Abstract
Ruscus is an evergreen shrub that offers dark-green glossy foliage used as green additions to bouquets and flower arrangements. One of the most significant ways to obtain new varieties of crop and ornamental plants is to induce mutations by radiation. Gamma radiation is most commonly used to obtain mutants in commercial food as well as feed crops and ornamental plants. In this study, we developed tissue culture methods for Ruscus proliferation from rhizomes to obtain rhizomes clusters. These clusters were subsequently irradiated with Gamma rays to obtain unique phenotypes, such as: elongated narrow phylloclades modified stem symmetry and dwarfed growth habit. Such Ruscus types can contribute to the expansion of the floral industry.
Keywords:
rhizome plants; growth regulators; mutations; new crops
Resumo
Ruscus é um arbusto sempre-verde que oferece folhagem verde-escura brilhante, utilizado como componente de buquês e arranjos de flores. Uma das formas mais significativas de obter novas variedades de plantas e plantas ornamentais é induzir mutações por radiação. A radiação Gama é mais comumente usada para obter mutantes em culturas comerciais, como as destinadas à alimentação e as plantas ornamentais. Neste estudo, foram desenvolvidos métodos de cultura de tecidos para a proliferação de Ruscus a partir de rizomas para obtenção de aglomerados de rizomas. Esses aglomerados foram subsequentemente irradiados com raios Gama para obter fenótipos únicos, tais como: filocládios estreitos e alongados modificando a simetria do caule e o hábito de crescimento anão. Esses tipos de Ruscus podem contribuir para a expansão da indústria da floricultura.
Palavras-chave:
plantas rizomatosas; reguladores de crescimento; mutações; novas culturas
Introduction
Ruscus hypoglossum L. is an evergreen rhizomatous herbaceous perennial of the Asparagaceae Juss family (Chase et al., 2009CHASE, M.W.; REVEAL, J.L.; FAY, M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Botanical Journal of the Linnean Society, v.161, n.2, p.132-136, 2009. DOI: 10.1111/j.1095-8339.2009.00999 ) with a native range from Italy north to Austria and Slovakia and east to Turkey and Crimea (Halada, 1994HALADA, L. Ruscus hypoglossum L. in Slovakia. Thaiszia - Journal of Botany, v.4, p.183-195, 1994.).There are few scientific works on Ruscus. Ruscus is indigenous to southwestern Europe, but different species are found in the wild, from Western Europe to Iran as well as in Israel. The plant’s apparent leaves, morphologically called phylloclades, are modified branches that look like leaves. Ruscus leaves are small, non-photosynthetic spikes, located at the center of the phylloclades, where the very small white flower develops on each phylloclade if conditions are favorable and a red fruit forms (Stamps, 1997). Fruits are red berries and fruiting is rare in this plant. Propagation of Ruscus plants is done by dividing the plant rhizomes into several small clumps that carry several branches and thick roots (Winarto, 2017WINARTO, B. In Vitro propagation of Ruscus: a Review. South Western Journal of Horticulture, Biology and Environment, v.8, n.2, p.103-121, 2017.). Ruscus plants exhibits high adaptability to diverse growing conditions (i.e. light, temperature, and various soil compositions) and has been widely cultivated in public areas of the Middle East since the 12th century (Veroese, 2015VEROESE, G. A study of the genus Ruscus and its horticultural value. 2015. Available at: Available at: https://www.slideshare.net/GiulioVeronese/a-study-on-the-genus-ruscus-and-its-horticultural-value . Accessed May 11th 2020.
https://www.slideshare.net/GiulioVerones...
).
