Abstract
This work aims to study the cationic miniemulsion polymerization of styrene catalyzed by iron-containing imidazolium-based ionic liquids. The polystyrenes had very high number-average molar mass around 1300 kg mol-1 at 85 °C, molar-mass dispersity close to 2.0 and glass transition temperature higher than 102 °C with average particle diameter that remained practically unchanged during the reaction, indicating that the monomer droplets correspond to the polymerization locus. First-order kinetics up to a limit conversion, along with the increase in molar mass as the temperature decreases, styrene polymerization at low temperatures and catalyst inability to polymerize monomers that react exclusively via free radical and/or anionic polymerization, indicate the cationic nature of polymerization. 1H-NMR and 13C-NMR spectra suggested the formation of polystyrene, allowing for tacticity distribution quantification: 10% isotactic, 20% atactic and 70% syndiotactic configurations. TEM micrographs confirmed the formation of spherical polymer nanoparticles and the presence of catalysts in the polymer matrix.
Keywords:
high molar mass; ionic liquid catalysts; cationic polymerization; miniemulsion polymerization; styrene
1. Introduction
Polymerizations performed in heterogeneous medium have many advantages, such as high heat removal capacity, low viscosity of the end product, ease of homogenization and manipulation, among others. For these reasons, polymerizations performed in water as a continuous phase are among the most widely used methods for large scale polymer synthesis[11 Distler, D., Neto, W. S., & Machado, F. (2017). Emulsion polymerization. In S. Hashmi (Eds.), Reference module in materials science and materials engineering (pp. 1-14). New York: Elsevier. http://dx.doi.org/10.1016/B978-0-12-803581-8.03746-2.
http://dx.doi.org/10.1016/B978-0-12-8035...
]. However, conventional ionic polymerizations are often performed under anhydrous conditions and traces of water should be avoided as catalysts react with water and become inactive[22 Bompart, M., Vergnaud, J., Strub, H., & Carpentier, J. F. (2011). Indium(III) halides as exceptionally active, water-tolerant catalysts for cationic polymerization of styrenics. Polymer Chemistry, 2(8), 1638-1640. http://dx.doi.org/10.1039/c1py00145k.
http://dx.doi.org/10.1039/c1py00145k...
]. In recent decades several efforts have been made in order to develop new catalysts very attractive to the ionic polymerization of different monomers in aqueous dispersed medium[33 Satoh, K., Kamigaito, M., & Sawamoto, M. (2000). Lanthanide triflates-mediated emulsion cationic polymerization of p-alkoxystyrenes in aqueous media. Macromolecules, 33(13), 4660-4666. http://dx.doi.org/10.1021/ma0000069.
http://dx.doi.org/10.1021/ma0000069...
4 Maitre, C., Ganachaud, F., Ferreira, O., Lutz, J. F., Paintoux, Y., & Hémery, P. (2000). Anionic polymerization of phenyl glycidyl ether in miniemulsion. Macromolecules, 33(21), 7730-7736. http://dx.doi.org/10.1021/ma0007132.
http://dx.doi.org/10.1021/ma0007132...
5 Kostjuk, S. V., & Ganachaud, F. (2010). Cationic polymerization of vinyl monomers in aqueous media: from monofunctional oligomers to long-lived polymer chains. Accounts of Chemical Research, 43(3), 357-367. http://dx.doi.org/10.1021/ar900198q. PMid:19957949.
http://dx.doi.org/10.1021/ar900198q...
6 Kostjuk, S. V., & Ganachaud, F. (2006). Cationic polymerization of styrene in solution and aqueous suspension using B(C6F5)3 as a water-tolerant Lewis acid. Macromolecules, 39(9), 3110-3113. http://dx.doi.org/10.1021/ma052650z.
http://dx.doi.org/10.1021/ma052650z...
7 Vasilenko, I. V., Ganachaud, F., & Kostjuk, S. V. (2016). New insights into the cationic polymerization in emulsion catalyzed by water-dispersible Lewis acid surfactant complexes: a case study with p-methoxystyrene. Macromolecules, 49(9), 3264-3273. http://dx.doi.org/10.1021/acs.macromol.6b00379.
http://dx.doi.org/10.1021/acs.macromol.6...
8 Cauvin, S., Sadoun, A., Dos Santos, R., Belleney, J., Ganachaud, F., & Hemery, P. (2002). Cationic polymerization of p-methoxystyrene in miniemulsion. Macromolecules, 35(21), 7919-7927. http://dx.doi.org/10.1021/ma0202890.
http://dx.doi.org/10.1021/ma0202890...
9 Touchard, V., Graillat, C., Boisson, C., D’Agosto, F., & Spitz, R. (2004). Use of a Lewis acid surfactant combined catalyst in cationic polymerization in miniemulsion: Apparent and hidden initiators. Macromolecules, 37(9), 3136-3142. http://dx.doi.org/10.1021/ma0355352.
http://dx.doi.org/10.1021/ma0355352...
10 Zhang, J., Wu, Y., Li, X., Yang, D., Zhang, M., Wang, H., Shang, Y., Ren, P., Mu, X., Li, S., & Guo, W. (2019). Characteristics and mechanism of styrene cationic polymerization in aqueous media initiated by cumyl alcohol/B(C6F5)3. Macromolecular Chemistry and Physics, 220(4), 1800419-1800427. http://dx.doi.org/10.1002/macp.201800419.
http://dx.doi.org/10.1002/macp.201800419...
11 Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a. PMid:15917929.
http://dx.doi.org/10.1039/b501489a...
-1212 Vasilenko, I. V., Yeong, H. Y., Delgado, M., Ouardad, S., Peruch, F., Voit, B., Ganachaud, F., & Kostjuk, S. V. (2015). A catalyst platform for unique cationic (co)polymerization in aqueous emulsion. Angewandte Chemie International Edition, 54(43), 12728-12732. http://dx.doi.org/10.1002/anie.201501157. PMid:26013180.
http://dx.doi.org/10.1002/anie.201501157...
]. However, these works use an excessive catalyst load (3 to 10 mol%) and, except for the work of Vasilenko et al.[1212 Vasilenko, I. V., Yeong, H. Y., Delgado, M., Ouardad, S., Peruch, F., Voit, B., Ganachaud, F., & Kostjuk, S. V. (2015). A catalyst platform for unique cationic (co)polymerization in aqueous emulsion. Angewandte Chemie International Edition, 54(43), 12728-12732. http://dx.doi.org/10.1002/anie.201501157. PMid:26013180.
http://dx.doi.org/10.1002/anie.201501157...
], there was the formation of polymers with low molar masses (less than 40 kg mol-1), similar to that obtained via free radical bulk polymerization. In the literature, there are few reports on cationic polymerization using the miniemulsion technique. Generally, polymerizations occur slowly and at the monomeric/water droplets interface resulting in low molar mass polymers and narrow molar mass distribution[88 Cauvin, S., Sadoun, A., Dos Santos, R., Belleney, J., Ganachaud, F., & Hemery, P. (2002). Cationic polymerization of p-methoxystyrene in miniemulsion. Macromolecules, 35(21), 7919-7927. http://dx.doi.org/10.1021/ma0202890.
http://dx.doi.org/10.1021/ma0202890...
