Abstract
Purple ipe (Handroanthus impetiginosus) is an important tree species in Cerrado biome conservation and very popular at the landscaping and urban afforestation. However, its micropropagation is affected by pathogens, such as Oidium sp. The aim this study was evaluate the efficiency of seed treatments in the control of powdery mildew of purple ipe obtained by micropropagation. The symptoms were observed during in vitro germination, a Koch’s postulates were performed for confirm the pathogenicity, colonization of the pathogen on the leaves was analyzed in optical and scanning microscopes and a scale to evaluate severity was proposed. Two experiments were realized to powdery mildew control using a completely randomized design, with 30 replicates. First experiment: Seeds were treated with ethanol (Et), chlorothalonil + thiophanate-methyl (C+TM), and sodium hypochlorite (NaOCl); second experiment: Seeds were treated with Et, NaOCl, C+TM, and neem oil. Disease severity and area under the disease progress curve (AUDPC) were assessed in both experiments. Disease symptoms and typical pathogen structures were observed, and the pathogenicity was confirmed. The disease severity was reduced by 30.78% in 1.5% neem oil for 10 min when compared with C+TM for 15 min. We conclude that neem oil can be a strategy sustainable for the control of powdery mildew in purple ipe in tissue culture.
Keywords:
alternative control; fungicide; Handroanthus impetiginosus; neem oil; tissue culture
Resumo
O ipê-roxo, Handroanthus impetiginosus, é uma espécie arbórea importante na conservação do bioma Cerrado e muito procurada no paisagismo e arborização urbana. No entanto, sua micropropagação é afetada por patógenos, como Oidium sp. O objetivo deste estudo foi avaliar a eficácia dos tratamentos em sementes no controle do oídio do ipê roxo por micropropagação. Os sintomas foram observados durante a germinação in vitro, os postulados de Koch foram realizados para confirmação da patogenicidade, a colonização do patógeno nas folhas foi analisada em microscópios ópticos e de varredura e uma escala para avaliação de severidade foi proposta. Foram realizados dois ensaios para controle do oídio em delineamento inteiramente casualizado, com 30 repetições. Primeiro experimento: sementes foram tratadas com etanol, clorotalonil + tiofanato-metílico (C+TM) e hipoclorito de sódio; segundo experimento: sementes foram tratadas com etanol, NaOCl, C+TM e óleo de neem. A severidade da doença e a área abaixo da curva de progresso da doença foram quantificadas. Os sintomas da doença e estruturas típicas do patógeno foram observados e a patogenicidade foi confirmada. A severidade da doença foi reduzida em 30,78% com 1,5% de óleo de neem por 10 min quando comparado com C + TM por 15 min. Concluímos que o óleo de neem pode ser uma estratégia sustentável para o controle do oídio em ipê roxo na cultura de tecidos.
Palavras-chave:
controle alternativo; cultura de tecidos; fungicidas; Handroanthus impetiginosus; óleo de nem
Introduction
Handroanthus impetiginosus (syn. Tabebuia avellanedae), popularly known as purple ipe, is a tree found in several Brazilian biomes; the conservation of this species is crucial since it is included in the official list of endangered flora species (Cncflora, 2012CNCFLORA. Handroanthus impetiginosus in Lista Vermelha da flora brasileira versão 2012.2. 2012. Available at: <Available at: http://cncflora.jbrj.gov.br/portal/pt-br/profile/Handroanthus impetiginosus > Accessed on: June 23, 2019.
http://cncflora.jbrj.gov.br/portal/pt-br...
).
The purple ipe has great beauty when in bloom, so it is one of the most popular species in Brazilian landscaping and in urban afforestation (Bassegio et al., 2017BASSEGIO, C.; FOGAÇA, L. A.; BALTAZAR, P.; EMMEL, E. Desenvolvimento de ipê-roxo em meios de cultura e concentrações de bap (6-benzilaminopurna) durante a etapa de multiplicação in vitro. Acta Iguazu, v.6, n.1, p.72-80, 2017. https://doi.org/10.48075/actaiguaz.v6i1.16878
https://doi.org/10.48075/actaiguaz.v6i1....
). Due to its importance, it was the first Cerrado tree to have its genome completely sequenced (Silva-Júnior et al., 2018SILVA-JÚNIOR, O.B.; GRATTAPAGLIA, D.; NOVAES, E.; COLLEVATTI, R.G. Genome assembly of the Pink Ipê (Handroanthus impetiginosus, Bignoniaceae), a highly valued, ecologically keystone Neotropical timber forest tree. Gigascience, v.7, n.1, p.1-16, 2018. https://doi.org/10.1093/gigascience/gix125
https://doi.org/10.1093/gigascience/gix1...
). The purple ipe is widely used in the reforestation of degraded areas and urban landscaping owing to its recognized beauty (Chaves, 2018CHAVES, P.M.S.; DA SILVA, J. R.; BRAGA, M.O.; MARQUES, N.S.; FREITAS, A.D.D. Qualidade fisiológica de sementes e crescimento inicial de mudas de Handroanthus impetiginosus sob diferentes sombreamentos e substratos. Revista Verde de Agroecologia e Desenvolvimento Sustentável, v.13, n.1, p.22-26, 2018. https://doi.org/10.18378/rvads.v13i1.5348
https://doi.org/10.18378/rvads.v13i1.534...
; Máximo, 2020MÁXIMO, W.; SANTOS, B.; MARTINS, J.; BEIJO, L.; BARBOSA, S. Multiplicação e enraizamentoin vitrodeHandroanthus impetiginosus(Mart. ex DC.) Mattos. Ciência Florestal, v.30, n.3, p.658-668, 2020. https://doi.org/10.5902/1980509827012
https://doi.org/10.5902/1980509827012...
