Abstracts
We described the reproductive behavior of the small South American cichlid Laetacara araguaiae in streams from Brazil. We predicted that this species will show reproductive cooperation and division of labor between males and females in a similar way presented by other substrate-spawner cichlids. Thus, we studied 34 pairs in the pre-spawning (n = 11), egg/wriggler (n = 11) and fry (n = 12) phases. In the pre-spawning phase both sexes become involved in nest building and territorial defense, but females emphasizes building nest (p = 0.03), while males invest more time in territorial defense (p = 0.04). After spawning, male and female alternate between rearing eggs and defending nest in the territory. In the egg/wriggler phase females devotes more time rearing the brood while males remain defending territory (p = 0.02). These differences disappear when young are in the fry stage, and parents jointly stay closer to fry (p = 0.98). However, at this phase, there is a reduction in the frequency of threats shown by males (p<0.01) and an increase in the frequency of attacks shown by female (p<0.01) that could be a response to an increased demand for parental defense. Our results indicate that the reproductive cooperation between males and females of L. araguaiae is marked by division of labor in the early reproductive phases and by sharing of parental duties as brood develops.
Descrevemos o comportamento reprodutivo do pequeno ciclídeo sul-americano Laetacara araguaiae em riachos do Brasil. Predizemos que essa espécie apresenta cooperação reprodutiva e divisão de trabalho entre machos e fêmeas, como ocorre em outras espécies de ciclídeos substrate-spawners. Assim, estudamos 34 casais nas fases pré-acasalamento (n = 11), ovo/larva (n = 11) e prole natante (n = 12). Na fase pré-acasalamento ambos os sexos envolvem-se na construção de ninhos e na defesa territorial, mas as fêmeas enfatizam a construção de ninho (p = 0,03), enquanto os machos investem mais na defesa do território (p = 0,04). Após a desova, machos e fêmeas alternam entre a manutenção dos ovos e a defesa do ninho. Na fase ovo/larva as fêmeas despendem mais tempo em atividades de manutenção da prole, enquanto os machos investem mais na defesa do território (p = 0,02). Essas diferenças desaparecem quando a prole atinge o estágio natante com ambos os pais permanecendo próximos aos filhotes (p = 0,98). Entretanto, há uma redução na frequência de ameaças emitidas pelos machos (p<0,01) e um aumento na frequência de ataques emitidos pelas fêmeas nessa fase (p<0,01), o que poderia ser uma resposta ao aumento da demanda por defesa da prole. Nossos resultados indicam que a cooperação reprodutiva entre machos e fêmeas de L. araguaiae é representada pela divisão de funções nas primeiras fases reprodutivas, seguida pelo compartilhamento de tarefas parentais à medida que a prole se desenvolve.
Bi-parental care; Cichlidae; Courtship; Division of labor; Ethogram; Nest building
ILaboratório de Ictiologia, Departamento de Zoologia e Botânica, Universidade Estadual Paulista (UNESP). Rua Cristóvão Colombo, 2265, 15054-000 São José do Rio Preto, SP, Brazil. fabricioteresa@yahoo.com.br
IILaboratório de Comportamento Animal, Departamento de Zoologia e Botânica, Universidade Estadual Paulista (UNESP), Aquaculture Center of UNESP (CAUNESP) and Research Center of Animal Welfare (RECAW, CNPq). elianeg@ibilce.unesp.br
ABSTRACT
We described the reproductive behavior of the small South American cichlid Laetacara araguaiae in streams from Brazil. We predicted that this species will show reproductive cooperation and division of labor between males and females in a similar way presented by other substrate-spawner cichlids. Thus, we studied 34 pairs in the pre-spawning (n = 11), egg/wriggler (n = 11) and fry (n = 12) phases. In the pre-spawning phase both sexes become involved in nest building and territorial defense, but females emphasizes building nest (p = 0.03), while males invest more time in territorial defense (p = 0.04). After spawning, male and female alternate between rearing eggs and defending nest in the territory. In the egg/wriggler phase females devotes more time rearing the brood while males remain defending territory (p = 0.02). These differences disappear when young are in the fry stage, and parents jointly stay closer to fry (p = 0.98). However, at this phase, there is a reduction in the frequency of threats shown by males (p<0.01) and an increase in the frequency of attacks shown by female (p<0.01) that could be a response to an increased demand for parental defense. Our results indicate that the reproductive cooperation between males and females of L. araguaiae is marked by division of labor in the early reproductive phases and by sharing of parental duties as brood develops.