,99 Touchard, V., Graillat, C., Boisson, C., D’Agosto, F., & Spitz, R. (2004). Use of a Lewis acid surfactant combined catalyst in cationic polymerization in miniemulsion: Apparent and hidden initiators. Macromolecules, 37(9), 3136-3142. http://dx.doi.org/10.1021/ma0355352.
http://dx.doi.org/10.1021/ma0355352...
].
In relation to cationic polymerization, recent researches in this area are focused on the development of new catalysts with reusability possibilities, the investigation of new methods using mild experimental conditions, avoiding the use of excess organic solvents, and which allow the more effective control of the final properties of the polymeric materials, such as: molecular structure and molar mass distribution, aiming its use in specific applications.
In this direction, it is worth highlighting the controlled cationic polymerizations of styrene in the presence of water[33 Satoh, K., Kamigaito, M., & Sawamoto, M. (2000). Lanthanide triflates-mediated emulsion cationic polymerization of p-alkoxystyrenes in aqueous media. Macromolecules, 33(13), 4660-4666. http://dx.doi.org/10.1021/ma0000069.
http://dx.doi.org/10.1021/ma0000069...
,1313 Radchenko, A. V., Kostjuk, S. V., Vasilenko, I. V., Ganachaud, F., & Kaputsky, F. N. (2007). Controlled/living cationic polymerization of styrene with BF3OEt2 as a coinitiator in the presence of water: improvements and limitations. European Polymer Journal, 43(6), 2576-2583. http://dx.doi.org/10.1016/j.eurpolymj.2007.03.026.
http://dx.doi.org/10.1016/j.eurpolymj.20...
] and the polymerizations of other cationically polymerized monomers in the presence of ionic liquids, such as p-methyl styrene[1414 Zhang, X., Guo, W., Wu, Y., Gong, L., Li, W., Li, X., Li, S., Shang, Y., Yang, D., & Wang, H. (2016). Cationic polymerization of p-methylstyrene in selected ionic liquids and polymerization mechanism. Polymer Chemistry, 7(32), 5099-5112. http://dx.doi.org/10.1039/C6PY00796A.
http://dx.doi.org/10.1039/C6PY00796A...
], vinyl ether and its derivatives[1515 Yoshimitsu, H., Kanazawa, A., Kanaoka, S., & Aoshima, S. (2016). Cationic polymerization of vinyl ethers with alkyl or ionic side groups in ionic liquids. Journal of Polymer Science. Part A, Polymer Chemistry, 54(12), 1774-1784. http://dx.doi.org/10.1002/pola.28039.
http://dx.doi.org/10.1002/pola.28039...
,1616 Wu, Y., Han, L., Zhang, X., Mao, J., Gong, L., Guo, W., Gu, K., & Li, S. (2015). Cationic polymerization of isobutyl vinyl ether in an imidazole-based ionic liquid: characteristics and mechanism. Polymer Chemistry, 6(13), 2560-2568. http://dx.doi.org/10.1039/C4PY01784F.
http://dx.doi.org/10.1039/C4PY01784F...
], and isobutylene[1717 Li, X., Wu, Y., Zhang, J., Li, S., Zhang, M., Yang, D., Wang, H., Shang, Y., Guo, W., & Yan, P. (2019). Synthesis of highly reactive polyisobutylenes via cationic polymerization in ionic liquids: characteristics and mechanism. Polymer Chemistry, 10(2), 201-208. http://dx.doi.org/10.1039/C8PY01141A.
http://dx.doi.org/10.1039/C8PY01141A...
18 Berezianko, I. A., Vasilenko, I. V., & Kostjuk, S. V. (2018). Acidic imidazole-based ionic liquids in the presence of diisopropyl ether as catalysts for the synthesis of highly reactive polyisobutylene: effect of ionic liquid nature, catalyst aging, and sonication. Polymer, 145, 382-390. http://dx.doi.org/10.1016/j.polymer.2018.04.059.
http://dx.doi.org/10.1016/j.polymer.2018...
-1919 Berezianko, I. A., Vasilenko, I. V., & Kostjuk, S. V. (2019). Cationic polymerization of isobutylene co-initiated by chloroferrate imidazole-based ionic liquid: the advantageous effect of initiator and aromatic compounds. European Polymer Journal, 121, 109307. http://dx.doi.org/10.1016/j.eurpolymj.2019.109307.
http://dx.doi.org/10.1016/j.eurpolymj.20...
]. We recently described the efficient encapsulation of hexadecane in high molar mass polystyrene nanoparticles obtained through cationic miniemulsion polymerization[2020 Agner, T., Zimermann, A., Machado, F., Silveira Neto, B. A., Araújo, P. H. H., & Sayer, C. (2020). Thermal performance of nanoencapsulated phase change material in high molecular weight polystyrene. Polímeros: Ciência e Tecnologia, 30(2), e2020013. http://dx.doi.org/10.1590/0104-1428.01320.
http://dx.doi.org/10.1590/0104-1428.0132...
], and the synthesis of several ILs catalysts intended to produce tailored polystyrene through bulk polymerization process[2121 Dutra, G. V. S., Teixeira, T. S., Medeiros, G. A., Abdelnur, P. V., Hermes de Araújo, P. H., Sayer, C., Neto, B. A. D., & Machado, F. (2020). On the role of metal-containing imidazolium-based ionic liquid catalysts in the formation of tailored polystyrene. Industrial & Engineering Chemistry Research, 59(50), 21685-21699. http://dx.doi.org/10.1021/acs.iecr.0c04327.
http://dx.doi.org/10.1021/acs.iecr.0c043...
].
Alves et al.[2222 Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035.
http://dx.doi.org/10.1016/j.eurpolymj.20...
] performed cationic miniemulsion polymerization of styrene using 1-butyl-3-methylimidazolium heptachlorodiferrate ionic liquid (BMI⋅Fe2Cl7) as cationic catalyst. Using a low molar ratio of 1:1000 of catalyst/monomer, the reactions showed colloidal stability and high conversion, 88%, using the temperature of 90 °C and 6 h of reaction. The particle size remained practically unchanged until the end of the polymerization process. The viscosimetric-average molar mass obtained at this temperature range, equal to 2231 kg mol-1, was higher than those usually found for cationic polymerization. Ayat et al.[2323 Ayat, M., Belbachir, M., & Rahmouni, A. (2017). Synthesis of block copolymers consists on vinylidene chloride and α- methylstyrene by cationic polymerization using an acid exchanged motmorillonite clay as heterogeneous catalyst (Algerian MMT). Journal of Molecular Structure, 1139, 381-389. http://dx.doi.org/10.1016/j.molstruc.2017.03.056.
http://dx.doi.org/10.1016/j.molstruc.201...
] used a modified natural clay initiator for the cationic copolymerization of vinylidene chloride (VDC) and α-methylstyrene (α-MS). The initiator was obtained by treating montmorillonite clay with sulfuric acid and proved to be an efficient, non-toxic, inexpensive, stable, and non-corrosive catalyst for cationic polymerization. Sang et al.[2424 Sang, W., & Yan, Q. (2018). Electro-controlled living cationic polymerization. Angewandte Chemie International Edition, 57(18), 4907-4911. http://dx.doi.org/10.1002/anie.201712270. PMid:29508512.
http://dx.doi.org/10.1002/anie.201712270...