One of the most prominent features of Ruscus branches is their long shelf life, even under difficult conditions. Ruscus brunches can look fresh after several weeks in a vase with only water. Attempts to market Ruscus branches, collected from gardens in Israel, dates to the early 1960s (Weizmann et al., 2014WEIZMANN, S.; HAZAN, A.; SHPIGEL, E.; BARKAI, N. Report to the Chief Scientist of the Ministry of Agriculture in Israel (In Hebrew). 2014. Available at: Available at: https://www.moag.gov.il/shaham/professionalinformation/documents/keren_mehkarim_shaham/risusi_alvaa_ruscus_lemniaat_clooza.pdf . Accessed May 11th 2020.
https://www.moag.gov.il/shaham/professio...
). Interest in Ruscus as a bouquet and flower arrangements filler intensified in Europe in the early 1970s. As the demand for new products, increased, Israeli farmers begun growing Ruscus commercially. The cultivated area of Ruscus in Israel grew from 25 hectares in late 1970’s to 200 hectares in 2000’s. The number of exported Ruscus stems remained high and stable in the last ten years, reaching 126 million stems per year (Weizmann et al., 2014).
The optimal condition for growing Ruscus for the European markets in Israel is under heavy shading (50-60% black nets) that improves the intensity of pigmentation of the phylloclades and increases their length (Stamps, 1997). The growth habit of Ruscus can be modified by the level and color of the shading nets (Oren-Shamir et al., 2001OREN-SHAMIR, M.; GUSSAKOVSKY, E.E.; SHPIEGEL, E.; NISSIM-LEVI, A.; RATNER, K.; OVADIA, R.; GILLER, Y.; SHAHAK, Y. Coloured shade nets can improve the yield and quality of green decorative branches of Pittosporum variegatum. The Journal of Horticultural Science and Biotechnology, v.76, n.3, p.353-361, 2001. DOI: https://doi.org/10.1080/14620316.2001.11511377
https://doi.org/10.1080/14620316.2001.11...
). Attempts to grow Ruscus commercially in other parts of the world have largely failed, and so far, there is almost no commercial competition to Israel. The success of this plant in Israel is due to; 1. Ruscus needs a warm dry summer to arrest the growth of branches and reset new meristems. Ruscus is very sensitive to fungi and nematodes thus high humidity during summer is devastating to the plant (Tamari, 2017TAMARI, Y. Growth instruction for Ruscus. Flower Growers Bulletin, 2017.; Elad, 2014ELAD, Y. 2014. Plant diseases in Israel - Ruscus. Available at: Available at: https://phytopathology.org.il/wp-content/uploads/2017/11/Ruscus.pdf . Accessed May 11th 2020.
https://phytopathology.org.il/wp-content...
); 2. For the maximum development of Ruscus plants, there is a need for moderate growth temperatures during winter because low temperatures impair the quality of the plant and high temperatures arrest development of new branches. The dry summer and moderate winter make Israel suited for cultivating Ruscus.
While Ruscus is a profitable crop, the numbers of varieties are very limited. Here we show for the first time that Ruscus can be modified to develop lines with new and unique shapes or growth habit of this ornamental crop.
Material and Methods
Plant material
The Ruscus plants (Ruscus hypoglossum L.) used in this study were taken from a commercial grower (Doron Haviv, Kfar Vitkin); the plants were uprooted and brought to the lab. Buds were excised from the base of the rhizomes and from the phylloclades. Both parts were separated and used as initial starting material. Flowers and phylloclades were also used as starting explant to establish a propagating culture.
Culture initiation
All segments of the underground and aerial parts were washed with soap (commercially available Palmolive) and water, then placed in water, and washed under running tap water for 1.5 hours. The Ruscus segments were then shaken in 96% v v-1 ethanol for 1 minute and placed for 15 minutes in 3% v v-1 sodium-hypochloride + 0.01% v v-1 Tween-20, and for 30 minutes in 1.5% v v-1 sodium-hypochlorite + 0.01% Tween-20 with vigorous mixing. Lastly, the segments were washed 3 times with sterile distilled water. Buds that were detached from the rhizome segments and segments of phylloclade with or without a flower were placed on MS (Murashige and Skoog salt medium) media differing in their plant growth regulators content.