RESUMO
Descrevemos o comportamento reprodutivo do pequeno ciclídeo sul-americano Laetacara araguaiae em riachos do Brasil. Predizemos que essa espécie apresenta cooperação reprodutiva e divisão de trabalho entre machos e fêmeas, como ocorre em outras espécies de ciclídeos substrate-spawners. Assim, estudamos 34 casais nas fases pré-acasalamento (n = 11), ovo/larva (n = 11) e prole natante (n = 12). Na fase pré-acasalamento ambos os sexos envolvem-se na construção de ninhos e na defesa territorial, mas as fêmeas enfatizam a construção de ninho (p = 0,03), enquanto os machos investem mais na defesa do território (p = 0,04). Após a desova, machos e fêmeas alternam entre a manutenção dos ovos e a defesa do ninho. Na fase ovo/larva as fêmeas despendem mais tempo em atividades de manutenção da prole, enquanto os machos investem mais na defesa do território (p = 0,02). Essas diferenças desaparecem quando a prole atinge o estágio natante com ambos os pais permanecendo próximos aos filhotes (p = 0,98). Entretanto, há uma redução na frequência de ameaças emitidas pelos machos (p<0,01) e um aumento na frequência de ataques emitidos pelas fêmeas nessa fase (p<0,01), o que poderia ser uma resposta ao aumento da demanda por defesa da prole. Nossos resultados indicam que a cooperação reprodutiva entre machos e fêmeas de L. araguaiae é representada pela divisão de funções nas primeiras fases reprodutivas, seguida pelo compartilhamento de tarefas parentais à medida que a prole se desenvolve.
Key words: Bi-parental care, Cichlidae, Courtship, Division of labor, Ethogram, Nest building.
Introduction
Reproductive strategies are highly variable among fishes, especially in the Cichlidae (Lowe-McConnell, 1969; Fryer & Iles, 1972). These fish are known for their complex reproductive behavior, with different mating system that encompass territorial defense, elaborate mechanisms of mate choice and prolonged parental care (e.g., Barlow, 1974, 2000; Ripley & Lobel, 2005; Balshine & Buston, 2008; Khoda et al., 2009). Among Neotropical cichlids, monogamy and biparental care are the most common strategies (Keenleyside, 1991), which involves male and female cooperation over the reproductive cycle (Timms & Keenleyside, 1975; Keenleyside & Bietz, 1981; Snekser & Itzkowitz, 2009), a less widespread behavior among teleost fishes (Balshine & Buston, 2008).
Cooperation may occur based on division of labor, mainly in the first reproductive phases (Barlow, 1974; Rogers, 1988), with females investing more time rearing brood while males invest more time in the territorial defense. These differences tend to reduce or disappear with brood development (Barlow, 1974; Neil, 1984; Keenleyside, 1991). The exchange of parental roles allow parents to alternate vigilance/feeding activities (Ward & Samarakoon, 1981) and as a consequence, keep better health conditions (Perrone & Zaret, 1979). Moreover, parental duty coordination also provides more effective defense of the offspring against predators (Nagoshi, 1987).
The parental roles may also be variable along reproductive phases and even within the same species, depending on the several factors such as brood development stage, number and proximity of potential predators, relative size between parents and sex ratio (Keenleyside et al., 1990; Itzkowitz et al., 2005; Richter et al., 2005). The variation is probably underestimated because the breadth of the behavioral diversity exhibited for Neotropical cichlids is neglected. This is evident among South American cichlids, whose registers of behavior are scarce (but see Yamamoto et al., 1999; Cacho et al., 2006; Rodrigues et al., 2009) in comparison with African cichlids (e.g., Barlow, 2000).
The genus Laetacara is an example of the limited knowledge about behavior of the South American cichlids. This genus has six species and there is no information about behavior for any of them. Laetacara araguaiae is a recently described species, being abundant especially in physically degraded streams (Casatti et al., 2006). Because of the ease of watching the species in such degraded habitats, it could be a good model for environmental studies and also to better understand cooperative behavior in fish. In this study we provide the description of the reproductive behavior of L. araguaiae and evaluate if this species presents division of parental roles. More specifically, the following main questions were addressed: (i) How are the behavioral patterns exhibited by parents along the reproductive phases? (ii) Are there qualitative and/or quantitative differences in the parental behavior related to sex of parents and/or to reproductive phases? As a substrate-spawner cichlid, we predicted that L. araguaiae shows some degree of labor division related to male and female reproductive roles. Additionally, as sex related differences in the profile of agonistic behaviors may emerge during parental care (Itzkowitz, 1985), we also predicted that this behavior can be showed in different ways over the reproductive cycle in males and females of L. araguaiae.