] synthesized homopolymers derived from vinyl ether and p-substituted styrene by electro-controlled living cationic polymerization. The new method used an organocatalyst, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), which through an electroredox process promoted the oxidation of the chain transfer agent, S-1-isobutoxylethyl S′-ethyl trithiocarbonate to form carbocations. The resulting polymers exhibited narrow and well-defined molar mass distribution and predictability of the functional groups present at the ends of the polymer chain. As a differential, the proposed method allows stopping the polymerization by removing the applied external potential.
In view of this, we extend here the use of different types of iron-containing imidazolium-based ionic liquids: 1-butyl-3-methylimidazolium heptachlorodiferrate (BMI⋅Fe2Cl7), 1-methyl-3-carboxymethylimidazolium heptachlorodiferrate (MAI⋅Fe2Cl7) and 1,2-Bis(methylimidazolium)ethane bis(heptachlorodiferrate) (bMIE⋅2Fe2Cl7), which efficiently acted as catalysts for styrene bulk polymerization[2525 Rodrigues, T. S., Medeiros, G. A., Silva, F. M., & Neto, B. A. D. (2011). Polimerização do estireno com líquidos iônicos que possuem ácidos de Lewis incorporados na estrutura. In 34° Reunião Anual da Sociedade Brasileira de Química (p. 1). Florianópolis: Sociedade Brasileira de Química.,2626 Bolner, F. M., Jensen, A. T., Sayer, C., Araújo, P. H. H., Machado, F., & Neto, B. A. D. (2017). Polimerização de estireno com catalisador a base de líquido iônico modificado. In 14° Congresso Brasileiro de Polímeros (pp. 1-5). Águas de Lindóia: Associação Brasileira de Polímeros.], expanding its applications as catalysts in a water-phase dispersed polymerization process. The application of these catalysts in this type of polymerization has the following advantages: i) the incorporation of iron into the anionic group of the ILs, which makes them relatively cheaper and more attractive from an economic point of view when compared to the Lewis acids commonly used in cationic polymerization; ii) the use of ILs, the low catalyst/monomer molar ratio (quite low loading) and the absence of traditional organic solvents favor more sustainable processes; iii) synthesis of polystyrene with very high molar mass; iv) and possibility of undergoing cationic polymerization[2222 Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035.
http://dx.doi.org/10.1016/j.eurpolymj.20...
].
2. Materials and Methods
2.1 Materials
Monomer (styrene, Merck, 99%) was purified before use by washing with a 10% (w/v) aqueous NaOH solution (Vetec, 99%). It was then allowed to stand with anhydrous sodium sulfate (Na2SO4) (Dynamic, 99%) for 24 h, filtered, vacuum distilled and stored in the refrigerator[2727 Ramos, J., & Forcada, J. (2010). The role of cationic monomers in emulsion polymerization. European Polymer Journal, 46(5), 1106-1110. http://dx.doi.org/10.1016/j.eurpolymj.2010.01.012.
http://dx.doi.org/10.1016/j.eurpolymj.20...
]. The reagents 1-methylimidazole (Sigma-Aldrich, 99%), ethyl acetate (Dynamic, 99.5%) and dichloromethane (Dynamic, 99.5%) were previously vacuum distilled. The other reagents used in the development of the experimental part were of analytical grade and without previous purification, 1-chlorobutane (99%), chloroacetic acid (99%), iron (III) chloride anhydrous (97%), hexadecyltrimethylammonium bromide (CTAB) (cationic surfactant, 99%) and hexadecane (co-stabilizer, 99%) were purchased from Sigma-Aldrich.
2.2 Synthesis
2.2.1. Synthesis of ionic liquids
The 1-butyl-3-methylimidazolium chloride (BMI⋅Cl) was synthesized as described by Dupont et al.[2828 Dupont, J., Consorti, C. S., Suarez, P. A. Z., Souza, R. F., Fulmer, S. L., Richardson, D. P., Smith, T. E., & Wolff, S. (2002). Preparation of 1-butyl-3-methyl imidazolium-based room temperature ionic liquids. Organic Syntheses, 79, 236-243. http://dx.doi.org/10.15227/orgsyn.079.0236.
http://dx.doi.org/10.15227/orgsyn.079.02...
]. In a two-neck round-bottom flask under an inert atmosphere of N2 was added 1.3 equiv (2.40 mol) of 1-chlorobutane, keeping the system under reflux, magnetic stirring and heating. Slowly, 1.0 equiv (1.85 mol) of 1-methylimidazole was added. The solution was heated at reflux at 80 °C for 48 h. The product was washed with ethyl acetate and a portion of acetonitrile and then dried under reduced pressure at 60 °C and crystallized to form a white solid. Figure S1 (Supporting Information
Supplementary Material
Supplementary Material accompanies this paper.
Figure S1. 1H-NMR spectrum of BMI⋅Cl (CDCl3, 600 MHz).
Figure S2. 1H-NMR spectrum of MAI⋅Cl (D2O, 600 MHz).
Figure S3. 13C-NMR spectrum of MAI⋅Cl (D2O, 600 MHz).
Figure S4. 1H-NMR spectrum of bMIE⋅2Cl (D2O, 600 MHz).
Figure S5. 13C-NMR spectrum of bMIE⋅2Cl (D2O, 600 MHz).
Figure S6. Photograph of the synthesized polymers using the catalysts BMI⋅Fe2Cl7, MAI⋅Fe2Cl7 and bMIE⋅2Fe2Cl7 (a) before and (b) after purification.
Figure S7. 1H-NMR spectrum of unpurified polystyrene sample synthesized using BMI⋅Fe2Cl7 catalyst at 85 °C (CDCl3, 600 MHz).
Table S1. Conversion, average diameter of the monomer droplets (initial Dp) and polymeric particles (final Dp) and polydispersity index for all polymerizations after 8 h of reaction.
Figure S8. 1H-NMR spectrum of the vinyl pivalate polymerization test in the presence of BMI⋅Fe2Cl7 (CDCl3, 600 MHz).
Table S2. Average molar masses and molar-mass dispersity as a function of conversion (Xp) of the polymers synthesized at 85 °C using ILs catalysts.
Figure S9. 1H-NMR spectra of purified polystyrene samples synthesized using (a) BMI⋅Fe2Cl7; (b) MAI⋅Fe2Cl7 and (c) bMIE⋅2Fe2Cl7 at 85 °C (CDCl3, 600 MHz).
Figure S10. 13C-NMR spectra of purified polystyrene samples synthesized using (a) BMI⋅Fe2Cl7; (b) MAI⋅Fe2Cl7 and (c) bMIE⋅2Fe2Cl7 at 85 °C (CDCl3, 600 MHz).
This material is available as part of the online article from http://www.scielo.br/po
) shows the 1H-NMR spectrum.
Yield: 82.1%. 1H-NMR (CDCl3, δ in ppm): 0.95 (3H, t, N(CH2)3CH3), 1.38 (2H, m, N(CH2)2CH2CH3), 1.90 (2H, m, NCH2CH2CH2CH3), 4.12 (3H, s, NCH3), 4.34 (2H, t, NCH2(CH2)2CH3), 7.58 (1H, t, CH3NCHCHN), 7.73 (1H, m, CH3NCHCHN) and 10.32 (1H, s, NCHN).
1-methyl-3-carboxymethylimidazolium chloride (MAI⋅Cl) was synthesized as it follows[2929 Ramos, L. M., Guido, B. C., Nobrega, C. C., Corrêa, J. R., Silva, R. G., Oliveira, H. C. B., Gomes, A. F., Gozzo, F. C., & Neto, B. A. D. (2013). The Biginelli reaction with an imidazolium–tagged recyclable iron catalyst: kinetics, mechanism, and antitumoral activity. Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4156-4168. http://dx.doi.org/10.1002/chem.201204314. PMid:23460474.
http://dx.doi.org/10.1002/chem.201204314...