). Furthermore, it is an arboreal species of economic value because of its wood presents good durability and resistance in addition to its ornamental and medicinal uses (Santos et al., 2020SANTOS, J.S.H.; SANTOS, K.T.H.; OLIVEIRA, V.S.; SANTOS, G.P.; MENEZES, L.F.T.; CZEPAK, M.P.; FALQUETO, A.R.; AOYAMA, E.M.; SCHMILDT, O.; SCHMILDT, E.R. Regression models for prediction of leaf area in purple ipe [Tabebuia impetiginosa (Mart.)]. Australian Journal of Crop Science, v.14, n.4, p.654-659, 2020. https://doi.org/10.21475/ajcs.20.14.04.p2291
https://doi.org/10.21475/ajcs.20.14.04.p...
) and a source of β-lapachone, one medicinal component (secondary metabolite), present both in stem periderm and woody part, which has anti-inflammatory, analgesic, antibiotic and antineoplastic properties (Bang et al., 2016BANG, W.; JEON, Y.; CHO, J. H.; LEE, R.H.; PARK, S.; SHIN, J.; CHOI, N.; CHOI, Y.H.; CHO, J.; SEO, J.; LEE, S.; SHIM, J.; CHAE, J. β-lapachone suppresses the proliferation of human malignant melanoma cells by targeting specificity protein 1. Oncology Reports, v.35, n.2, p.1109-1116, 2016. https://doi.org/10.3892/or.2015.4439
https://doi.org/10.3892/or.2015.4439...
; Campanholi et al., 2018CAMPANHOLI, K.S.S.; GEROLA, A.P.; VILSINSKI, B.H.; OLIVEIRA, E.L.; MORAIS, F.A.P.; RABELLO, B.R.; BRAGA, G.; CALORI, I.R.; SILVA, E.L.; HIOKA, N.; CAETANO, W. Development of Pluronic® nanocarriers comprising Pheophorbide, Zn-Pheophorbide, Lapachol and β-lapachone combined drugs: Photophysical and spectroscopic studies. Dyes and Pigments, v.157, p.238-250, 2018. https://doi.org/10.1016/j.dyepig.2018.04.057
https://doi.org/10.1016/j.dyepig.2018.04...
).
Oidium sp., a biotrophic pathogen is the causal agent of powdery mildew in horticultural, fruit, ornamental, and forest species, including purple ipe and causing significant losses (Liyanage et al., 2017LIYANAGE, K.K.; KHAN, S.; BROOKS, S.; MORTIMER, P.E.; KARUNARATHNA, S.C.; XU, J.; HYDE, K.D. Taxonomic revision and phylogenetic analyses of rubber powdery mildew fungi. Microbial Pathogenesis, v.105, p.185-195, 2017. https://doi.org/10.1016/j.micpath.2017.01.054
https://doi.org/10.1016/j.micpath.2017.0...
; Li et al., 2020). The disease occurs in all regions of the world and causes physiological changes such as reducing photosynthesis and perspiration (Suthaparan et al., 2016SUTHAPARAN, A.; SOLHAUG, K. A.; STENSVAND, A.; GISLERØD, H.R. Determination of UV action spectra affecting the infection process of Oidium neolycopersici, the cause of tomato powdery mildew. Journal of Photochemistry and Photobiology B: Biology, v.156, p.41-49, 2016.https://doi.org/10.1016/j.jphotobiol.2016.01.009
https://doi.org/10.1016/j.jphotobiol.201...
; Zhai et al., 2020ZHAI, D.L.; WANG, J.; THALER, P. Contrasted effects of temperature during defoliation vs. refoliation periods on the infection of rubber powdery mildew (Oidium heveae) in Xishuangbanna, China. International Journal of Biometeorology, v.64, p.1835-1845, 2020. https://doi.org/10.1007/s00484-020-01969-y
https://doi.org/10.1007/s00484-020-01969...
;).
In vitro propagation is highlighted as a tool for the deployment of clonal forests, because enables to rapid production of standardized plants of selected genotypes on large scale (Mushtaq at al., 2017MUSHTAQ, T.; BANYAL, R.; MUGLOO, J.; MUSHTAQ, T.; AZIZ, M. A. Clonal forestry: An effective technique for increasing the productivity of plantations. SKUAST Journal of Research, v.19, n.1, p.22-28, 2017.). The contamination by microorganisms and pathogens represents one of the major problems of tissue culture and may become a limiting factor in establishing protocols for some species (Orlikowska et al., 2017ORLIKOWSKA, T.; NOWAK, K.; REED, B. Bacteria in the plant tissue culture environment. Plant Cell Tissue and Organ Culture, v.128, p.487-508, 2017. https://doi.org/10.1007/s11240-016-1144-9
https://doi.org/10.1007/s11240-016-1144-...
; Kim et al., 2017KIM, D.H.; GOPAL, J.; SIVANESAN, I. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Advances, v.7, n.58, p.36492-36505, 2017. https://doi.org/10.1039/c7ra07025j
https://doi.org/10.1039/c7ra07025j...
).
One of the pathogens of purple ipe is powdery mildew which occurs in natural habitat and conventional system. The pathogen is recognized by the plasma membrane, overcoming the initial and later defense responses. Subsequently, they secrete effector proteins that alter the resistance and manifestation of defense responses in the cytosol through the haustoria, their feeding structures (Li et al., 2020LI, X.; LIU, Y.; HE, Q.; LI, S.; LIU, W.; LIN, C.; MIAO, W. A candidate secreted effector protein of rubber tree powdery mildew fungus contributes to infection by regulating Plant ABA biosynthesis. Frontiers in microbiology, v.11, 591387, 2020. https://doi.org/10.3389/fmicb.2020.591387
https://doi.org/10.3389/fmicb.2020.59138...
), and the analysis of the colonization of the interaction becomes important to reveal the forms of control.
In conventional agriculture the disease control is performed only with fungicides such as chlorothalonil + thiophanate-methyl (C+TM), which have a systemic and contact action (Ihara, 2019IHARA. Iharabras S.A. Indústrias Químicas. 2019. Available at: <Available at: http://www.ihara.com.br/upload/produtos/bula/1557518279.pdf > Accessed on: June 28, 2019.
http://www.ihara.com.br/upload/produtos/...