Material and Methods
Study site and the species
The study was conducted in two first order streams located in the Vitória Brasil and Dolcinópolis Municipalities, northwest of São Paulo State, Brazil (20º10'05.7"S 50º29'49.9"W and 20º09'38.2"S 50º32'02.6"W, respectively). Both environments are silted shallow waters inserted in pasture matrix, with deforested banks, which are composed predominantly by grasses. These sites were chosen because water transparency facilitates observation from banks.
Laetacara araguaiae is a small cichlid species (Fig. 1) recently described from Araguaia drainage (Ottoni & Costa, 2009), but has been also collected in streams from upper Paraná River. It lives in marginal shallow slow waters of streams and rivers (Casatti et al., 2006; Souza-Filho & Casatti, 2010) and pairs in reproductive activities are easily observed from banks.
Adult fish were previously sampled to check size and sex (by macroscopic inspection of gonads). Males were always the largest member of the pair (mean ± SD: Males = length: 5.78 ± 0.42 cm, n = 17; Females = 4.83 ± 0.38 cm, n = 17).
Behavioral records
Field observations were done in spring and early summer, from September/2005 to January/2006 and in October/2006. Pairs bonding were located during slow walking along banks and the larger individual of the pair was, then, assumed to be the male.
In a first step we described the reproductive behavior by observing 54 h of ad libitum sampling (Altmann, 1974) (n = 23 pairs). In a second step, the male and female parental behavior was studied to test the prediction of male and female division of labor along the reproductive period featured by three phases: pre-spawning, egg/wriggler, and fry. We recorded the frequency of aggressive acts showed by male and female during territorial and brood defense, the frequency of digging behavior during nesting, and the time spent near of brood (less than a fish's body length distance from the brood).
Male and female behavior was quantified by focal animal sampling (Altmann, 1974) (15 min sessions) in the pre-spawning (n = 11), egg/wriggler (n = 11) and fry (n = 12) reproductive phases. Behavior was recorded in an audio portable recorder device. Frequency of nest digging and agonistic interactions were quantified according to the ethograms described in the first observation phase. As sex related differences in the profile of agonistic behaviors may exist during parental care (Itzkowitz, 1985), the agonistic profile over reproductive cycle was also evaluated. Behavioral units were grouped according to the gradient of aggressiveness. Acts involving direct physical contact were referred to as "attacks" and acts with no physical contact were referred to as "threats".
Data analysis
Shapiro-Wilk's test was used to assess data normality and Fmax for homoscedasticity (Zar, 1999). Parametric and non-parametric analyses were applied accordingly. We compared the frequency of agonistic acts and the time spent near the brood between sexes and among reproductive phases by using a two-way ANOVA. In this case, data were previously transformed to ((x + 0.5) ½). As parents can modulate each other's behavior (Itzkowitz et al., 2003), we treated sex as a repeated measure.
Because ANOVA assumptions of normality and homoscedasticity were violated, nonparametric tests were carried out to analyze agonistic behavior and also digging behavior. Thus, we used a Wilcoxon signed-rank test to compare agonistic acts showed between sexes and the Kruskall-Wallis completed with Dunn post hoc test (Zar, 1999) to analyze differences among reproductive phases for each sex. The nest digging behavior was compared between sexes by Wilcoxon signed-rank test (Zar, 1999).
Results
Qualitative description of reproductive behavior repertoire Pair formation
The establishment of territory by males preceded pair formation. Adult males established territories and defended them with attacks and threats against intruder fish, especially of conspecific ones. Each male was observed defending only one territory. Pairs were assumed to be formed when female also engaged in territorial defense. After that, male and female alternated among courtship behaviors, nest digging and territorial defense.
Territorial defense
After the pair formation, the territory was defended by both parents. Male and female alternated patrolling around territory, repelling intruder fish by using the following agonistic acts:
Threats
1. Opercular opening: a fish approach to another with the head directed to it and spread down the branchiostegal membrane. A more aggressive posture may be adopted by curling the body laterally, with the fish assuming a "S" shape of the body.
2. Lateral threat: a fish approaches to an opponent, shows its body laterally and spreads its fins. This behavioral act may also be performed with opercular opening.
3. Simultaneous frontal threat: two fish face each other and may partially open the operculae. They may remain in this position even though far apart (~600 mm).
