considering the population densities recorded for E. vicentespelaea in the São Vicente II cave stream (see means and abundance per month, Table 2) and the total stream area of ca. 2,100 m2, we estimate a mean abundance of 270 ± 89.1 individuals (95% confidence interval) for E. vicentespelaea in the São Vicente II cave.
The PCA analysis shows that axis 1 (PC1) explains 32.42% of the variation and axis 2 (PC2) explains 21.76 % of variation (Table 4). This means that the variables with highest influence on PC1 are depth, water current and substrate type. On PC2, plant debris and the conductivity had the highest influence, and in a less proportion, the pH (Table 4; Fig. 5).
Eigenvalues and percentual of variance resulting from Principal Component Analysis of population densities of Eigenmannia vicentespelaea and seven environmental variables.
Biplot resulting from Principal Component Analysis with seven variables. Dark circles represent sampling units.
Although field observations indicated that these fish occur preferentially in places with large rocks on the benthos, we observed a variable substrate type in most of sampled reaches (Table 1).
Based on the 16 counts performed during the underwater observations in rio da Lapa, the population densities of E. trilineata varied from 0.01 ind.m-2 (period of less activity, twilight) to 0.13 ind.m-2 (peak of activity, middle of the night) (Fig. 6), representing values lower than those recorded for E. vicentespelaea. Individuals of Eigenmannia trilineata showed a preference for places with silt, sand and rocky substrate, with little plant debris, logs or trunks.
Population densities of Eigenmannia trilineata from rio da Lapa, São Domingos karst area, central Brazil, observed from dusk until night phase during two nights (April and August 2001). Asterisk indicates that no sample was recorded in this period at April 2001
Environmental variables. There was little variation in pH in the cave stream, which ranged from 6.1 to 7.8, with the highest pH recorded in the middle of the dry season each year (July, Fig. 7a). For conductivity, we observed greater variation (from 15 to 34 µS.cm-1), with the highest values observed in the beginning of the dry months each year (Fig. 7b). Seasonal variations in temperature were very small (from 23.2 to 24.7°C, Fig. 7c) and, thus similar across years. In the sampled reach of rio da Lapa, pH was similar to that in the cave habitat, but varied greatly considering the measurements were made on one night in April and one night in August of 2001 (5.9-7.5 in April and 6.4 to 7.6 in August). The highest values were recorded in the colder/dryer month. Variation in temperature was also high (23.8 to 25.8°C in April and 22.4 to 24.0°C in August), with the highest temperatures recorded in the beginning of dry season (April). On average, conductivity was significantly lower than in the SVII cave stream (10-16 µS.cm-1 in April and 5 µS.cm-1 in August, with no variation), and showed a marked decrease from April to August towards the beginning to the middle of dry season. Interestingly, the rio da Lapa has a large influence on the headspring waters where the substrate is composed of sandstone rocks and not limestone and carbonatic rocks such as is observed in the SVII cave stream, which influences the conductivity values.
Box-plots showing means and standard deviations of pH (a) conductivity (b) and temperature (c) from the São Vicente II cave stream in the dry seasons of 1999, 2000 and 2001 (circle and asterisk represent outliers).