]: In a round bottom flask was added 1.0 equiv (0.20 mol) of 1-methylimidazole, 50 mL of acetonitrile and 1.3 equiv (0.26 mol) of chloroacetic acid. The solution was heated at reflux at 80 °C under magnetic stirring and in an inert atmosphere for 48 h. The solid was washed with ethyl acetate until the filtrate was colorless, and then washed with aliquots of acetonitrile. The obtained white solid was vacuum dried at 80 °C. 1H-NMR and 13C-NMR spectra are shown in Figures S2 and S3, Supporting Information.
Yield: 50.4%. 1H-NMR (D2O, δ in ppm): 3.93 (3H, s, NCH3), 5.08 (2H, s, NCH2COOH), 7.48 (2H, m, CH3NCHCHN) and 8.78 (1H, s, NCHN). 13C-NMR (D2O, δ in ppm): 38.84 (NCH3), 53.10 (NCH2COOH), 126.43 (CH3NCHCHN), 140.25 (NCHN) and 173.26 (NCH2COOH).
1,2-Bis(methylimidazolium)ethane dichloride (bMIE⋅2Cl) was obtained as described by Ahrens et al.[3030 Ahrens, S., Zeller, A., Taige, M., & Strassner, T. (2006). Extension of the alkane bridge in bisNHC−palladium−chloride complexes. Synthesis, structure, and catalytic activity. Organometallics, 25(22), 5409-5415. http://dx.doi.org/10.1021/om060577a.
http://dx.doi.org/10.1021/om060577a...
]. In a round bottom flask was added 1.5 equiv (0.3 mol) of 1-methylimidazole, 1.0 equiv (0.2 mol) of 1,2-dichloroethane and 100 mL of acetonitrile. The solution was heated at reflux at 80 °C for 48 h under magnetic stirring and in an inert atmosphere. The solid was washed with ethyl acetate and small portions of acetonitrile and vacuum dried at 80 °C, resulting in a pale yellow solid. NMR spectra are shown in Figures S4 and S5 in the Supporting Information.
Yield: 67.8%. 1H-NMR (D2O, δ in ppm): 3.91 (6H, s, NCH3), 4.77 (4H, s, NCH2), 7.45 (2H, d, CH3NCHCHN) and 7.53 (2H, d, CH3NCHCHN). 13C-NMR (D2O, δ in ppm): 38.83 (NCH3), 51.59 (NCH2), 124.97 (CH3NCHCHN), 127.41 (CH3NCHCHN) and 139.46 (NCHN).
2.2.2. Synthesis of iron-containing ionic liquid catalysts
Previously synthesized ILs were mixed with anhydrous FeCl3 to form the iron-containing ILs catalysts: 1-butyl-3-methylimidazolium heptachlorodiferrate (BMI⋅Fe2Cl7), 1-methyl-3-carboxymethylimidazolium heptachlorodiferrate (MAI⋅Fe2Cl7) and 1,2-Bis(methylimidazolium)ethane bis(heptachlorodiferrate) (bMIE⋅2Fe2Cl7), keeping the following ratio: 2.0 equiv. FeCl3/1.0 equiv. IL-chloride. Initially, each IL was added to a schlenk and then a defined amount of FeCl3 was added. The reactions were kept under heating, magnetic stirring and inert atmosphere, as shown in Scheme 1. The catalysts were not purified, obtaining a quantitative yield, where BMI⋅Fe2Cl7 is a dark liquid and the others are dark solids at room temperature.
2.2.3. Miniemulsion polymerization
The polymerizations were carried in a 150 mL jacketed glass reactor, integrated with a thermostatic bath at the desired temperature (85, 70 or 55 °C), remaining for 8 h under constant mechanical stirring of 400 rpm and N2 bubbling. Polymerizations were performed in triplicates, maintaining a catalyst/styrene molar ratio of 1:1000. The formulations used were adapted from Alves et al.[2222 Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035.
http://dx.doi.org/10.1016/j.eurpolymj.20...
]. Initially, the aqueous phase, made up of 0.36 g of the cationic surfactant CTAB, and 66 g of deionized water, and the organic phase, 0.90 g of hexadecane and 18 g of styrene, were prepared separately under magnetic stirring at 300 rpm for 20 min and at room temperature. Then the organic phase was added to the aqueous phase, while maintaining magnetic stirring at 800 rpm. After 20 min, the coarse emulsion was miniemulsified using an ultrasonic homogenizer for 1 min in a 70% amplitude ice bath (10 s on/ 5 s off).
Subsequently, a solution consisting of catalyst and 6 g of water was prepared, leaving it to stir until complete solubilization of the catalyst. First, the miniemulsion and, later the catalytic solution were transferred, in a single step, to the reactor, kept under stirring for 5 min and N2 bubbling. After the mixing time, the thermostatic bath was integrated with the reactor and a condenser was adapted at one end and the system was kept under N2 bubbling. Aliquots of latex were collected during the reaction. The latex obtained has a light yellow color and the dry polymers are yellow color (Figure S6a, Supporting Information). The dry polymers were purified by solubilization in dichloromethane and then submitted to extraction with distilled water. The organic phase was allowed to stand with anhydrous Na2SO4 and then filtered and the purified polymer was obtained after evaporation of the solvent (Figure S6b, Supporting Information). 1H-NMR (600 MHz, CDCl3) (Figure S7, Supporting Information) δ (ppm): 0.88 (6H, CH3(CH2)14CH3 of hexadecane), 1.26 (28H, CH3(CH2)14CH3 of hexadecane), 1.30-1.55 (2H, -CH2CH(Ph)-), 1.83 (1H, -CH2CH(Ph)-), 6.30-7.08 (m, 5H, Ar) - Polystyrene obtained using BMI⋅Fe2Cl7/styrene molar ratio of 1:1000 at 85 °C and 8 h of synthesis; = 1266 kg mol-1 and ĐM = 1.88.
2.3 Material Characterization
The mass-average molar mass () and the number-average molar mass () and the dispersity (ĐM) of the polymers were determined using a gel permeation chromatograph (Malvern, model Viscotek RImax) equipped with a refractive index detector with a set of three columns of 300 x 8 mm mounted in series (KF-802.5, KF-804L and KF-805L) operating at 40 °C. The system was calibrated using polystyrene standards with molar mass ranging from 1.20 kg mol-1 to 4500 kg mol-1 and monodisperse (ĐM close to 1.0). Tetrahydrofuran solvent (THF), HPLC grade, was used as the mobile phase with a flow rate of 1 mL⋅min-1. Prior to injection, previously prepared solutions (1.5 mg sample/1.0 mL THF) were filtered through hydrophobic polytetrafluoroethylene (PTFE) membranes with 0.45 μm pore size and the injection volume was 100 μL.
The differential scanning calorimetry (DSC) curves were obtained using a Shimadzu model DSC-60 equipment. The initial masses used were approximately 5.0 mg and aluminum crucibles were used. The measurements were made under a helium atmosphere at a flow rate of 30 mL min−1, with heating rate of 10 °C min−1 and two heating ramps (−40 to 180 °C). The second heating cycle was used to determine the Tg of the polymers.
Nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were obtained using a Bruker 600 Ascend spectrometer, equipped with a 5 mm probe operating at 600 MHz. About 20 mg of the samples were dissolved in 0.5 mL of deuterated solvent and the spectra were acquired expressing chemical shifts in parts per million (ppm) and tetramethylsilane (TMS) was used as internal standard.
The average sizes of the monomer droplets (initial Dp) and latex polymer particles (final Dp) and their respective polydispersity indexes (PdI) were determined using the dynamic light scattering technique (DLS) using a Malvern equipament, Zetasizer Nano ZS model. Measurements were made by direct dispersion of a drop in approximately 2.5 mL of water.
Transmission electron micrographs (TEM) were obtained using a JEOL JEM-2100 microscope operating at 200 kV. The samples were initially dispersed in distilled water and sonicated for 15 min. Then a drop of the suspension was applied to a 200 mesh copper containing a supported carbon film and dried at room temperature.
The conversions were obtained by gravimetric data. At predetermined time intervals, aliquots of the reaction medium, about 1.0 g, were removed and added to pre-weighed aluminum foil capsules treated with 5 drops of NaOH solution (4 g L-1)[88 Cauvin, S., Sadoun, A., Dos Santos, R., Belleney, J., Ganachaud, F., & Hemery, P. (2002). Cationic polymerization of p-methoxystyrene in miniemulsion. Macromolecules, 35(21), 7919-7927. http://dx.doi.org/10.1021/ma0202890.
http://dx.doi.org/10.1021/ma0202890...
]. The capsules were dried in an oven at 60 °C for approximately 48 h. The conversion was obtained by the ratio of the dry polymer mass to the monomer mass used. The polymer mass was obtained by subtracting the added NaOH fraction and the non-polymer species (initiator, surfactant and stabilizer).
3. Results and Discussion
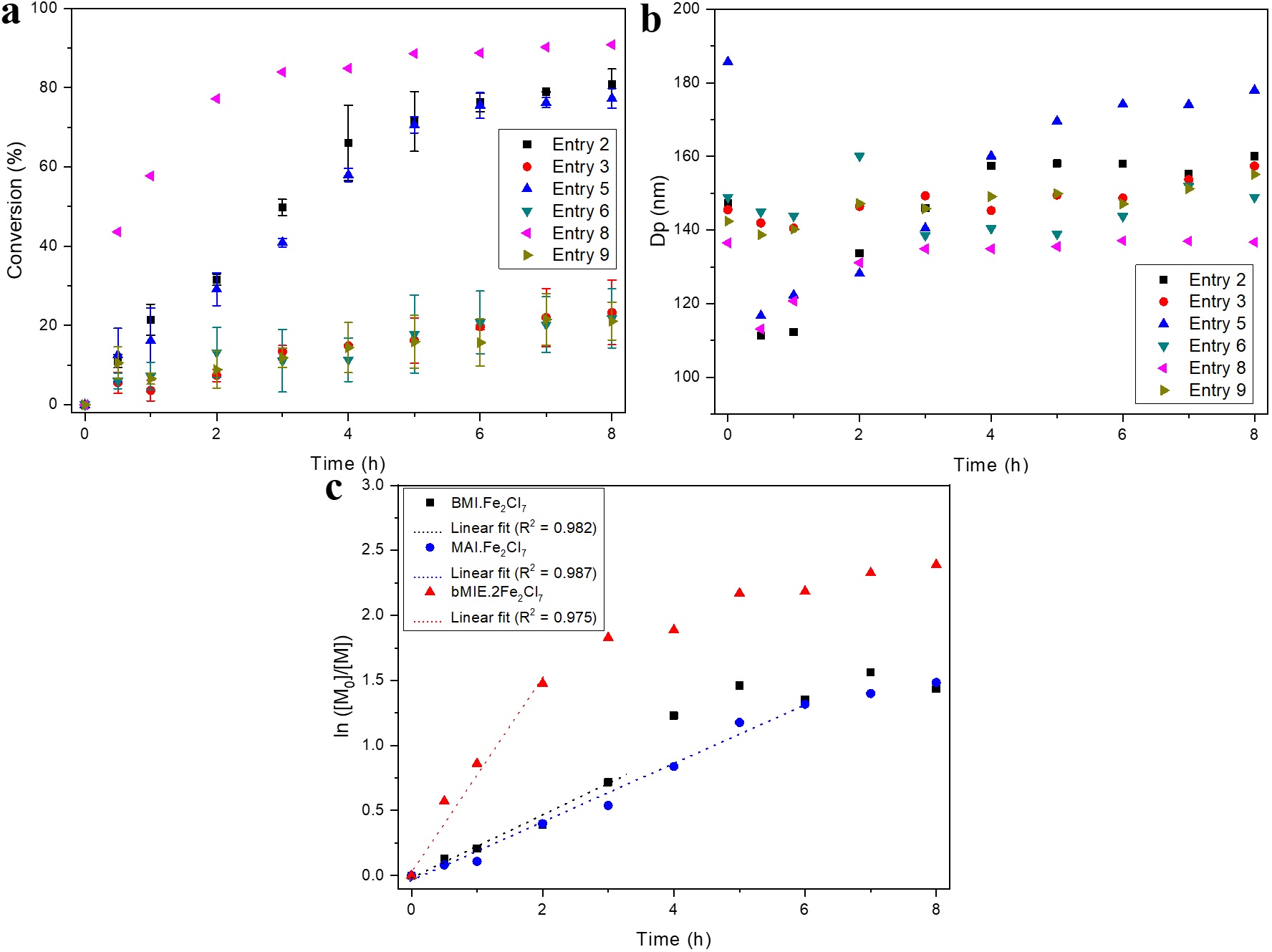
The polymerizations were performed in accordance with the experimental procedure previously described and aliquots were collected throughout the polymerization process in order to evaluate performance of iron-containing ILs catalysts incorporated in their anionic structure, BMI⋅Fe2Cl7, MAI⋅Fe2Cl7 and bMIE⋅2Fe2Cl7, and the effect of the reaction temperature on final polymer properties, nanoparticle size and conversion. Table 1 shows the conversion and diameter values of monomer droplets (initial Dp) and polymeric particles (final Dp), including polydispersity index (PdI), corresponding to the mean and standard deviation of the essays performed in triplicate, and the values obtained for each synthesis are presented in Table S1 in the Supporting Information. The polymerizations were carried out in triplicate in order to evaluate the reproducibility of the polymerization process and minimize experimental scattering due to gravimetry measurements. Figure 1 shows the evolution of conversion, mean particle diameter and the semilogarithmic plot of monomer concentration for the different synthesis conditions.
Conversion, average diameter of the monomer droplets (initial Dp) and polymeric particles (final Dp) and polydispersity index for polymerizations after 8 h of reaction.
Evolution of (a) conversion; (b) mean diameter of latex particles during reactions at 85 and 70 °C and; (c) dependence of semilogarithmic of the monomer concentration with synthesis time at 85 °C.