Culture media and culture conditions
The above explants were placed on several basic media that are standard in our lab, containing MS salts (Murashige and Skoog, 1962MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum, v.15, p.473-497, 1962.) supplemented with 88.2 mM sucrose and 0.8% agar. Growth regulators supplements were various ratios of NAA (naphthalene acetic acid) and BA (6-Benzyladenine). The developing explants were sub-cultured onto various multiplication media for propagation and elongation every 4-6 weeks.
Five to nine single shoots were sub-cultured to polypropylene Vitro-Vent containers (Duchefa, 9x9 cm and 9 cm height) or round polypropylene food grade vessels. In each treatment, five to nine single shoots were sub-cultured in a box and the number of shoots that emerged after 4-6 weeks were subdivided and counted. Statistical analysis was done using JMP (SAS institute, Cary, North Carolina, USA) of at least three replicates in each treatment and each experiment was repeated at least twice.
Ruscus cultures were maintained in culture room at 250C + 10C under 16h light (cool white fluorescent lamps giving 50 mmol m-2 s-1) regime.
Rooting and acclimatization
Single Ruscus shoots clumps were sub-cultured into magenta vessels or baby food jars with rooting basic salt media was MS (Murashige and Skoog, 1962MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum, v.15, p.473-497, 1962.) supplemented with 58.8 mM sucrose and 0.8 % agar. Growth regulators supplements were a two concentrations of IBA (3-Indole butyric acid), NAA and IAA (Indole-3-acetic acid) concentrations, rooting can sporadically occur in the absence of auxins. For acclimatization, rooted plants were planted in pots filled with peat and tuff mixture or send or pure perlite or vermiculite. The plants were maintained at a temperature-controlled room under high humidity for at least two weeks before transfer to a regular greenhouse.
Irradiation of plant material
The explant we chose to irradiate were dividing meristems. Two days prior to irradiation, single meristems about two to 5 mm in diameter from dividing rhizomes in culture, in culture and clustered closely on agar plates supplemented with propagation medium. The plates were exposed to various levels of gamma irradiation from a source of Co60 in Soreq Nuclear Research Center, Israel. Radiation doses were 80, 40, 20, 15, 10, 5 Gy. Three plates, each with 15 clusters were irradiated. Because radiation level drops as a square root of the distance from the source, radiation level was determined by keeping the plates with the explant material at different distances from the Co60 source at the same time. Radiation flux was measured to be 1.64 Gy/h down to 0.71 Gy/h. A day after the irradiation, the explants were transferred to fresh medium and the growth and the development of the plantlet were analyzed over time.
Results
Regeneration of plantlets from various plant tissues
Direct regeneration from buds was obtained on MS medium containing only NAA and BA. There was no development of callus from the buds that were placed on the medium above instead the bud expanded and then produced multiple small buds (Figures 1A-D) and those small buds sprouted phylloclades (Figures 1A-D).
Shoot regeneration from Ruscus rhizomes. Ruscus buds from sterilized rhizomes placed on agar medium containing BA and NAA and produced multiple small buds (arrowheads in B to D) and those small buds sprouted phylloclades. Shoot meristems initiated from flower buds (C and D).
We tested several cytokinins and auxins in the regeneration medium. BA was the sole cytokinin combined with NAA as auxin source that produced shoot multiplication in Ruscus at various combinations while, flower buds responded to only one combination of BA and NAA (1 mg L-1 NAA + 2 mg L-1 BA) and started to initiate shoots (Figures 1C-D). The best combination for producing Ruscus shoots from rhizome meristems was 1 mg L-1 NAA + 3 mg L-1 BA (Figure 2A). Flower buds and buds from rhizome produced similar number of new shoot on the same media (Figure 2B).
The effect of cytokinin and auxin on shoot regeneration from rhizomes and flower buds. A: Variations of BA and NAA affected the number of shoots regenerated from each treatment. B: Comparing the number of shoots regenerated from rhizomes or flower buds on the same medium. The SD for the data in the figure of each data point is shown using JMP statistical program. A Tukey test using the JMP program. The Tukey Test is a post-hoc test based on the ranking range distribution.