Attacks
4. Lateral attack: a fish approaches and beats with its mouth opened on the opponent's body.
5. Mouth fighting: two fish approach each other with mouth opened and touch or bite their jaws. This behavior stops immediately after mouth contact, but in some cases it can lasts about a minute.
6. Undulation: the fish performs antero-posterior waving of the body at the side of the opponent.
7. Chasing: the fish swims toward the opponent, which swims in an opposite direction.
Territorial fish repelled conspecifics and also heterospecifics from the territory. Contests with conspecifics usually follow a sequence, beginning with simultaneous frontal threat. Thereafter, fish usually alternated between opercular opening and lateral threats. Contests ended when one of the opponents retreated or escalated aggressive interactions, showing lateral attacks and frontal confrontation.
Nest building
Males and females built circular nests (50 to 100 mm of diameter) inside the territory on the sand bottom in shallow waters near roots or macrophyte leaves. Nests were used as a spawning site and also for care of egg and wriggler stages of the brood. At the beginning, the pairs dug several nests (two to four), but spawning occurred only in one of them. We observed, in some cases, parental fish moving brood to another previously built nest.
The digging behavior to build nest included two behavioral acts:
1. Mouth digging: Fish dig the substrate, catching substrate particles with its mouth and releasing them at the edge of the nest.
2. Undulation digging: The ventral portion of the body is pressed against the substrate as the fish moves forward with fast tail beating and body undulations causing dispersion of the substrate. This was the more frequent behavioral unit of nest digging.
Courtship
Courtship begins near or within the nest and ends with spawning. We observed three courtship behavioral acts performed by both male and female:
1. Lateral display: fish spread totally or partially its dorsal and anal fins, exposing the lateral portion of the body toward to the mate.
2. Quivering: fish performing repeated short and quick body undulations when near to the mate.
3. Quivering with tail beating: male and female side by side spread their fins and assume an "S" shape body posture with their head turned away from the mate. This posture is showed while fish quiver their bodies and beat their tail against each other. These movements occur repeatedly and can be showed by one or both mates simultaneously.
Spawning
The pair's behavior was synchronized during spawning. The female followed a circular path, pressed the belly against the substrate and vibrated the posterior portion of the body when entering in the nest. During these movements, female released the adhesive oocytes. Thereafter, the male entered in the nest performing similar movements to those of the female, but with the body farther from the substrate, passing over of the oocytes, apparently releasing sperm. Male and female alternated the entering in the nest, and rarely did it simultaneously. All spawning observed occurred between 04:30 and 07:00 pm (n = 4) and lasted about an hour.
Brood caring
Egg/wriggler rearing
The brood remained in the nest during egg and wriggler development stage. In this phase the most common behavior performed by parents were fanning and cleaning, which ceased when the offspring began to swim freely.
1. Fanning: one parent moves its pectoral and pelvic fins near the brood, apparently improving water flow to them.
2. Cleaning: parents remove odd particles out of the nest by mouth.
Egg/wriggler defense
One parent remained close to the offspring while the other patrolled the boundaries of the territory, repelling intruder fish by agonistic behavior (as described in the pre-spawning phase). The brood defense was alternated with rearing activities by male and female.
Fry defense
When the offspring began to swim freely and to move through the territory, the pair remained closer to it and started to defend a smaller space around. This phase lasted about 4 weeks, during which the family moved slowly around the territory and its surroundings, usually along the banks of the stream in places with high density of macrophytes. The fry formed shoals and foraged on the substrate. During this phase, the parents moved away from them only to attack another approaching fish.
Parental roles
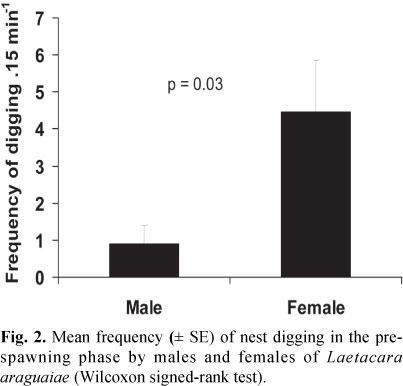
In the pre-spawning phase, the pair engaged in courtship activities, nest building and territorial defense. Females exhibited higher frequency of nest digging behavior than the males did (Wilcoxon signed-rank test, Z = -2.11, p = 0.03, Fig. 2).