Discussion
Population densities recorded for E. vicentespelaea varied between 0.04 and 0.17 ind.m2. According to the classification proposed by Trajano (2001) for troglobitic fish, these densities fall between low (< 0.1 ind.m-2) and medium (0.1-1.0 ind.m-2), tending toward low. Among Brazilian troglobitic fishes, low densities have been recorded for the heptapterids Rhamdiopsis krugi and Rhamdiopsis sp. from Toca do Gonçalo, northeastern Brazil (Trajano & Bichuette, 2010Trajano E. & M. E. Bichuette. 2010. Subterranean fishes of Brazil. Pp. 331-355. In: Trajano, E., M. E. Bichuette & B. G. Kapoor (Eds.). Biology of subterranean fishes. Enfield, Science Publishers.), similar to the densities recorded for the epigean species, E. trilineata in the rio da Lapa (0.01 - 0.13 ind.m-2). Some authors state that Gymnotiformes in general (including Eigenmannia spp.) have low densities (Steinbach, 1970Steinbach, A. B. 1970. Diurnal movements and discharge characteristics of eletric gymnotid fishes in the Rio Negro, Brazil. Biological Bulletin, 138: 200-210.; Marrero, 1987Marrero, C. 1987. Notas preliminares acerca de la historia natural de los peces del Bajo Llano. I- Comparación de los hábitos alimentarios de tres especies de peces Gymnotiformes, en Rio Apure (edo Apure, Venezuela). Revista de Hydrobiologia Tropical, 20: 57-63.; Peretti, 1997Peretti, D. 1997. Alimentação de Eigenmannia trilineata (Lopez & Castello, 1966) (Pisces, Sternopygidae) em distintos biótopos da planície de inundação do alto rio Paraná, Brasil. Unpublished Monography, Universidade Estadual de Maringá, Maringá, 31p.), even lower than those recorded herein. However, these authors were basing these reports on data acquired with indirect methods, such as apparatuses to detect electric-fishes (electrodes with amplifiers or collections with trawls). To our knowledge, no study other than ours used visual censuses along transects, a method that, although less precise than mark-recapture (not viable for small to medium sized gymnotiforms), is more reliable than indirect methods to accurately determine population parameters.
The highest population densities recorded for E. vicentespelaea are closely associated with deep water, slow water current and a large amount of plant debris. This latter variable may represent more food availability because diverse benthic invertebrates live in detritus banks in river bottoms (Allan, 1996Allan, J. D. 1996. Stream ecology: structure and function of running waters. Oxford, Alden Press.). Corroborating this idea, Bichuette (2003)Bichuette, M. E. 2003. Distribuição, biologia, ecologia populacional e comportamento de peixes subterrâneos, gêneros Ituglanis (Siluriformes: Trichomycteridae) e Eigenmannia (Gymnotiformes: Sternopygidae) da área cárstica de São Domingos, Nordeste de Goiás. Umplubished Ph.D. Thesis, Universidade de São Paulo, São Paulo, 330p. showed, through stomach content analysis, that E. vicentespelaea is an invertivorous and benthivorous fish, with a tendency toward insectivory, and the individuals forage in the plant debris banks in the SVII cave. This is consistent with the results of other studies indicating that Eigenmannia fish prefer habitats with accumulated plant debris and slow-moving waters (Alves-Gomes, 1997Alves-Gomes, J. A. 1997. Informações preliminares sobre a bio-ecologia de peixes-elétricos (Ordem Gymnotiformes) em Roraima. Pp. 509-555. In: Barbosa, R.I., E. J. G. Ferreira & E. G. Castellón (Eds.). Homem, ambiente e ecologia no estado de Roraima. Manaus, INPA.). However, contrasting to reports of other epigean Eigenmannia species (Alves-Gomes, 1997Alves-Gomes, J. A. 1997. Informações preliminares sobre a bio-ecologia de peixes-elétricos (Ordem Gymnotiformes) em Roraima. Pp. 509-555. In: Barbosa, R.I., E. J. G. Ferreira & E. G. Castellón (Eds.). Homem, ambiente e ecologia no estado de Roraima. Manaus, INPA.), even when riparian vegetation, trunks and logs are available, the epigean species, E. trilineata from rio da Lapa, occur in places with rocky substrate, occupying microhabitats similar to that of E. vicentespelaea, which are also associated with substrate type (PCA analysis, 13.03%). The weak influence of the physicochemical water variables (conductivity, pH and temperature) is most likely related to the very small variation recorded in this study. The pH values recorded for the São Vicente II cave, approximately 7.0, are typical of karstic drainages (Culver, 1982Culver, D. C. 1982. Cave life: evolution and ecology. Cambridge, Harvard University Press.). The low variation in the water temperature throughout the study is expected due to the general environmental stability typical of most subterranean ecosystems (Vandel, 1964Vandel, A. 1964. Biospéologie. La biologie des animaux cavernicoles. Paris, Gauthier-Villars.; Moore & Sullivan, 1997Moore, G. W. & N. Sullivan. 1997. Speleology. Caves and the cave environment. St. Louis, Cave Books.). A decrease in the mean population density was observed in the dry seasons of 1999 and 2000. In 2001, a low population density was observed in May that could be explained by flash floods occurring in March. In May of 1999 and 2001, unusually heavy rains caused very intense flooding events in the São Vicente II cave, which drastically changed some of the study reaches, especially in May 2001. These events most likely washed the fishes in the cave downstream. The lower density observed in May 2001 is likely a consequence of this drastic flood, along with plant debris on the ceiling of the cave. Apparently, 2001 was an atypical year, with precipitation values from March and April 2001 together (303.4 mm) higher than those recorded for the same period in 1999 (276.0 mm) and 2000 (239.8 mm). A re-establishment of the population was observed in the following months (see August 2001). The fast recovery of the population to the levels observed by the end of the dry season in the previous years may have occurred by recruitment of adults and sub-adults that had hidden in protected microhabitats. This indicates that the variations observed in climatic factors (such as precipitation and the subsequent flash floods), strongly regulates the abundance of E. vicentespelaea.