We tested the possibility of regenerating Ruscus shoots from roots in culture. Roots failed to regenerate shoots under all the conditions we tested. The only tissue that produced and multiplied shoots were the buds isolated from the rhizomes and flowers.
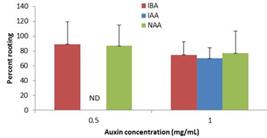
Rooting of tissue culture derived shoots
Rooting was observed on all MS media with auxin (Figure 3). As rooting was so easy, we tested two concentrations of auxins and no differences were observed between the two (Figure 3). Lowering the MS salt concentration to half in the rooting medium had no deleterious effect on rooting (not shown). Rooted plants were transferred to four growth substrates: soil, Jiffy, Perlite and sand. Regenerated plants did not survive in sand, but the other three substrates were proven equally efficient.
The effect of auxin concentration on shoot rooting. The concentration of IBA, NAA and IAA was varied in the presence 1/2 MS and number of rooted shoots was recorded. Each point is the mean of at least three boxes in each treatment and each box contained five to nine single shoots as starting material. Percent survival + SD for the data point. The SD for the data in the figure of each data point is shown using JMP statistical program.
Survival and propagation of Gamma ray irradiated tissue
Nine weeks after first irradiation trial with 20 Gy h-1 to 80 Gy h-1 of gamma rays, the survival rate of Ruscus clusters was examined. While most of the clusters seem greening and alive, only at dose of 80 Gy h-1 there was a decrease in viability of Ruscus clusters (Figure 4).
The effect of Gamma radiation dose on shoot regeneration. Survival of Ruscus rhizome explants treated with increasing amounts of Gamma radiation. Each point is the mean of at least three plates in each treatment and each plate contained nine single rhizome cluster without the phylloclades as shown in Fig, 1. Percent survival + SD for the data point. The SD for the data in the figure of each data point is shown using JMP statistical program.
Ruscus shoots were separated every 8 weeks from irradiated clusters, transferred to multiplication media, and counted. The original radiation dose was tracked all the time. The number of shoots that reproduced from the irradiated clusters decreased after irradiation dose of 15 Gy and remained around three shoots per meristem compared to around eight shoots per meristem at radiation dose of five Gy (Figure 5). All shoots rooted after Gamma ray radiation treatment with the exception of clusters treated with 80 Gy dose. After applying a dose of 80 Gy, some clusters propagated shoots but failed to produce roots.
The effect Gamma radiation on shoot regeneration. Rhizome explants treated with increasing amounts of Gamma radiation and the number of shoots regenerated from each treatment recorded over time. At each transfer for three years, the number of shoots was recorded. The SD for the data in the figure of each data point is shown using JMP statistical program. The SD for the data in the figure of each data point is shown using JMP statistical program. A Tukey test using the JMP program. The Tukey Test is a post-hoc test based on the ranking range distribution.
After growth in a greenhouse, the irradiated plants were transferred to commercial growers for continued growth and evaluation. Some of the plants showed altered phenotype such as narrow phylloclades or three phylloclades emerging from an internode (Figure 6).
Photos of regenerated Ruscus plants in the soil. A: Control plants; B and C: plants with elongated phylloclades; D and E: plants with three phylloclade per internode. Arrow head point to the internodes. Bar indicates 2 cm.
Highest percentage of altered plants was obtained after 10-20 Gy irradiation. In the pictures depicting the phenotyping there are control-regenerated plants that were treated as the irradiated ones. Non-irradiated plant showed no altered phylloclade indication that somaclonal variation was at least less frequent than mutation induced by irradiation.