There was significant interaction between reproductive phase and sex (F(1,20) = 6.84, p = 0.017, Fig. 3), considering time spent near of the offspring. Females remained near the egg/wriggler longer than males (Tukey post hoc test, p = 0.017, Fig. 3). However, this difference between sexes was not observed in the fry phase (p = 0.98). In this case, both males and females remained with the offspring longer than in the early phases (p<0.001, Fig. 3).
We observed significant interaction between reproductive phase and sex for the total of agonistic acts (F(2,31) = 4.97, p = 0.013). Males showed higher frequency of agonistic acts than females in the pre-spawning phase (Tukey post hoc test, p = 0.04), but the frequency was similar between sexes after spawning (p>0.92, Fig. 4). The frequency of agonistic acts did not differ over the reproductive phases for both males and females (p>0.10, Fig. 4).
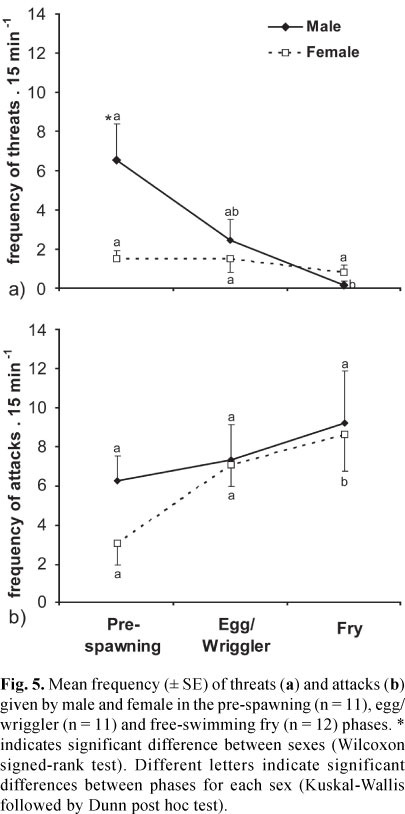
We observed a higher frequency of threats emitted by males than by females, but it happened only in the pre-spawning phase (Wilcoxon signed-rank test, Z = -2.18, p = 0.02, Fig. 5). Moreover, there was an effect of reproductive phase on the frequency of threats by males (Kruskal-Wallis, H = 10.15, p = 0.006, Fig. 5), which decreased from pre-spawning to fry phase (Dunn post hoc test, p<0.05). The frequency of threats emitted by females did not differ over reproductive phases (H = 1.23, p = 0.54, Fig. 5).
The frequency of attacks did not differ between males and females in the different reproductive phases (Wilcoxon signed-rank test, p>0.06, Fig. 5). There was no effect of the reproductive phase among males (Kruskal-Wallis, H = 0.003, p = 0.99, Fig. 5), but the frequency of attacks given by females increased from the pre-spawning phase to fry phase (H = 6.76, Dunn post hoc test, p<0.05, Fig. 5).
Discussion
The reproductive behavior of L. araguaiae described in this study is typical of the substrate brooder Neotropical cichlids, beginning with territory establishment and extending to parental care until the fry phase. The parental care in this species is a cooperative activity between male and female, where each member of the pair performs similar behaviors and alternates between rearing offspring and territorial defense. However, there is a division of labor at the beginning of the reproductive phase.
Biparental cichlids are known to perform relatively long parental care periods (Keenleyside, 1991), resulting in a high investment devoted by the parents (Rogers, 1988). However, the reproductive activities preceding spawning represent an important parental investment as well (Mackereth & Keenleyside, 1993). In this way, this study is one of the few exploring parental investment in the pre-spawning phase (see Timms & Keenleyside, 1975; Rogers, 1988). In this phase, L. araguaiae females invest more in nest building, while males invest in territorial defense. A similar pattern was observed in the egg/wriggler phase, when males devoted less time near offspring than did females, indicating higher involvement in territorial defense despite higher investment in rearing duties by females. This division of labor is common among substrate brooding cichlids, mainly during brood stationary developmental stage (Itzkowitz & Nyby, 1982; Townshend & Wootton, 1985; Rogers, 1988) and may be the result of the differential significance that parental roles represent to each sex (Trivers, 1972). The higher investment of females in the offspring care (e.g., Keenleyside et al., 1991; this study) is well correlated with evolutionary explanations based on female's relatively low reproductive potential (Trivers, 1972). This inference could be supported by the higher investment in the nest building and rearing activities during pre-spawning and egg/wriggler phase, respectively. Moreover, the possession of a territory may have higher value for males, since they could to ensure attraction of other females and to have other spawning chances if they fail in a first attempt to reproduce (Schwanck, 1989). This is especially true to species whose males establish a territory before couple formation (Perrone, 1978; Neil, 1984). Alternatively, the specialization in the execution of parental duties may be the result of differential ability to perform some particular tasks. For example, males usually are bigger than females and could be more effective in the territorial defense and, therefore, they would emphasize this activity (Barlow, 1974; Schwanck, 1989; Awata & Khoda, 2004; Itzkowitz et al., 2005).