Conservation status. Eigenmannia vicentespelaea is endemic to a single cave system (São Vicente), has low population densities, smaller even than those of several other troglobitic fishes, occurs in specific areas in the subterranean stream and depends on food carried from the surface by floods. Such attributes characterize this species as vulnerable to anthropogenic disturbance. One of the caves forming the system, the São Vicente II cave studied herein is difficult to access and is barred from tourism, which, thus, does not represent a direct threat. Nevertheless, the streams that form the cave systems, including the São Vicente River, cross several farms where deforestation and burning occur periodically. Thus, one of the main threats to the subterranean ecosystem in the area is habitat destruction, especially at the headwaters. Deforestation causes siltation of springs, leading to a decrease in the food supply for the hypogean communities located downstream. In January 2013, a large scale erosion event destroyed part of the São Vicente spring, muddying the water for two months. This type of impact may greatly harm fish that communicate through electrical signals in the water, such as Eigenmannia species, most likely causing disturbances in the electro-communication. Eigenmannia vicentespelaea has been categorized as VU D2 on the Brazilian Red List since 2002 (Bichuette, 2008Bichuette, M. E. 2008. Eigenmannia vicentespelaea. Pp. 176-177. In: Machado, A. B. M., G. Fonseca & A. P. Paglia (Orgs.). Livro vermelho da fauna brasileira ameaçada de extinção. Belo Horizonte, Ministério do Meio Ambiente & Fundação Biodiversitas. v. 2.; Gallão & Bichuette, 2012Gallão, J. E. & M. E. Bichuette. 2012. The list of endangered fauna and impediments to inclusion of species - the example of Brazilian troglobitic fish. Natureza & Conservação,10: 83-87.), however; so far no action has been taken to effectively protect this species.
The population densities of E. vicentespelaea may be considered low for troglobitic fish in general, following the ranking proposed by Trajano (2001)Trajano, E. 2001. Ecology of subterranean fishes: an overview. Pp. 133-160. In: Romero, A. (Ed.). The Biology of hypogean fishes. Dordrecht, Kluwer Academic Publishers. and similar to those recorded for the epigean species occurring in the same area, E. trilineata. Further, the mean population size for E. vicentespelaea, ca. 270 individuals may be considered small. We observed significant fluctuations in the population densities during the course of the dry season (annually), which suggests seasonality for E. vicentespelaea. The densities of E. vicentespelaea are influenced primarily by depth (greater depths), water current (slow current) and amount of plant debris (high). The relatively low population densities along with small population sizes and small geographic range (endemic to a single cave-system) justify its permanence in the Brazilian Red List of Threatened Fauna (included since 2002). We recommend, as urgent actions for its effective conservation, the protection of the headsprings within the boundaries of the Terra Ronca State Park.