The high conversion values indicate that all ILs catalysts were efficiently capable of producing polystyrene. The highest conversions, generally under the same experimental conditions, were achieved using the bMIE⋅2Fe2Cl7 catalyst (comparing Entries 2, 5 and 8 and Entries 4, 7 and 10 of Table 1). This behavior was not observed at 70 °C, as Entries 3 and 6 of Table 1 showed the largest standard deviations. This difference in reactivity of the catalysts is due to the higher proportion of the anionic species Fe2Cl7 in the bMIE⋅2Fe2Cl7 than in the others, since according to Rodrigues et al.[3131 Rodrigues, T. S., Machado, F., Lalli, P. M., Eberlin, M. N., & Neto, B. A. D. (2015). Styrene polymerization efficiently catalyzed by iron-containing imidazolium-based ionic liquids: reaction mechanism and enhanced ionic liquid effect. Catalysis Communications, 63, 66-73. http://dx.doi.org/10.1016/j.catcom.2014.11.002.
http://dx.doi.org/10.1016/j.catcom.2014....
] these species are responsible for initiating styrene polymerization. In addition, these polymerizations showed a limit conversion of 80 to 90%, related to monomer loss due to nitrogen drag in the bubbling system, besides the occurrence of the glass effect which is associated with the increase of viscosity inside the monomer droplets, leading to a reduction of the reaction rates[3232 Machado, F., Lima, E. L., & Pinto, J. C. (2007). Uma revisão sobre os processos de polimerização em suspensão. Polímeros: Ciência e Tecnologia, 17(2), 166-179. http://dx.doi.org/10.1590/S0104-14282007000200016.
http://dx.doi.org/10.1590/S0104-14282007...
].
The polymerizations were performed at different temperatures of 55, 70 and 85 °C with catalyst/monomer molar ratio of 1:1000. For BMI⋅Fe2Cl7, the conversions increased from 7% to 81% (Table 1 and Figure 1a) when synthesis temperatures were increased from 55 to 85 °C. Similar behavior was observed for MAI⋅Fe2Cl7 with an increase from 8% to 77%, whereas for bMIE⋅2Fe2Cl7, it was observed an increase in the conversion from 15% to 90%. This is because catalytic species become more reactive at higher temperatures[3333 Aoshima, S., & Kanaoka, S. (2009). A renaissance in living cationic polymerization. Chemical Reviews, 109(11), 5245-5287. http://dx.doi.org/10.1021/cr900225g. PMid:19803510.
http://dx.doi.org/10.1021/cr900225g...
].
In order to evaluate if competitive thermal polymerization via radical initiation is favored by the reaction temperature (85 °C), a blank reaction was performed under the same experimental conditions, but without the addition of ILs or any other initiator (Entry 1, Table 1). This blank reaction presented a much lower conversion, about 30%. In addition, average molar masses are relatively different from those obtained by using ILs catalysts. Also, under the same experimental conditions, polymerization was carried out in the presence of BMI⋅Fe2Cl7 and radical polymerization inhibitors, as the synthesis was performed with unpurified styrene containing approximately 50 ppm of 4-tert-butylcatechol as stabilizer (Entry 11, Table 1). This reaction presented a relatively high conversion, about 64% and molar masses very close to those obtained using BMI⋅Fe2Cl7 with purified styrene. Although these reactions were conducted at relatively high temperatures, which might favor the self-initiation of styrene, the polymerizations carried out in presence of ILs catalysts are favorably governed by the cationic route due to the extremely high reactivity of the ILs catalysts.
The effect of temperature was also investigated at 24 h of reaction, and the results can be observed in Entries 12-14 of Table 1. Again, a low monomer conversion is observed for the blank reactions, around 18% at 60 °C (Entry 14, Table 1). On the other hand, the polymerization carried out in the presence of BMI⋅Fe2Cl7 (Entry 12, Table 1) showed conversion of 72% and even when reactions where conducted at lower temperature of 50 °C, polystyrene is formed, with conversion of 33% (Entry 13, Table 1) and molar masses quite different from that obtained in the blank reaction. In addition, the decrease in temperature favored the formation of polystyrene with higher number-average molar mass and with narrower dispersity.
As a matter of fact, the above-mentioned results suggest that cationic polymerization is undoubtedly taking place. Additionally, experiments were carried out to verify the ability of other vinyl monomers [methyl methacrylate (MMA), vinyl pivalate (VPi) and butyl acrylate (BuA)] to polymerize via bulk polymerization using BMI⋅Fe2Cl7 at 70 °C (Entries 15-17, Table 1). It is well-known that these monomers cannot be polymerized in accordance with the cationic mechanism due to the instability of the formed carbocation and, therefore, the absence of polymerization gives more evidence that the reaction mechanism is purely cationic. Figure S8 (Supporting Information) presents the 1H-NMR spectrum of the experiment in which VPi was added (Entry 16, Table 1) and the absence of a signal at 1.7 ppm, attributed to methylene (–CH2–) protons in the polymer backbone[3434 Islam, M. N., Haldorai, Y., Nguyen, V. H., & Shim, J.-J. (2014). Synthesis of poly(vinyl pivalate) by atom transfer radical polymerization in supercritical carbon dioxide. European Polymer Journal, 61, 93-104. http://dx.doi.org/10.1016/j.eurpolymj.2014.09.003.
http://dx.doi.org/10.1016/j.eurpolymj.20...
], indicating that poly(vinyl pivalate) was not synthesized.
In comparison, experiment using only styrene (Entry 18, Table 1), led to a rapid polymerization, forming a highly viscous solution in only 15 min, which is highly unlikely to happen through self-initiated thermal polymerization of styrene. Polymerization also succeeded at lower temperatures, as for instance, i) 30 °C (Entry 19, Table 1), occurring more slowly and achieving similar conversion after 3 h and ii) 0 °C, achieving a 9.9% conversion in 6 h of reaction (Entry 20, Table 1), which clearly indicates that the polymerization of styrene mediated by this kind of catalyst follows predominantly a cationic mechanism.
These experimental results agree very well with the one described by Alves and coauthors[2222 Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035.
http://dx.doi.org/10.1016/j.eurpolymj.20...
], in which replacing ILs catalysts with benzoyl peroxide, conversion around 30% has been achieved, after 5 h, under the same operating conditions. One should also bear in mind that in the particular case of the ILs catalysts used here, styrene homopolymerization takes place due to the formation of a key styrene chloronium cation, which is believed to be stabilized by the catalyst through ion-pairing effects[3131 Rodrigues, T. S., Machado, F., Lalli, P. M., Eberlin, M. N., & Neto, B. A. D. (2015). Styrene polymerization efficiently catalyzed by iron-containing imidazolium-based ionic liquids: reaction mechanism and enhanced ionic liquid effect. Catalysis Communications, 63, 66-73. http://dx.doi.org/10.1016/j.catcom.2014.11.002.
http://dx.doi.org/10.1016/j.catcom.2014....
].
Bulk polymerizations (Entry 18, Table 1)[3131 Rodrigues, T. S., Machado, F., Lalli, P. M., Eberlin, M. N., & Neto, B. A. D. (2015). Styrene polymerization efficiently catalyzed by iron-containing imidazolium-based ionic liquids: reaction mechanism and enhanced ionic liquid effect. Catalysis Communications, 63, 66-73. http://dx.doi.org/10.1016/j.catcom.2014.11.002.
http://dx.doi.org/10.1016/j.catcom.2014....
] achieved limit conversions in much shorter reaction times than miniemulsion polymerizations. This difference can be attributed to the high water content of the miniemulsion system, leading to a reduced reaction rate and thus decreasing conversion. Generally, one of the main obstacles of cationic polymerization has been the high reaction rates that make polymerization control difficult. Thus, the water effect resulted in lower rates, allowing better control of polymerization[2222 Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035.
http://dx.doi.org/10.1016/j.eurpolymj.20...