Besides quantitative differences between males and females in the parental activities found in this study for L. araguaiae, the sexes differed also qualitatively in how they perform these activities. Despite the fact that females spent less time patrolling territory in the egg/wriggler phase than did males, they showed a similar frequency of agonistic acts against intruders. This fact suggests that females exhibit more agonistic acts per time unit and reveal differences in the fighting tactics between sexes. Therefore the agonistic acts performed by females would be faster and probably at closer range with intruders than did males, as observed for Cichlasoma cyanoguttatum (=Herichthys cyanoguttatus) and Lamprologus toae (=Neolamprologus toae) (Itzkowitz, 1985; Nakano & Nagoshi, 1990). Moreover, as males are bigger than females, intruders may be more likely to flee from a large male before it has performed any agonistic act.
The sex differences in the parental care in the early reproductive phases disappeared in the fry phase, which is a tendency already observed for biparental cichlids (Keenleyside, 1991) and seems to be a response to changes in the behavior of the offspring. When the young begin swimming freely, they leave the nest and move in shoals around the territory. This is the phase when young are more vulnerable to attacks from predators because they are more conspicuous (Fitzgerald & Keenleyside, 1978; Nagoshi, 1987). In this sense, the permanence of both parents near the young, as observed in this study, would guarantee a larger area free of predators around them (Annett et al., 1999).
The increased frequency of agonistic acts should also be a response to a phase that demands more parental defense (Smith-Grayton & Keenleyside, 1978; Neil, 1984; Itzkowitz, 1985). Although males of L. araguaiae have reduced the frequency of threats during the fry phase, females increased the frequency of attacks as the offspring developed. The threats are less aggressive agonistic units and are important for signaling the territory, ensuring the defense of the territory while providing less risk of injuries (Clutton-Brock et al., 1979; Itzkowitz, 1985) and less energetic cost than overt fights (Ros et al., 2006). The higher frequency of threats showed by males is compatible with higher investment devoted by them patrolling territory in the early reproductive phases (Itzkowitz & Nyby, 1982; Townshend & Wootton, 1985; Rogers, 1988). The reduction in the frequency of threats emitted by males during the fry phase overlaps disappearance of the territory, during which the pair jointly stays close to the fry defending a moving space around the offspring, and not a well delimited physical space. In the early reproductive phase, displays exhibited in the territory boundaries represent an effective way to keeping intruders away (Itzkowitz & Nyby, 1982; Itzkowitz, 1985), differently of fry stage when both parents stay near young allowing greater approaching of potential predators, which represent eminent risk to fry survival. In this case more aggressive acts would be expected. However, the increased aggressiveness, which is common in parental Neotropical cichlids during the fry stage (Smith-Grayton & Keenleyside, 1978; Neil, 1984; Itzkowitz, 1985), was not shown by increase in total agonistic acts, but through of the increase of attacks emitted by females. This change in the female agonistic behavior during reproductive cycle probably is related to reduction of the demand by rearing activities and more independence of the young in the fry stage (Keenleyside et al., 1990). Therefore, the increased level of aggressiveness would be a compensatory response to increase of the fry vulnerability resulting from its higher dispersion and mobility.
In summary, the present study is one of the few detailed descriptions of the reproductive repertoire of the South American cichlids. Laetacara araguaiae shows reproductive cooperation between males and females, and such strategy is marked by division of labor in the early reproductive phases and by sharing of parental duties as brood develops, following the general pattern of Neotropical substrate-spawner cichlids.
ACKNOWLEDGEMENTS
The authors thank Fernando R. Carvalho (IB/UFRGS, RS) for operational support during data collecting; Mr. Luciro Franco and Valmir Ognibeni for permission to carry out the study on their property; Gilson L. Volpato (IB/UNESP, SP), Regina H. F. Macedo (IB/UNB, DF), Thaís B. Carvalho (UFAM, AM) for critical reading and suggestions in the early version of the manuscript; John W. Wenzel (Ohio State University) for critical review of the manuscript and helping with the English language. FBT received a grant from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) during the period when this research was done.
Literature Cited
Accepted February 20, 2011
- Altmann, J. 1974. Observational study of behavior: sampling methods. Behaviour, 49(3/4): 227-267.