). Alternative control, such as the use of oils and plant extracts of neem (Azadirachta indica A. Juss), is widely used in organic crops, with acaricide, nematicide, and fungicide effects (Mishra et al., 2017MISHRA, V.; LAL, A.A.; SIMON, S. Efficacy of botanicals and bio-agents against powdery mildew disease of garden pea (Pisum sativum L.). Journal of Pharmacognosy and Phytochemistry, v.6, p.1125-1126, 2017.), however, no reporting of your use in micropropagation has been reported.
Therefore, the aim this study was evaluate the efficiency of seed treatments in the control of powdery mildew of purple ipe obtained by micropropagation.
Material and Methods
Seeds of purple ipe from a matrix located in the Leolídio di Ramos Caiado municipal park in Goiânia, Goiás State, Brazil (16°37’46”S 49°15’23”W) were used.
In one previously experiment well-formed seeds with no visible signs of injury or disease had their membrane wings removed. For first cultivation the seeds were disinfested or not with ethanol and NaOCl for 10 and 20 min. After the treatment of seeds in a laminar flow cabinet, they were rinsed three times with autoclaved H2O and inoculated in sterile flasks of 300 mL containing 30 mL of MS medium (Murashige and Skoog, 1962MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioexperiments with tobacco tissue culture. Physiologia Plantarum, v.15, n.3, p.473-497, 1962.), supplemented with inositol (100 mg L-1) and sucrose (30 g L-1), and solidified with agar (7 g L-1). The pH of the medium was adjusted to 5.8 before autoclaving at 120 °C for 20 min. The cultures were kept in a growing room at a temperature of 25 ± 2 °C, photoperiod of 16 h light, and irradiance of 36 mmol m-2 s-1 (Abbade et al., 2007ABBADE, L.C.; PAIVA, P.D.O.; PAIVA, R. Efeito do GA3 e meios de cultura na germinação in vitro de sementes de Ipê-Branco (Tabebuia roseo-alba). Ornamental Horticulture, v.13, p.487-489, 2007.; Paiva et al., 2007PAIVA, P.D.O.; ABBADE, L. C.; CENTOFANTE, A.R.; PAIVA, R. Desinfestação de sementes de Ipê-Branco (Tabebuia roseo-alba) para estabelecimento in vitro. Ornamental Horticulture , v.13, p.1631-1633, 2007. https://doi.org/10.14295/oh.v13i0.1804
https://doi.org/10.14295/oh.v13i0.1804...
). The design was entirely randomized, with 50 replicates and one seed per experimental plot.
After the presence of a pathogen in the in vitro plants was detected in all plants, the identification was performed on the basis morphological characters (type and form of conidiophores and conidia) using the mycological key by Barnett and Hunter (2003BARNETT, H.L.; HUNTER, B.B. Illustrated genera of imperfect fungi. Minnesota: The American Phytopatological Society Press, 2003. 218p.). At 60 days, 12 leaf samples of purple ipe with or without symptoms of the disease were collected and fixed in FAA70 (Formalin -70% alcohol - acetic acid) (Johansen, 1940JOHANSEN, D. A. Plant Microtechnique. New York: McGraw-Hill Book Company Inc, 1940. 523p.) for 24-h and conserved in 70% ethanol for further observation using optical microscopy and scanning electron microscopy (SEM).
For observation under optical microscope, transversal sections were clarified with 6% NaOCl, washed in distilled water, and subjected to double coloration with 0.1% basic fuchsin and 0.3% Astra blue in the ratio 1:3 for 3 min. Then, they were mounted between the slide and a coverslip with hydrating glycerin. The observation was performed using a Leica DM 500 optical microscope with a digital camera attached. For observation using SEM, transversal and longitudinal sections approximately 1 cm in length were dehydrated in increasing ethanolic series (70%, 80%, 90%, and 99.1% ethanol, 15 min each); adapted from Chaibub et al. (2020CHAIBUB, A.A.; SOUSA, T.P.; ARAÚJO, L.G.; FILIPPI, M.C.C.Cladosporium cladosporioidesC24G modulates gene expression and enzymatic activity during leaf blast suppression in rice plants. Journal of Plant Growth Regulation, v.39, p.1140-1152, 2020. https://doi.org/10.1007/s00344-019-10052-9
https://doi.org/10.1007/s00344-019-10052...
) subjected to CO2 critical point drying, and covered with gold films in a Denton Vacuum film deposition system. The images were obtained using a Jeol (JSM-6610) scanning electron microscope equipped with EDS System (Thermo Scientific NSS Spectral Imaging), operated at 4 Kv, and installed at the High-Resolution Microscopy Multiuser Laboratory (LABMIC).
In this case, in which powdery mildew is a mandatory and biotrophic parasite, in which it needs its living host and cannot be cultivated, Koch’s postulate is accomplished by exposing a sick plant to a healthy plant. A Koch’s postulate was performed on the healthy leaves, detached from purple ipe cultivated in vitro for 15 days, to determine and confirm the host-pathogen association. Three detached healthy leaves were exposed to a diseased leaf and incubation in a germination chamber at 25 ± 2 °C, photoperiod of 16 h light. The evaluation of the presence of powdery mildew symptoms was performed at 10 days after inoculation.
Two experiments were performed in a completely randomized design, with 30 replicates to evaluate the efficiency of other seed disinfectants in the control of powdery mildew and each experimental plot consisted of one seed. In the first experiment, the seeds were subjected to the following treatments: control (no treatment), 70% ethanol for 2 min, C+TM for 5 min, and 2% NaOCl for 10 min; 70% ethanol for 2 min, C+TM for 10 min, and 2% NaOCl for 10 min; 70% ethanol for 2 min, C+TM for 15 min, and 2% NaOCl for 10 min. The fungicide (C+TM) was used at the dose recommended by the manufacturer to control powdery mildew.
In the second experiment, the following treatments were tested: control (no treatment), 70% ethanol for 2 min and 2% NaOCl for 10 min; 70% ethanol for 2 min, C+TM for 5 min, and 2% NaOCl for 10 min; C+TM for 15 min, and 1.5% neem oil for 10 min. In both experiments, after the treatments, the seeds were inoculated in MS medium, kept under the same conditions for in vitro germination as described in the previous experiment.