].
It was observed that the mean particle diameter (Dp) remained practically unchanged, as shown in Figure 1b and Table S1 of the Supporting Information, that is, the average diameter of the polymeric particles (final Dp) shows values close to the monomer droplets (initial Dp), indicating that nucleation occurs preferentially within the monomeric droplets, and that both effects of coalescence and diffusional degradation were properly minimized during polymerization. Thus, the polymeric latexes were obtained with high colloidal stability and particle diameter between 113 nm and 197 nm, presenting low polydispersity indexes (PdI) (Table S1, Supporting Information), indicating the formation of polymer nanoparticles with narrow size distribution.
The semilogarithmic plot of monomer concentration versus synthesis time using the ILs catalysts shown in Figure 1c, indicates that polymerization does not follow first-order kinetics throughout the reaction. Kostjuk et al.[3535 Kostjuk, S. V., Dubovik, A. Y., Vasilenkol, I. V., Mardykin, V. P., Gaponik, L. V., Kaputsky, F. N., & Antipin, L. M. (2004). Novel initiating system based on AlCl3 etherate for quasiliving cationic polymerization of styrene. Polymer Bulletin, 52(3), 227-234. http://dx.doi.org/10.1007/s00289-004-0280-2.
http://dx.doi.org/10.1007/s00289-004-028...
] observed the same behavior when studying cationic polymerization of styrene using AlCl3-based initiators. The authors explained that this curvature (similar to the one depicted in Figure 1c indicates that the concentration of propagating and reversibly terminated chains decreases more rapidly than the formation of new chains by slow initiation. Theoretically, for a truly living polymerization system, this plot should be linear. Thus, a more detailed analysis shows a linear dependence of the semilogarithmic plot up to a certain polymerization period, indicating that the reactions follow a first-order kinetic up to a limit conversion of about 51% (BMI⋅Fe2Cl7), 73% (MAI⋅Fe2Cl7) and 77% (bMIE⋅2Fe2Cl7). Semilogarithmic linear dependence has been reported up to a 51% conversion[3636 Banerjee, S., Paira, T. K., & Mandal, T. K. (2013). Control of molecular weight and tacticity in stereospecific living cationic polymerization of α-methylstyrene at 0 °C using FeCl3-based initiators: effect of tacticity on thermal properties. Macromolecular Chemistry and Physics, 214(12), 1332-1344. http://dx.doi.org/10.1002/macp.201300092.
http://dx.doi.org/10.1002/macp.201300092...
] and a 23% conversion[3737 Paira, T. K., Banerjee, S., Raula, M., Kotal, A., Si, S., & Mandal, T. K. (2010). Peptide−polymer bioconjugates via atom transfer radical polymerization and their solution aggregation into hybrid micro/nanospheres for dye uptake. Macromolecules, 43(9), 4050-4061. http://dx.doi.org/10.1021/ma1001904.
http://dx.doi.org/10.1021/ma1001904...
] in the cationic polymerization of α-methylstyrene using FeCl3-based initiators and in the reaction of a peptide-poly(methyl methacrylate) (peptide-PMMA) hybrid bioconjugates by atom transfer radical polymerization (ATRP), respectively. Therefore, polymerization systems using ILs catalysts are possibly cationic in nature, following a first-order kinetics to a limit conversion.
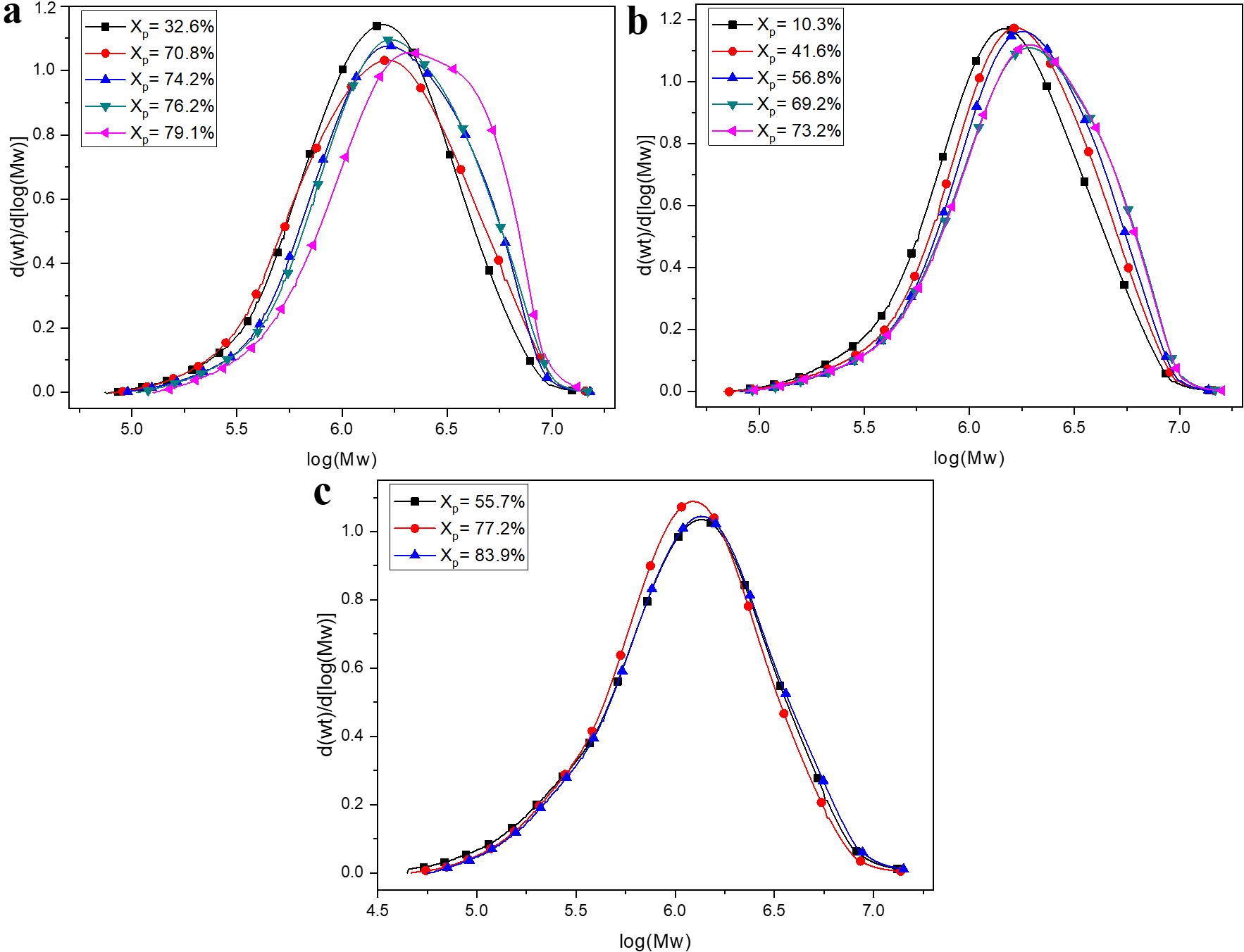
Figure 2 shows the molar mass distribution curves of the polystyrenes obtained as a function of conversion using the different ILs catalysts at 85 °C, these values are shown in Table S2 in the Supporting Information. It was possible to synthesize polystyrene with high number-average molar mass, around 1300 kg mol-1, much higher than those obtained by Cauvin et al.[88 Cauvin, S., Sadoun, A., Dos Santos, R., Belleney, J., Ganachaud, F., & Hemery, P. (2002). Cationic polymerization of p-methoxystyrene in miniemulsion. Macromolecules, 35(21), 7919-7927. http://dx.doi.org/10.1021/ma0202890.
http://dx.doi.org/10.1021/ma0202890...