- Annett, C. A., R. Pierotti & J. R. Baylis. 1999. Male and female parental roles in the monogamous cichlid, Tilapia mariae, introduced in Florida. Environmental Biology of Fishes, 54(3): 283-293.
- Awata, S. & M. Kohda. 2004. Parental roles and the amount of care in a bi-parental substrate brooding cichlid: The effect of size differences within pairs. Behaviour, 141: 1135-1149
- Balshine, S. & P. M. C. Buston. 2008. Cooperative behaviour in fishes. Pp. 437-484. In: Maghagen, C., V. Braithwaite, E. Forsgren & B. G. Kapoor (Eds.). Fish Behaviour, New Hampshire, Science Publishers, 648p.
- Barlow, G. W. 1974. Contrasts in social behaviour between Central American cichlids and coral-reef surgeon fishes. American Zoologist, 14(1): 09-34.
- Barlow, G. W. 2000. The cichlid fishes: nature´s grand experiment in evolution. Massachusetts, Perseus Publishing, 335p.
- Cacho, M. S. R. F., S. Chellappa & M. E. Yamamoto. 2006. Reproductive success and female preference in the amazonian cichlid angel fish, Pterophyllum scalare (Lichtenstein, 1823). Neotropical Ichthyology, 4(1): 87-91.
- Casatti, L., F. Langeani, A. M. Silva & R. M. C. Castro. 2006. Stream fish, water and habitat quality in a pasture dominated basin, Southeastern Brazil. Brazilian Journal of Biology, 66(2B): 681-696.
- Clutton-Brock, T. H., S. D. Albon, R. M. Gibson & F. E. Guinness. 1979. The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Animal Behaviour, 27(1): 211-225.
- Fitzgerald, G. J. & M. H. A. Keenleyside. 1978. The effects of numerical density of adult fish on reproduction and parental behavior in the convict cichlid fish Cichlasoma nigrofasciatum (Günther). Canadian Journal of Zoology, 56(6): 1367-1371.
- Fryer, G. & T. D. Iles. 1972. The cichlid fishes of the great lakes of Africa. New Jersey, Neptune City, T. F. H. Publications, 641p.
- Itzkowitz, M. 1985. Sexual differences in offspring defense in a monogamous cichlid fish. Zeitschrift für Tierpsychologie, 70(3): 247-255.
- Itzkowitz, M. & J. Nyby. 1982. Field observations of parental behaviour of the Texas cichlid Cichlasoma cyanoguttatum American Midland Naturalist, 108(2): 364-368.
- Itzkowitz, M., N. Santangelo & M. Richter. 2003. How does a parent respond when its mate emphasizes the wrong role? A test using a monogamous fish. Animal Behaviour, 66(5): 863-869.
- Itzkowitz, M., N. Santangelo, A. Cleveland, A. Bockelman & M. Richter. 2005. Is the selection of sex-typical parental roles based on an assessment process? A test in the monogamous convict cichlid fish. Animal Behaviour, 69(1): 95-105.
- Keenleyside, M. H. A. 1991. Cichlid fishes: behavior, ecology and evolution. Great Britain, Chapman & Hall, 376p.
- Keenleyside, M. H. A. & B. F. Bietz. 1981. The reproductive behaviour of Aequidens vittatus (Pisces, Cichlidae) in Surinam, South America. Environmental Biology of Fishes, 6(1): 87-94.
- Keenleyside, M. H. A., R. C. Bailey & V. H. Young. 1990. Variation in the mating system and associated parental behaviour of captive and free-living Cichlasoma nigrofasciatum (Pisces, Cichlidae). Behaviour, 112(3-4): 202-221.
- Kohda, M., D. Heg, Y. Makino, T. Takeyama, J. Shibata, K. Watanabe, H. Munehara, M. Hori & S. Awata. 2009. Living on the wedge: female control of paternity in a cooperatively polyandrous cichlid. Proceedings of the Royal Society of London Biological Sciences, 276(1676): 4207-4214.
- Lowe-McConnell, R. H. 1969. The cichlid fishes of Guyana, South America, with notes on their ecology and breeding behaviour. Zoological Journal of the Linnean Society, 48(2): 255-302.
- Mackereth, R. W. & M. H. A. Keenleyside. 1993. Breeding territoriality and pair formation in the convict cichlid (Cichlasoma nigrofasciatum: Pisces, Cichlidae). Canadian Journal of Zoology, 71(5): 960-967.