Acknowledgements
We would like to thank the field assistants, Danilo Allegrini, Leandro D. Bertoni, Amazonas Chagas-Jr., Flávio C.T. Lima, Flávio D. Passos, Marcelo M. Bezerra, Hertz F. Santos, Dirk Möller, Yvonne Meyer-Lucht and Jens Poschadel; Marco A. Batalha for statistical support; Ramiro H. Santos and Mr. Gustavo, the guides from São Domingos; Mr. José and Ms. Balbina, owners of Poção farm, for support on the trails to the São Vicente II cave; Regina B. Schultz and Arnor V. Mello, Park officials, for the infrastructure and support to carry out this work; Diego M. von Schimonsky for map creation; the Secretaria do Meio Ambiente e Recursos Hídricos - SEMARH and Instituto Brasileiro do Meio Ambiente (IBAMA) for collection permits; the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), for financial support to the senior author (Doctorate scholarship, process number 98/13858-1). We also thank the three anonymous reviewers for their careful reading and valuable contributions to this work. Native speaker revised English.
References
- Albert, J. S. 2001. Species diversity and phylogenetic systematics of american knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications Museum of Zoology, University of Michigan, 190: 1-127.
- Albert, J. S. 2003. Family Sternopygidae. Pp. 487-491. In: Reis R. E., S. O. Kullander & C. J. Ferraris Jr (Eds.). Checklist of the Freshwater Fishes of South and Central America. Porto Alegre, Edipucrs.
- Albert, J. S. & R. Campos-da-Paz. 1998. Phylogenetic systematics of american knifefishes: a review of the available data. Pp. 409-435. In: Malabarba, L., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (Eds.). Phylogeny and classification of Neotropical fishes. Porto Alegre, Edipucrs.
- Allan, J. D. 1996. Stream ecology: structure and function of running waters. Oxford, Alden Press.
- Alves-Gomes, J. A. 1997. Informações preliminares sobre a bio-ecologia de peixes-elétricos (Ordem Gymnotiformes) em Roraima. Pp. 509-555. In: Barbosa, R.I., E. J. G. Ferreira & E. G. Castellón (Eds.). Homem, ambiente e ecologia no estado de Roraima. Manaus, INPA.
- Alves-Gomes, J. A., G. Ortí, M. Haygood, A. Meyer & W. Heiligenberg. 1995. Phylogenetic analysis of the South American electric fishes (Order Gymnotiformes) and the evolution of their electrogenic system: a synthesis based on morphology, electrophysiology, and mitochondrial sequence data. Molecular Biology and Evolution, 12: 298-318.
- Auler, A. & A. R. Farrant. 1996. A brief introduction to karst and caves in Brazil. Proceedings of the University of Bristol Spelaeological Society, 20: 187-200.
- Bennett, M. V. L. 1971a. Electric organs. Pp. 349-491. In: Hoar, W. S. & D. J. Randall (Eds.). Fish physiology. New York, Academic Press.
- Bennett, M. V. L. 1971b. Electroreception. Pp. 493-574. In: Hoar, W. S. & D. J. Randall (Eds.). Fish physiology. New York, Academic Press.
- Bichuette, M. E. 2003. Distribuição, biologia, ecologia populacional e comportamento de peixes subterrâneos, gêneros Ituglanis (Siluriformes: Trichomycteridae) e Eigenmannia (Gymnotiformes: Sternopygidae) da área cárstica de São Domingos, Nordeste de Goiás. Umplubished Ph.D. Thesis, Universidade de São Paulo, São Paulo, 330p.
- Bichuette, M. E. 2008. Eigenmannia vicentespelaea. Pp. 176-177. In: Machado, A. B. M., G. Fonseca & A. P. Paglia (Orgs.). Livro vermelho da fauna brasileira ameaçada de extinção. Belo Horizonte, Ministério do Meio Ambiente & Fundação Biodiversitas. v. 2.
- Bichuette, M. E. & E. Trajano. 2003. Epigean and subterranean ichthyofauna from the São Domingos karst area, Upper Tocantins River basin, Central Brazil. Journal of Fish Biology, 63: 1100-1121.
- Bichuette, M. E. & E. Trajano. 2004. Three new subterranean species of Ituglanis from Central Brazil (Siluriformes: Trichomycteridae). Ichthyological Exploration of Freshwaters, 15: 243-256.
- Bichuette, M. E. & E. Trajano. 2006. Morphology and distribution of the cave knifefish Eigenmannia vicentespelaea Triques, 1996 (Gymnotiformes: Sternopygidae) from Central Brazil, with an expanded diagnosis and comments on subterranean evolution. Neotropical Ichthyology, 4: 99-105.