In the first experiment, powdery mildew severity was measured at 60, 80, and 100 days and in the second experiment from 14 to 28 days with two-day intervals between the evaluations to determine the area under the disease progress curve (AUDPC), according to Shaner and Finney (1977CARDOSO, J.E.; MARTINS, M.V.V.; LIMA, J.S.; VIANA, F.M.P.; SILVA, L.G.C. Controle químico do oídio do cajueiro. Fortaleza: Embrapa Agroindústria Tropical, 2012. 4p.). In both experiments, severity evaluation was performed using a scale adapted from Paz Lima and Café Filho (2004LIMA, M.L.; CAFÉ FILHO, A.C. Estabilidade da resistência de Capsicum spp. ao oídio em telado e casa de vegetação. Fitopatologia Brasileira, v.29, n.5, p.519-525, 2004. https://doi.org/10.1590/S0100-41582004000500008
https://doi.org/10.1590/S0100-4158200400...
) developed for this work (Table 1), ranging from 0 (no symptoms) to 5 (very severe infection) and the data were transformed using arcsen √x/100. Subsequently, the data were subjected to analysis of variance and means compared using the Tukey test at 5% probability with the R software.
Severity assessment scale developed for powdery mildew in purple ipe plants obtained by in vitro germination.
Results and Discussion
In the present study, the occurrence of powdery mildew in purple ipe plants may have been favored by in vitro culture conditions such as controlled temperature, light intensity, and the culture medium used. According to Rana et al. (2018RANA, A.; MALANNAYAR, A.B.; BANYAL, D.K. Studies on biology and environmental factors affecting the development of tomato powdery mildew under protected cultivation. Indian Phytopathology, v.71, p.385-391, 2018. https://doi.org/10.1007/s42360-018-0049-4
https://doi.org/10.1007/s42360-018-0049-...
) and Gu et al. (2020GU, Y.; CHU, B.; WANG, C.; LI, L.; ZHOU, Y.; LUO, Y.; MA, Z. Spore concentrations of Blumeria graminis f. sp. tritici in relation to weather factors and disease development in Gansu, China. Canadian Journal of Plant Pathology, v.42, n.1, p.52-61, 2020. https://doi.org/10.1080/07060661.2019.1630011
https://doi.org/10.1080/07060661.2019.16...
) these variables influence spore production. For biotrophic parasites like Oidium sp. the sporulation is high when conditions favorable to photosynthesis (long photoperiods, high luminous intensity, and broad light spectrum) during the colonization period. Such advantages for the reproduction and development of the fungus are found during the process of in vitro seed germination.
This is the first report of the occurrence and control of powdery mildew in plants obtained by in vitro germination of purple ipe seeds. The scale adapted from Lima and Café Filho (2004LIMA, M.L.; CAFÉ FILHO, A.C. Estabilidade da resistência de Capsicum spp. ao oídio em telado e casa de vegetação. Fitopatologia Brasileira, v.29, n.5, p.519-525, 2004. https://doi.org/10.1590/S0100-41582004000500008
https://doi.org/10.1590/S0100-4158200400...
) developed for this work (Table 1, Figure 1), ranging from 0 (no symptoms) to 5 (very severe infection) was efficient to evaluate of disease.
Symptoms observed in purple ipe plants during in vitro germination used to adapt the scale proposed by Lima and Café Filho (2004LIMA, M.L.; CAFÉ FILHO, A.C. Estabilidade da resistência de Capsicum spp. ao oídio em telado e casa de vegetação. Fitopatologia Brasileira, v.29, n.5, p.519-525, 2004. https://doi.org/10.1590/S0100-41582004000500008
https://doi.org/10.1590/S0100-4158200400... ). A) leaves without symptoms, B) sporulation with less than 25% of affected leaf area, C) sporulation with more than 25% and less than 50% of affected leaf area, D) sporulation with more than 50% of affected leaf area, E) rolling, F) stem necrosis, G) leaf necrosis, and H) sporulation with total coverage of the affected leaf area.
The main symptoms observed and described on the scale that were illustrated are leaves without symptoms (Figure 1A), leaves with different degrees of sporulation (Figures 1B, 1C, 1D, 1H), deformations, curling and leaf fall (Figures 1E, 1F), necrosis on the stem (Figure 1F) and on the leaves (Figure 1G).
After the symptoms description and observed, Koch’s postulates were carried out to confirm the association of the pathogen to the host and with this we confirm this association. When selecting a diseased plant (Figure 2A) and placing it in contact with a healthy plant (Figure 2B), we observe the reproduction of symptoms (Figure 2C) and therefore, we confirm the association of the pathogen with the host.
Koch’s postulates performed to confirm the powdery mildew association in purple ipe plants during in vitro germination. A) symptomatic leaf, B) leaf without symptoms, and C) leaf with symptom after contact with first symptomatic leaf.
Botelho et al. (2008BOTELHO, L.S; MORAES, M.H.D.; MENTEN, J.O.M. Fungos associados às sementes de ipê-amarelo (Tabebuia serratifolia) e ipê-roxo (Tabebuia impetiginosa): incidência, efeito na germinação e transmissão para as plântulas. Summa phytopathologica, v.34, n.4, p.343-348, 2008. https://doi.org/10.1590/S0100-54052008000400008
https://doi.org/10.1590/S0100-5405200800...
) highlighted the importance of pathogens associated with purple ipe seeds since they are harmful to the production of this species’ plants. Our study demonstrated and confirmed that powdery mildew is a potential pathogen for purple ipe and is carried by the seed.