- Nagoshi, M. 1987. Survival of broods under parental care and parental roles of the cichlid fish, Lamprologus toae, in Lake Tanganyika. Japanese Journal of Ichthyology, 34(1): 71-75.
- Nakano, S. & M. Nagoshi. 1990. Brood defense and parental roles in a biparental cichlid fish Lamprologus toae in Lake Tanganyika. Japanese Journal of Ichthyology, 36(4): 468-476.
- Neil, S. J. 1984. Field studies of the behavioral ecology and agonistic behavior of Cichlasoma meeki (Pisces: Cichlidae). Environmental Biology of Fishes, 10(1-2):59-68.
- Ottoni, F. P. & W. J. E. M. Costa. 2009. Description of a new species of Laetacara Kullander, 1986 from central Brazil and re-description of Laetacara dorsigera (Heckel, 1840) (Labroidei: Cichlidae: Cichlasomatinae). Vertebrate Zoology, 59(1): 41-48.
- Perrone, M. J. 1978. Mate size and breeding success in a monogamous cichlid fish. Environmental Biology of Fishes, 3(2): 193-201.
- Perrone, M. J. & T. M. Zaret. 1979. Parental care of fishes. American Naturalist, 113(3): 351-361.
- Richter, M., N. Santangelo & M. Itzkowitz. 2005. Biparental division of roles in the convict cichlid fish: influence of the intruder numbers and locations. Ethology, Ecology and Evolution, 17(1): 1-15.
- Ripley, J. L. & P. S. Lobel. 2005. Reproductive behavior of the Lake Malawi cichlid fish, Tramitichromis intermedius Environmental Biology of Fishes, 73(2): 171-180.
- Rodrigues, R. R., L. N. Carvalho, J. Zuanon & K. Del-Claro. 2009. Color changing and behavioral context in the Amazonian dwarf cichlid Apistogramma hippolytae (Perciformes). Neotropical Ichthyology, 7(4): 641-646.
- Rogers, W. 1988. Parental investment and division of labor in the Midas Cichlid (Cichlasoma citrinellum). Ethology, 79(2): 126-142.
- Ros, A. F. H., K. Becker & R. F. Oliveira. 2006. Aggressive behaviour and energy metabolism in a cichlid fish, Oreochromis mossambicus Physiology & Behavavior, 89(2): 164-170.
- Schwanck, E. J. 1989. Parental care of Tilapia mariae in the field and in aquaria. Environmental Biology of Fishes, 24(4): 251-265.
- Smith-Grayton, P. K. & M. H. A. Keenleyside. 1978. Male-female parental roles in Herotilapia multispinosa (Pisces: Cichlidae). Animal Behaviour, 26(2): 520-526.
- Snekser, J. L. & M. Itzkowitz. 2009. Sex differences in retrieval behavior by the biparental convict cichlid. Ethology, 115(5): 457-464.
- Souza-Filho, P. S. & L. Casatti. 2010. História de vida de Laetacara aff. araguaiae Ottoni & Costa, 2009 (Perciformes, Cichlidae) em dois riachos no Noroeste do Estado de São Paulo, Brasil. Biota Neotropica, 10(2): 153-158.
- Timms, A. M. & M. H. A. Keenleyside. 1975. The reproductive behaviour of Aequidens paraguayensis (Pisces, Cichlidae). Zeitschrift für Tierpsychologie, 39: 8-23.
- Townshend, T. J. & R. J. Wootton. 1985. Variation in the mating system of a biparental cichlid fish, Cichlasoma pananense Behaviour, 95: 181-197.
- Trivers, R. L. 1972. Parental investment and sexual selection. Pp. 136-179. In: Campbell, B. G. (Ed.). Sexual selection and descent of man, 1871-1971. Chicago, Aldine, 378p.
- Ward, J. A. & J. I. Samarakoon. 1981. Reproductive tactics of the Asian cichlids of the genus Etrolopus in Sri Lanka. Environmental Biology of Fishes, 6(1): 95-103.
- Yamamoto M. E., S. Chellappa, M. S. R. F. Cacho & F. A. Huntingford. 1999. Mate guarding in an Amazonian cichlid, Pterophyllum scalare Journal of Fish Biology, 55(4): 888-891.
- Zar, J. H. 1999. Biostatistical analysis. New Jersey, Prentice Hall, 663p.
Reproductive behavior and parental roles of the cichlid fish Laetacara araguaiae
Publication Dates
-
Publication in this collection
10 June 2011 -
Date of issue
June 2011