Discussion
Ruscus regenerated shoots on media that contained BA as cytokinin and NAA as auxin source (Figures 1 and 2). In a review of Ruscus propagation, Winarto (2017WINARTO, B. In Vitro propagation of Ruscus: a Review. South Western Journal of Horticulture, Biology and Environment, v.8, n.2, p.103-121, 2017.) quotes studies that used BA and IAA as well as 2,4D and kinetin and TDZ with NAA all for direct regeneration from Ruscus rhizomes (Winatro, 2017). Winarto (2017) also mentions that there is a decline in explants vigor during successive transfers under the above media. Our regeneration protocol seems to be fast and simple and rather robust at it can regenerate 6-8 shoots in a single transfer without a decline in shoot regeneration after transferring the Ruscus cultures for three years (about 36 subcultures), and without an observed decrease in vigor and/or necrosis of the explants material.
The regenerated shoots rooted on half MS salts media with the presence of 0.5 or 1 mg L-1 auxin (IBA, IAA or NAA) with no differences between them (Figure 3). All of the rooted shoots survived upon transfer to various soils with the exception of sand that was detrimental to the regenerated shoots and to non-irradiated plants. Jha and Sen (1985JHA, S.; SEN, S. In vitro regeneration of Ruscus hypophyilum L. plants. Plant Cell Tissue and Organ Culture, v.5, p.79-87, 1985. DOI: https://doi.org/10.1007/BF00033573
https://doi.org/10.1007/BF00033573...
) reported survival rate of 80% and our results show similar results. Ruscus seems to be a very sturdy plant and can adjust too many soils and radiation treatments. High doses of Gamma radiation were not detrimental to the plant (Figure 4), but just reduce its ability to proliferate (Figure 5), and it was clear that increasing radiation intensity affect rooting adversely. Rooting seemed to be more sensitive to irradiation than shoot proliferation. These observations combined with the use of Ruscus as a green filler in bouquets and its ability to stay green for extended periods indicate not only to the hardiness of the plant but that it’s physiologically unique. There is no physiological data available on Ruscus such as light intensity requirements and photosynthetic ability, but to successfully grow Ruscus crop in Israel, light intensity must be reduced dramatically by black nets. Thus, due to its hardiness Ruscus makes an ideal plant for mutagenesis by radiation to induce variability.
A genome-wide study on mammalian germline showed that ionizing radiation markedly increases the frequency and spectrum of de novo mutations (Adeolu el al., 2015ADEOLU, A.B.; LINDSAY, A.J.; DUBROVA, A.; HURLES, M.E. The genome-wide effects of ionizing radiation on mutation induction in the mammalian germline. Nature Communications, v.6, n.1, p.1-8, 2015. DOI: https://doi.org/10.1038/ncomms7684
https://doi.org/10.1038/ncomms7684...
). We used this type of radiation because of its ability to produce beneficial mutations on vegetative tissue. We have managed to obtain some visual mutants of Ruscus by Gamma radiation (Figure 6). However, only nine visual phenotypes out of one thousand plants that were planted turned out to be useful. Thus, to increase the number of useful mutants, screening of more plants is needed. We are currently producing more irradiated plants to increase the number of possible mutants. The stability of the mutated rhizomes we obtained will be tested over the following years in order to confirm that these rhizomes produce sellable stems.
The main advantage of irradiation treatment to induce variation in horticultural crops is the hygiene of the procedure, it is safe (as all done remotely) and it can be done on closed vessels (like petri dishes or jars). The irradiation treatment is performed on green multiplying tissue such as meristems, woody branches, corms and dry seeds as well. The procedure is easy and cheap if there is a near research or medical nuclear facility. In comparison chemical induction of variation is messy and dangerous and cannot be used on woody or hard plant material. The main disadvantage of irradiation is the availability of research or medical nuclear facility nearby that is trained to work with radiation emitting materials.
Conclusions
We present a rapid all year in vitro propagation for Ruscus via tissue culture, with a protocol by Gamma radiation to obtain phenotypic variation. Here we show that rapid tissue culture is suitable for mutagenesis.
We present the obtained visual phenotypes induced by radiation in Ruscus.