- Crampton, W. G. R. 1998. Electric signal design and habitat preferences in a species rich assemblage of gymnotiform fishes from the Upper Amazon basin. Anais da Academia Brasileira de Ciências, 70: 805-847.
- Culver, D. C. 1982. Cave life: evolution and ecology. Cambridge, Harvard University Press.
- Culver, D. C. & T. Pipan. 2009. The biology of caves and other subterranean habitats. New York, Oxford University Press.
- Fernández, L. & M. E. Bichuette. 2002. A new cave dwelling species of Ituglanis from the São Domingos karst, central Brazil (Siluriformes: Trichomycteridae). Ichthyological Exploration of Freshwaters, 13: 273-278.
- Ford, D. C & P. W. Williams. 2007. Karst hydrogeology and geomorphology. West Sussex, Wiley.
- Gallão, J. E. & M. E. Bichuette. 2012. The list of endangered fauna and impediments to inclusion of species - the example of Brazilian troglobitic fish. Natureza & Conservação,10: 83-87.
- Gibert, J. & L. Deharveng. 2002. Subterranean ecosystems: a truncated functional biodiversity. BioScience, 52: 473-481.
- Giora, J. & C. B. Fialho. 2009. Reproductive biology of weakly electric fish Eigenmannia trilineata López and Castello, 1966 (Teleostei, Sternopygidae). Brazilian Archives of Biology and Technology, 52: 617-628.
- Giora, J., C. B. Fialho & A. P. Dufech. 2005. Feeding habit of Eigenmannia trilineata Lopez & Castello, 1966 (Teleostei: Sternopygidae) of Parque Estadual de Itapuã, RS, Brazil. Neotropical Ichthyology, 3: 291-298.
- Giora, J., H. M. Tarasconi & C. B. Fialho. 2011. Reproduction and feeding habits of the highly seasonal Brachyhypopomus bombilla (Gymnotiformes: Hypoppomidae) from southern Brazil, with evidence for a dormancy period. Environmental Biology of Fishes, 94: 649-662.
- Gotelli, N. J. & A. M. Ellison. 2011. Princípios de estatística em ecologia. Porto Alegre, Artmed.
- Hagedorn, M. 1988. Ecology and behavior of a pulse-type electric fish, Hypopomus occidentalis (Gymnotiformes, Hypopomidae), in a fresh-water stream in Panama. Copeia, 1988: 324-335.
- Hammer, Ø., D. A. T. Harper & P. D. Ryan. 2001. PAST. Paleontological statistics software package for education and data analysis. Paleontologia Eletronica, 4: 9 pp.
- Holsinger, J. R. & D. C. Culver. 1988. The invertebrate cave fauna of Virginia and part of eastern Tennessee: zoogeography and ecology. Brimleyana, 14: 1-162.
- Hopkins, C. D. 1974. Electric communication in fish. American Scientist, 62: 426-437.
- Juberthie, C. 2000. The diversity of the karstic and pseudokarstic hypogean habitats in the world. Pp. 17-39. In: Wilkens, H., D. C. Culver & W. F. Humphreys (Eds.). Ecosystems of the world, subterranean ecosystems. Amsterdan, Elsevier Academic Press.
- Kramer, B. 1990. Sexual signs in electric fishes. Trends in Ecology and Evolution, 5: 247-250.
- Kramer, B. 1996. Electric signaling and communication in weakly electric fishes (Gymnotiformes) of South America. Pp. 23-30. In: Val, A. L. & D. T. Randall (Eds.). Physiology and biochemistry of the fishes of the Amazon. Manaus, INPA.
- Lissmann, H. W. 1961. Ecological studies on gymnotids. Pp. 215-226. In: Chagas, C. & A. P. Carvalho (Eds.). Bioelectrogenesis. Amsterdan, Elsevier.
- Lopez, R. B. & H. P. Castello. 1966. Eigenmannia trilineata (Teleostomi, Sternopygidae), nueva especie hallada en el Río de La Plata. Comunicaciones del Museo Argentino de Ciencias Naturales Bernardino Rivadavia, 4: 7-12.