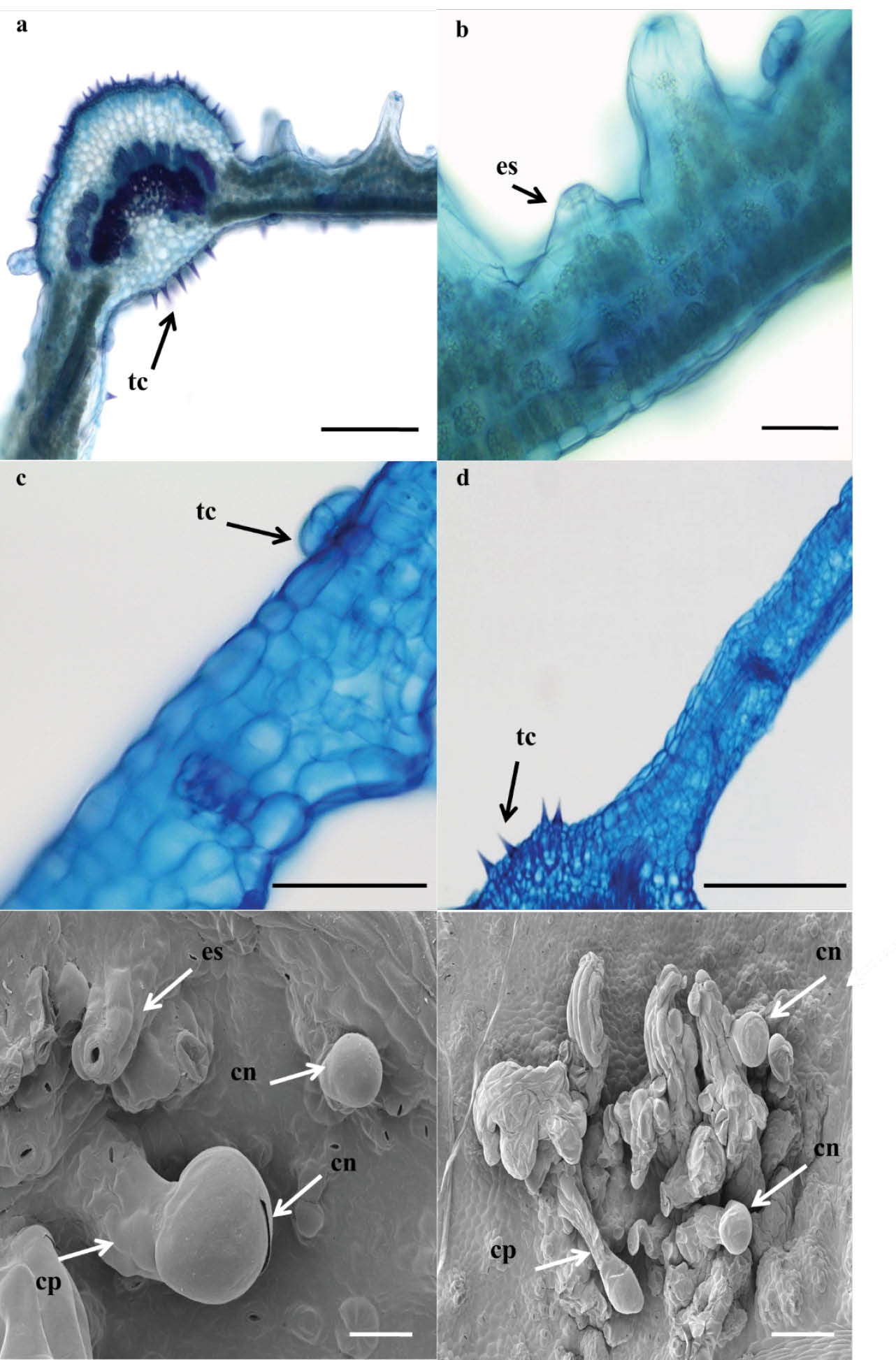
Transverse and longitudinal sections of the leaves with symptoms observed under optical microscope (Figures 3A, 3B) and SEM (Figures 3E, 3F), respectively showed the presence of typical conidiophores of the genus Oidium: simple, erect, and increased in length as the conidia are formed in the chain.
Description of the occurrence of powdery mildew in purple ipe plants during in vitro germination. A and B) optical microscope of purple ipe plants sections with conidiophores of powdery mildew (cp) and typical structures of leaves as trichomes (tc) and stomata (es) (Bars = 100 and 50 µm, respectively), C and D) leaves without symptoms with typical structures of leaves as trichomes (tc) and without conidiophores of powdery mildew (Bars = 50 and 500 µm, respectively), E and F) elevation of the epidermal region under the stomata seen in scanning electron microscopy (es) and formation of conidia (cn) and conidiophore (cp) of powdery mildew on leaves (Bars = 50 and 100 µm, respectively).
The conidia are typical of the genus Oidium: cylindrical, hyaline, and produced in basipetal chains (older conidia at the apex and younger at the base of the chain). Transverse and longitudinal sections of leaves without symptoms observed under optical microscope demonstrated absence of powdery mildew structures and the presence of characteristic leaf structures such as trichomes (tc) (Figures 3C, 3D) also observed in symptomatic leaves (Figures 3A, 3B). The colonization of the pathogen altered the development of the stomas on the abaxial side of the leaf. Although the stomata normally develop at the same height as the other epidermal cells, i.e., on the symptomatic leaves, the stomas near the regions of colonization developed above the other cells (Figure 3E). In the samples analysed using SEM, conidiophores that formed from the differentiation of hyphae in regions near the stomas were also identified (Figure 3E and 3F).
In both experiments the treatment control without seed treatment not germinated because the high occurrence of powdery mildew, others fungi, and bacteria.
In the first experiment at 60, 80 and 100 days after the in vitro establishment, severity was high in plants subjected to 2 min in 70% ethanol, 15 min in C+TM, and 10 min in 2% NaOCl) by 2.92, 2.92 and 3.15, respectively when compared with 70% ethanol for 2 min, C+TM for 5 min, and 2% NaOCl for 10 min (1.57, 1.57 and 1.65); and 70% ethanol for 2 min, C+TM for 10 min, and 2% NaOCl for 10 min (2.75, 2.75 and 2.95)
Severity at 60, 80, and 100 days of powdery mildew in purple ipe plants during in vitro germination in seed treatments: 70% Ethanol for 2 min, C+TM for 5 min, and 2% NaOCl for 10 min; 70% Ethanol for 2 min, C+TM for 10 min, and 2% NaOCl for 10 min; and 70% Ethanol for 2 min, C+TM for 15 min, and 2% NaOCl for 10 min.