Acknowledgements
Funding by Ministry of Agriculture, Chief Scientist, Israel.
References
- ADEOLU, A.B.; LINDSAY, A.J.; DUBROVA, A.; HURLES, M.E. The genome-wide effects of ionizing radiation on mutation induction in the mammalian germline. Nature Communications, v.6, n.1, p.1-8, 2015. DOI: https://doi.org/10.1038/ncomms7684
» https://doi.org/10.1038/ncomms7684 - CHASE, M.W.; REVEAL, J.L.; FAY, M.F. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Botanical Journal of the Linnean Society, v.161, n.2, p.132-136, 2009. DOI: 10.1111/j.1095-8339.2009.00999
- ELAD, Y. 2014. Plant diseases in Israel - Ruscus Available at: Available at: https://phytopathology.org.il/wp-content/uploads/2017/11/Ruscus.pdf Accessed May 11th 2020.
» https://phytopathology.org.il/wp-content/uploads/2017/11/Ruscus.pdf - HALADA, L. Ruscus hypoglossum L. in Slovakia. Thaiszia - Journal of Botany, v.4, p.183-195, 1994.
- JHA, S.; SEN, S. In vitro regeneration of Ruscus hypophyilum L. plants. Plant Cell Tissue and Organ Culture, v.5, p.79-87, 1985. DOI: https://doi.org/10.1007/BF00033573
» https://doi.org/10.1007/BF00033573 - MURASHIGE, T.; SKOOG, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum, v.15, p.473-497, 1962.
- OREN-SHAMIR, M.; GUSSAKOVSKY, E.E.; SHPIEGEL, E.; NISSIM-LEVI, A.; RATNER, K.; OVADIA, R.; GILLER, Y.; SHAHAK, Y. Coloured shade nets can improve the yield and quality of green decorative branches of Pittosporum variegatum The Journal of Horticultural Science and Biotechnology, v.76, n.3, p.353-361, 2001. DOI: https://doi.org/10.1080/14620316.2001.11511377
» https://doi.org/10.1080/14620316.2001.11511377 - STAMPS, R.H. Florida/Holland/Israeli Ruscus production and use. Apopka: Extension, Institute of Food and Agriculstural Sciences, 2001.
- TAMARI, Y. Growth instruction for Ruscus Flower Growers Bulletin, 2017.
- VEROESE, G. A study of the genus Ruscus and its horticultural value. 2015. Available at: Available at: https://www.slideshare.net/GiulioVeronese/a-study-on-the-genus-ruscus-and-its-horticultural-value Accessed May 11th 2020.
» https://www.slideshare.net/GiulioVeronese/a-study-on-the-genus-ruscus-and-its-horticultural-value - WEIZMANN, S.; HAZAN, A.; SHPIGEL, E.; BARKAI, N. Report to the Chief Scientist of the Ministry of Agriculture in Israel (In Hebrew). 2014. Available at: Available at: https://www.moag.gov.il/shaham/professionalinformation/documents/keren_mehkarim_shaham/risusi_alvaa_ruscus_lemniaat_clooza.pdf Accessed May 11th 2020.
» https://www.moag.gov.il/shaham/professionalinformation/documents/keren_mehkarim_shaham/risusi_alvaa_ruscus_lemniaat_clooza.pdf - WINARTO, B. In Vitro propagation of Ruscus: a Review. South Western Journal of Horticulture, Biology and Environment, v.8, n.2, p.103-121, 2017.
Abbreviations
- BA 6-Benzyladenine
- IAA Indole-3-acetic acid
- NAA Naphthalene Acetic Acid
- IBA 3-Indole butyric acid
- MS Murashige and Skoog salt medium
-
Area Editor: Fernanda Carlota Nery
Publication Dates
-
Publication in this collection
15 July 2020 -
Date of issue
Apr-Jun 2020
History
-
Received
25 Dec 2019 -
Accepted
07 May 2020 -
Published
25 May 2020