- Mago-Leccia, F. 1994. Electric fishes of the continental waters of America. Classification and catalogue of the electric fishes of the Order Gymnotiformes (Teleostei: Ostariophysi), with descriptions of new genera and species. Biblioteca de la Academia de Ciencias Fisicas, Matematicas y Naturales, Caracas, 29: 1-225.
- Marrero, C. 1987. Notas preliminares acerca de la historia natural de los peces del Bajo Llano. I- Comparación de los hábitos alimentarios de tres especies de peces Gymnotiformes, en Rio Apure (edo Apure, Venezuela). Revista de Hydrobiologia Tropical, 20: 57-63.
- Moore, G. W. & N. Sullivan. 1997. Speleology. Caves and the cave environment. St. Louis, Cave Books.
- Nimer, E. 1989. Climatologia do Brasil. Rio de Janeiro, Supren.
- Peretti, D. 1997. Alimentação de Eigenmannia trilineata (Lopez & Castello, 1966) (Pisces, Sternopygidae) em distintos biótopos da planície de inundação do alto rio Paraná, Brasil. Unpublished Monography, Universidade Estadual de Maringá, Maringá, 31p.
- Poulson, T. L. & W. B. WHITE. 1969. The cave environment. Science, 165: 971-980.
- Proudlove, G. S. 2010. Biodiversity and distribution of the subterranean fishes of the world. Pp. 41-64. In: Trajano, E., M. E. Bichuette & B. G. Kapoor (Eds.). Biology of subterranean fishes. Enfield, Science Publishers.
- R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available from: http://www.R-project.org.
» http://www.R-project.org - Schaan, A. B.; J. Giora & C. B. Fialho. 2009. Reproductive biology of the Neotropical electric fish Brachyhypoppomus draco (Teleostei: Hypoppomidae) from southern Brazil. Neotropical Ichthyology, 7: 737-744.
- Sket, B. 1999. The nature of biodiversity in hypogean waters and how it is endangered. Biodiversity Conservation, 8: 1319-1338.
- Steinbach, A. B. 1970. Diurnal movements and discharge characteristics of eletric gymnotid fishes in the Rio Negro, Brazil. Biological Bulletin, 138: 200-210.
- Trajano, E. 2001. Ecology of subterranean fishes: an overview. Pp. 133-160. In: Romero, A. (Ed.). The Biology of hypogean fishes. Dordrecht, Kluwer Academic Publishers.
- Trajano, E. 2012. Ecological classification of subterranean organisms. Pp. 275-277. In: White, W. B. & D. C. Culver (Eds.). Encyclopedia of caves. Oxford, Elsevier Academic Press.
- Trajano E. & M. E. Bichuette. 2010. Subterranean fishes of Brazil. Pp. 331-355. In: Trajano, E., M. E. Bichuette & B. G. Kapoor (Eds.). Biology of subterranean fishes. Enfield, Science Publishers.
- Trajano, E., R. E. Reis & M. E. Bichuette. 2004. Pimelodella spelaea: A new cave catfish from Central Brazil, with data on ecology and evolutionary considerations (Siluriformes: Heptapteridae). Copeia, 2004: 315-325.
- Triques, M. L. 1996. Eigenmannia vicentespelea, a new species of cave dwelling electrogenic neotropical fish (Ostariophisi: Gymnotiformes: Sternopygidae). Revue Française d'Aquariologie, 23: 1-4.
- Vandel, A. 1964. Biospéologie. La biologie des animaux cavernicoles. Paris, Gauthier-Villars.
- Westby, G. W. M. 1988. The ecology, discharge diversity and predatory behavior of gymnotiform electric fish in coastal streams of French Guiana. Behavioral Ecology and Sociobiology, 22: 341-354.
- Wilkens, H. 2010. Genes, modules and the evolution of cave fish. Heredity, 105: 413-422.
- Zar, J. H. 1996. Biostatistical analysis. New Jersey, Prentice-Hall.
Publication Dates
-
Publication in this collection
Jan-Mar 2015
History
-
Received
19 Dec 2013 -
Reviewed
05 Nov 2014 -
Accepted
31 Mar 2015