The aim of the chemical treatment of seeds of a forest species is to control or reduce diseases efficiently. Besides ethanol and NaOCl, some treatments used in this study included systemic and contact fungicide (C+TM). This product is recommended for the control of powdery mildew in field conditions in several crops such as beans, melon, watermelon, and roses (IHARA, 2019).
Treatment with 2 min in 70% ethanol, 5 min in C+TM, and 10 min in 2% NaOCl promoted the lowest values of severity. In general, plants from seeds exposed for a less time to the disinfecting agents were less fragile, presenting lower severity than that of other treatments. The shorter exposure time of fungicide (C+TM) meant that the tissues of the seeds were less degraded, leading to less physiological alterations. Thus, the higher severity rates observed in in other treatments may be related to the toxic effects of the exposure time to the disinfecting agents used.
In the second experiment, from 14 (disease emergence) to 22 evaluation days, the treatment with 1.5% neem oil for 10 min was the only one that differed from the others from 16 days to the last evaluation day. The treatments with 70% ethanol for 2 min and 2% NaOCl for 10 min); 70% ethanol for 2 min, C+TM for 5 min, and C+TM for 15 min presented 3.56, 3.43, and 3.8 of severity on the last evaluation day, respectively, while treatment with 10 min in 1.5% neem oil presented 2.63
The AUDPC in the treatment 10 min in 1.5% neem oil was 19.63, and was significantly lower than 70% ethanol for 2 min and 2% NaOCl for 10 min; 70% Et for 2 min, C+TM for 5 min and C+TM for 15 min with 36.90, 29.90, and 40.46 respectively (Figure 4). The disease severity was reduced by 30.78% in the treatment with 1.5% neem oil for 10 min when compared with C+TM for 15 min.
Area under disease progress curve of powdery mildew in purple ipe plants during in vitro germination at 14, 16, 18, 20, 22, 24, 26, and 28 days of seed treatments 1 (70% Ethanol for 2 min and 2% NaOCl for 10 min), 2 (70% Ethanol for 2 min, C+TM for 5 min, and 2% NaOCl for 10 min), 3 (C+TM for 15 min), and 4 (1.5% neem oil for 10 min). Means followed by the same letters were not significantly different from each other according to Tukey’s test (p < 0.05).
Cardoso et al. (2012CARDOSO, J.E.; MARTINS, M.V.V.; LIMA, J.S.; VIANA, F.M.P.; SILVA, L.G.C. Controle químico do oídio do cajueiro. Fortaleza: Embrapa Agroindústria Tropical, 2012. 4p.) aimed to control Oidium anacardii by using C+TM, and observed a reduction in the severity and AUDPC in cashew; however, the authors found that it was not the most efficient treatment for disease control, as also observed in our study. In the second experiment (A2), the best treatment was with the neem oil, which showed a significant difference in severity of powdery mildew and AUDPC compared to those of other treatments.
Alternative products, such as neem oil, probably leave no residues on plant tissues and have low production costs, and thus are more advantageous. Neem oil as an alternate control agent has proven to be efficient, including for powdery mildew. The neem oil reduced powdery mildew severity in rose (Ramos et al., 2020RAMOS, S.M.B; ALMEIDA, E.F.A.; ROCHA, F.S.; FERNANDES, M.F.G.; SANTOS, E.B. Organic fertilization and alternative products in the control of powdery mildew. Ornamental Horticulture , v.26, n.1, p.57-68, 2020. https://doi.org/10.1590/2447-536x.v26i1.2109
https://doi.org/10.1590/2447-536x.v26i1....
), garden pea (Mishra et al., 2017MISHRA, V.; LAL, A.A.; SIMON, S. Efficacy of botanicals and bio-agents against powdery mildew disease of garden pea (Pisum sativum L.). Journal of Pharmacognosy and Phytochemistry, v.6, p.1125-1126, 2017.) and in Cannabis sativa L. of over 50% (Scott and Punja, 2020SCOTT, C.; PUNJA, Z.K. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L. (marijuana) plants. Canadian Journal of Plant Pathology , p.1-19, 2020. https://doi.org/10.1080/07060661.2020.1836026
https://doi.org/10.1080/07060661.2020.18...
). Spraying neem oil on C. sativa with powdery mildew was also efficient and the results did not differ statistically from that fluopyram treatment (Scott and Punja, 2020).
There are four registered products based on neem oil in Brazil, however, it has an indication for the control of powdery mildew in cashew and beans. Our results, thus, show potential use of the product registered also for the purple ipe (Agrofit, 2021AGROFIT. The Agrofit Phytosanitary pesticide systems. 2021. Available at: Available at: http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons . Access on: January 8, 2021.
http://extranet.agricultura.gov.br/agrof...
).
The effects that contributed to the efficiency of neem oil in the control of powdery mildew may have been direct through the action of toxic compounds in the death and inactivation of the pathogen and indirect through the induction of resistant plants eliciting pathways signaling defense responses.
In our study will contribute to future micropropagation for this specie on the selection of superior genotypes, including the evaluation of in vitro resistance in many genotypes, with improved environmental control and in less physical space. In addition, this study provides a promising method of seed treatment that could be used ex vitro.
Conclusions
We conclude that Oidium sp. is a potential pathogen to the purple ipe and the neem oil can be a strategy sustainable for the control by seed treatment of powdery mildew in tissue culture.
Acknowledgments
We thank the Federal University of Goiás and the Brazilian National Council for Scientific and Technological Development (CNPq) for financial support and scholarship.
References
- ABBADE, L.C.; PAIVA, P.D.O.; PAIVA, R. Efeito do GA3 e meios de cultura na germinação in vitro de sementes de Ipê-Branco (Tabebuia roseo-alba). Ornamental Horticulture, v.13, p.487-489, 2007.
- AGROFIT. The Agrofit Phytosanitary pesticide systems. 2021. Available at: Available at: http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons Access on: January 8, 2021.
» http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons - BANG, W.; JEON, Y.; CHO, J. H.; LEE, R.H.; PARK, S.; SHIN, J.; CHOI, N.; CHOI, Y.H.; CHO, J.; SEO, J.; LEE, S.; SHIM, J.; CHAE, J. β-lapachone suppresses the proliferation of human malignant melanoma cells by targeting specificity protein 1. Oncology Reports, v.35, n.2, p.1109-1116, 2016. https://doi.org/10.3892/or.2015.4439
» https://doi.org/10.3892/or.2015.4439 - BASSEGIO, C.; FOGAÇA, L. A.; BALTAZAR, P.; EMMEL, E. Desenvolvimento de ipê-roxo em meios de cultura e concentrações de bap (6-benzilaminopurna) durante a etapa de multiplicação in vitro. Acta Iguazu, v.6, n.1, p.72-80, 2017. https://doi.org/10.48075/actaiguaz.v6i1.16878
» https://doi.org/10.48075/actaiguaz.v6i1.16878 - BARNETT, H.L.; HUNTER, B.B. Illustrated genera of imperfect fungi. Minnesota: The American Phytopatological Society Press, 2003. 218p.
- BOTELHO, L.S; MORAES, M.H.D.; MENTEN, J.O.M. Fungos associados às sementes de ipê-amarelo (Tabebuia serratifolia) e ipê-roxo (Tabebuia impetiginosa): incidência, efeito na germinação e transmissão para as plântulas. Summa phytopathologica, v.34, n.4, p.343-348, 2008. https://doi.org/10.1590/S0100-54052008000400008
» https://doi.org/10.1590/S0100-54052008000400008 - CAMPANHOLI, K.S.S.; GEROLA, A.P.; VILSINSKI, B.H.; OLIVEIRA, E.L.; MORAIS, F.A.P.; RABELLO, B.R.; BRAGA, G.; CALORI, I.R.; SILVA, E.L.; HIOKA, N.; CAETANO, W. Development of Pluronic® nanocarriers comprising Pheophorbide, Zn-Pheophorbide, Lapachol and β-lapachone combined drugs: Photophysical and spectroscopic studies. Dyes and Pigments, v.157, p.238-250, 2018. https://doi.org/10.1016/j.dyepig.2018.04.057
» https://doi.org/10.1016/j.dyepig.2018.04.057 - CNCFLORA. Handroanthus impetiginosus in Lista Vermelha da flora brasileira versão 2012.2. 2012. Available at: <Available at: http://cncflora.jbrj.gov.br/portal/pt-br/profile/Handroanthus impetiginosus > Accessed on: June 23, 2019.
» http://cncflora.jbrj.gov.br/portal/pt-br/profile/Handroanthus impetiginosus - CARDOSO, J.E.; MARTINS, M.V.V.; LIMA, J.S.; VIANA, F.M.P.; SILVA, L.G.C. Controle químico do oídio do cajueiro. Fortaleza: Embrapa Agroindústria Tropical, 2012. 4p.
- CHAIBUB, A.A.; SOUSA, T.P.; ARAÚJO, L.G.; FILIPPI, M.C.C.Cladosporium cladosporioidesC24G modulates gene expression and enzymatic activity during leaf blast suppression in rice plants. Journal of Plant Growth Regulation, v.39, p.1140-1152, 2020. https://doi.org/10.1007/s00344-019-10052-9
» https://doi.org/10.1007/s00344-019-10052-9 - CHAVES, P.M.S.; DA SILVA, J. R.; BRAGA, M.O.; MARQUES, N.S.; FREITAS, A.D.D. Qualidade fisiológica de sementes e crescimento inicial de mudas de Handroanthus impetiginosus sob diferentes sombreamentos e substratos. Revista Verde de Agroecologia e Desenvolvimento Sustentável, v.13, n.1, p.22-26, 2018. https://doi.org/10.18378/rvads.v13i1.5348
» https://doi.org/10.18378/rvads.v13i1.5348 - GU, Y.; CHU, B.; WANG, C.; LI, L.; ZHOU, Y.; LUO, Y.; MA, Z. Spore concentrations of Blumeria graminis f. sp. tritici in relation to weather factors and disease development in Gansu, China. Canadian Journal of Plant Pathology, v.42, n.1, p.52-61, 2020. https://doi.org/10.1080/07060661.2019.1630011
» https://doi.org/10.1080/07060661.2019.1630011 - IHARA. Iharabras S.A. Indústrias Químicas. 2019. Available at: <Available at: http://www.ihara.com.br/upload/produtos/bula/1557518279.pdf > Accessed on: June 28, 2019.
» http://www.ihara.com.br/upload/produtos/bula/1557518279.pdf - JOHANSEN, D. A. Plant Microtechnique. New York: McGraw-Hill Book Company Inc, 1940. 523p.
- KIM, D.H.; GOPAL, J.; SIVANESAN, I. Nanomaterials in plant tissue culture: the disclosed and undisclosed. RSC Advances, v.7, n.58, p.36492-36505, 2017. https://doi.org/10.1039/c7ra07025j
» https://doi.org/10.1039/c7ra07025j - LI, X.; LIU, Y.; HE, Q.; LI, S.; LIU, W.; LIN, C.; MIAO, W. A candidate secreted effector protein of rubber tree powdery mildew fungus contributes to infection by regulating Plant ABA biosynthesis. Frontiers in microbiology, v.11, 591387, 2020. https://doi.org/10.3389/fmicb.2020.591387
» https://doi.org/10.3389/fmicb.2020.591387 - LIMA, M.L.; CAFÉ FILHO, A.C. Estabilidade da resistência de Capsicum spp. ao oídio em telado e casa de vegetação. Fitopatologia Brasileira, v.29, n.5, p.519-525, 2004. https://doi.org/10.1590/S0100-41582004000500008
» https://doi.org/10.1590/S0100-41582004000500008 - LIYANAGE, K.K.; KHAN, S.; BROOKS, S.; MORTIMER, P.E.; KARUNARATHNA, S.C.; XU, J.; HYDE, K.D. Taxonomic revision and phylogenetic analyses of rubber powdery mildew fungi. Microbial Pathogenesis, v.105, p.185-195, 2017. https://doi.org/10.1016/j.micpath.2017.01.054
» https://doi.org/10.1016/j.micpath.2017.01.054 - MÁXIMO, W.; SANTOS, B.; MARTINS, J.; BEIJO, L.; BARBOSA, S. Multiplicação e enraizamentoin vitrodeHandroanthus impetiginosus(Mart. ex DC.) Mattos. Ciência Florestal, v.30, n.3, p.658-668, 2020. https://doi.org/10.5902/1980509827012
» https://doi.org/10.5902/1980509827012 - MISHRA, V.; LAL, A.A.; SIMON, S. Efficacy of botanicals and bio-agents against powdery mildew disease of garden pea (Pisum sativum L.). Journal of Pharmacognosy and Phytochemistry, v.6, p.1125-1126, 2017.
- MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioexperiments with tobacco tissue culture. Physiologia Plantarum, v.15, n.3, p.473-497, 1962.
- MUSHTAQ, T.; BANYAL, R.; MUGLOO, J.; MUSHTAQ, T.; AZIZ, M. A. Clonal forestry: An effective technique for increasing the productivity of plantations. SKUAST Journal of Research, v.19, n.1, p.22-28, 2017.
- ORLIKOWSKA, T.; NOWAK, K.; REED, B. Bacteria in the plant tissue culture environment. Plant Cell Tissue and Organ Culture, v.128, p.487-508, 2017. https://doi.org/10.1007/s11240-016-1144-9
» https://doi.org/10.1007/s11240-016-1144-9 - PAIVA, P.D.O.; ABBADE, L. C.; CENTOFANTE, A.R.; PAIVA, R. Desinfestação de sementes de Ipê-Branco (Tabebuia roseo-alba) para estabelecimento in vitro Ornamental Horticulture , v.13, p.1631-1633, 2007. https://doi.org/10.14295/oh.v13i0.1804
» https://doi.org/10.14295/oh.v13i0.1804 - RAMOS, S.M.B; ALMEIDA, E.F.A.; ROCHA, F.S.; FERNANDES, M.F.G.; SANTOS, E.B. Organic fertilization and alternative products in the control of powdery mildew. Ornamental Horticulture , v.26, n.1, p.57-68, 2020. https://doi.org/10.1590/2447-536x.v26i1.2109
» https://doi.org/10.1590/2447-536x.v26i1.2109 - RANA, A.; MALANNAYAR, A.B.; BANYAL, D.K. Studies on biology and environmental factors affecting the development of tomato powdery mildew under protected cultivation. Indian Phytopathology, v.71, p.385-391, 2018. https://doi.org/10.1007/s42360-018-0049-4
» https://doi.org/10.1007/s42360-018-0049-4 - SANTOS, J.S.H.; SANTOS, K.T.H.; OLIVEIRA, V.S.; SANTOS, G.P.; MENEZES, L.F.T.; CZEPAK, M.P.; FALQUETO, A.R.; AOYAMA, E.M.; SCHMILDT, O.; SCHMILDT, E.R. Regression models for prediction of leaf area in purple ipe [Tabebuia impetiginosa (Mart.)]. Australian Journal of Crop Science, v.14, n.4, p.654-659, 2020. https://doi.org/10.21475/ajcs.20.14.04.p2291
» https://doi.org/10.21475/ajcs.20.14.04.p2291 - SCOTT, C.; PUNJA, Z.K. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L. (marijuana) plants. Canadian Journal of Plant Pathology , p.1-19, 2020. https://doi.org/10.1080/07060661.2020.1836026
» https://doi.org/10.1080/07060661.2020.1836026 - SHANER, G.; FINNEY, R.F. The effects of nitrogen fertilization on the expression of show-mildwing in knox wheat. Phytopathology, v.67, n.8, p.1051-1055, 1977. https://doi.org/10.1094/Phyto -67-1051
» https://doi.org/10.1094/Phyto -67-1051 - SUTHAPARAN, A.; SOLHAUG, K. A.; STENSVAND, A.; GISLERØD, H.R. Determination of UV action spectra affecting the infection process of Oidium neolycopersici, the cause of tomato powdery mildew. Journal of Photochemistry and Photobiology B: Biology, v.156, p.41-49, 2016.https://doi.org/10.1016/j.jphotobiol.2016.01.009
» https://doi.org/10.1016/j.jphotobiol.2016.01.009 - SILVA-JÚNIOR, O.B.; GRATTAPAGLIA, D.; NOVAES, E.; COLLEVATTI, R.G. Genome assembly of the Pink Ipê (Handroanthus impetiginosus, Bignoniaceae), a highly valued, ecologically keystone Neotropical timber forest tree. Gigascience, v.7, n.1, p.1-16, 2018. https://doi.org/10.1093/gigascience/gix125
» https://doi.org/10.1093/gigascience/gix125 - ZHAI, D.L.; WANG, J.; THALER, P. Contrasted effects of temperature during defoliation vs. refoliation periods on the infection of rubber powdery mildew (Oidium heveae) in Xishuangbanna, China. International Journal of Biometeorology, v.64, p.1835-1845, 2020. https://doi.org/10.1007/s00484-020-01969-y
» https://doi.org/10.1007/s00484-020-01969-y
-
Area Editor: José Eudes de Morais Oliveira
Author Contribution
-
TCM: Preparation, implementation, data collection, evaluation, and scientific writing; AAC: Statistical analysis of sample data and intellectual contributions to scientific research; KCIS: Implementation, data collection and evaluation; MTF: Performed optical microscopy of the anatomical sections; LAG: Performed scanning microscopy of the anatomical sections; MCCF: Intellectual contributions to scientific research and scientific writing; LGA: Research co-orientation and scientific writing; STS: Research orientation and scientific writing.
Publication Dates
-
Publication in this collection
08 July 2022 -
Date of issue
Apr-Jun 2022
History
-
Received
26 Feb 2021 -
Accepted
22 Feb 2022 -
Published
04 Apr 2022