,1111 Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a. PMid:15917929.
http://dx.doi.org/10.1039/b501489a...
] and Touchard et al.[99 Touchard, V., Graillat, C., Boisson, C., D’Agosto, F., & Spitz, R. (2004). Use of a Lewis acid surfactant combined catalyst in cationic polymerization in miniemulsion: Apparent and hidden initiators. Macromolecules, 37(9), 3136-3142. http://dx.doi.org/10.1021/ma0355352.
http://dx.doi.org/10.1021/ma0355352...
] who studied the cationic miniemulsion polymerization of p-methoxystyrene, obtaining between 1.0-39.4 kg mol-1. These results are indicative that the polymerization mechanism occurs preferably inside monomer droplets rather than at the monomer droplets/water interface, commonly obtained in miniemulsion ionic polymerizations[55 Kostjuk, S. V., & Ganachaud, F. (2010). Cationic polymerization of vinyl monomers in aqueous media: from monofunctional oligomers to long-lived polymer chains. Accounts of Chemical Research, 43(3), 357-367. http://dx.doi.org/10.1021/ar900198q. PMid:19957949.
http://dx.doi.org/10.1021/ar900198q...
,88 Cauvin, S., Sadoun, A., Dos Santos, R., Belleney, J., Ganachaud, F., & Hemery, P. (2002). Cationic polymerization of p-methoxystyrene in miniemulsion. Macromolecules, 35(21), 7919-7927. http://dx.doi.org/10.1021/ma0202890.
http://dx.doi.org/10.1021/ma0202890...
,99 Touchard, V., Graillat, C., Boisson, C., D’Agosto, F., & Spitz, R. (2004). Use of a Lewis acid surfactant combined catalyst in cationic polymerization in miniemulsion: Apparent and hidden initiators. Macromolecules, 37(9), 3136-3142. http://dx.doi.org/10.1021/ma0355352.
http://dx.doi.org/10.1021/ma0355352...
,1111 Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a. PMid:15917929.
http://dx.doi.org/10.1039/b501489a...
].
Molar mass distribution curves of the polystyrenes synthesized by using ILs catalysts at 85 °C (a) BMI⋅Fe2Cl7; (b) MAI⋅Fe2Cl7; (c) bMIE⋅2Fe2Cl7.
Table S2 in the Supporting Information shows the average molar masses and ĐM of the polystyrenes obtained in bulk polymerization (shown in Entries 18-20, Table 1). These polymers had number-average molar mass, around 15 kg mol-1, much lower than those achieved by miniemulsion polymerization. These differences can be attributed to the characteristics of the two synthesis processes, since in the bulk polymerization there is an increase in viscosity as the conversion increases, causing the interruption of the homogenization of the system and delaying the mobility of the monomer to the growing chains. In addition, there is a much higher concentration of reactive species at the bulk polymerization locus, resulting in lower molar masses[2222 Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035.
http://dx.doi.org/10.1016/j.eurpolymj.20...
].
The values of the average molar masses of polystyrene obtained after 8 h of synthesis showed significant differences when using different ILs catalysts, and the polymers obtained in the presence of bMIE⋅2Fe2Cl7 showed the lowest number-average molar mass. This difference is attributed to the higher concentration of the catalytic species, Fe2Cl7, in the polymerization locus. As a result, the initiation step is favored, resulting in the formation of several polymer chains with smaller molar mass (less than 800 kg mol-1) and with different sizes, consequently, increasing the molar-mass dispersity (higher than 2.1). The samples obtained with the other catalysts, BMI⋅Fe2Cl7 and MAI⋅Fe2Cl7, presented similar number-average molar mass and molar-mass dispersity, since they have relatively equal concentrations of the catalytic species. These results support the assumption that cationic polymerization takes place, since the increase in the concentration of catalytic species, using bMIE⋅2Fe2Cl7, led a decrease in the molar masses. Rodrigues et al.[3131 Rodrigues, T. S., Machado, F., Lalli, P. M., Eberlin, M. N., & Neto, B. A. D. (2015). Styrene polymerization efficiently catalyzed by iron-containing imidazolium-based ionic liquids: reaction mechanism and enhanced ionic liquid effect. Catalysis Communications, 63, 66-73. http://dx.doi.org/10.1016/j.catcom.2014.11.002.
http://dx.doi.org/10.1016/j.catcom.2014....
] had already observed a reduction in the average molar masses with the increase in the concentration of the catalyst BMI⋅Fe2Cl7 in the styrene bulk polymerization.
The molar mass distribution curves of the obtained polymers, Figure 2, are unimodal and a slight displacement of the curves is observed for higher average molar masses values as the conversion increases. Nevertheless, under these conditions, molar-mass dispersity remained relatively high (ĐM ≅ 1.9) throughout the reaction, suggesting that polymerization is not as well controlled as in other studies[3838 Yamada, K., Minoda, M., & Miyamoto, T. (1999). Controlled synthesis of amphiphilic block copolymers with pendant N-acetyl-D-glucosamine residues by living cationic polymerization and their interaction with WGA lectin. Macromolecules, 32(11), 3553-3558. http://dx.doi.org/10.1021/ma9816315.
http://dx.doi.org/10.1021/ma9816315...
]. However, the samples synthesized here have narrower distribution compared to other studies that exemplified the formation of polymer with high molar mass.
The molar mass distribution curves of the blank reaction performed at 85 °C (Entry 1, Table 1), and of the polymerizations in the presence of BMI⋅Fe2Cl7 at 85 °C with purified (Entry 2, Table 1) and unpurified styrene (Entry 11, Table 1) are shown in Figure 3a. The values of average molar masses and ĐM are shown in Table S2 (Supporting Information). The blank reaction showed lower molar masses than those obtained using ILs catalysts and the profile of the molar mass distribution curves are relatively different. On the other hand, the molar mass distribution, as well as, the average molar masses using BMI⋅Fe2Cl7 with purified and non-purified styrene are very similar.
Molar mass distribution curves of the polystyrenes obtained by (a) blank reaction and using BMI⋅Fe2Cl7 in the presence of purified and unpurified styrene at 85 °C and (b) reactions conducted at 60 and 50 °C for 24 h.
Figure 3b shows the molar mass distribution curves of reactions performed at lower temperatures and 24 h of reaction, including polymerizations in the presence of BMI⋅Fe2Cl7 at 60 °C (Entry 12, Table 1) and 50 °C (Entry 13, Table 1) and the blank reaction at 60 °C (Entry 14, Table 1). Table S2 (Supporting Information) shows the values of average molar masses and ĐM. Again, the blank reaction showed lower molar masses than the one observed for the reaction conducted in the presence of the catalyst BMI⋅Fe2Cl7 and the decrease in the synthesis temperature caused a slight increase in the number-average molar mass and a decrease in the molar-mass dispersity. This behavior is characteristic of cationic polymerization.
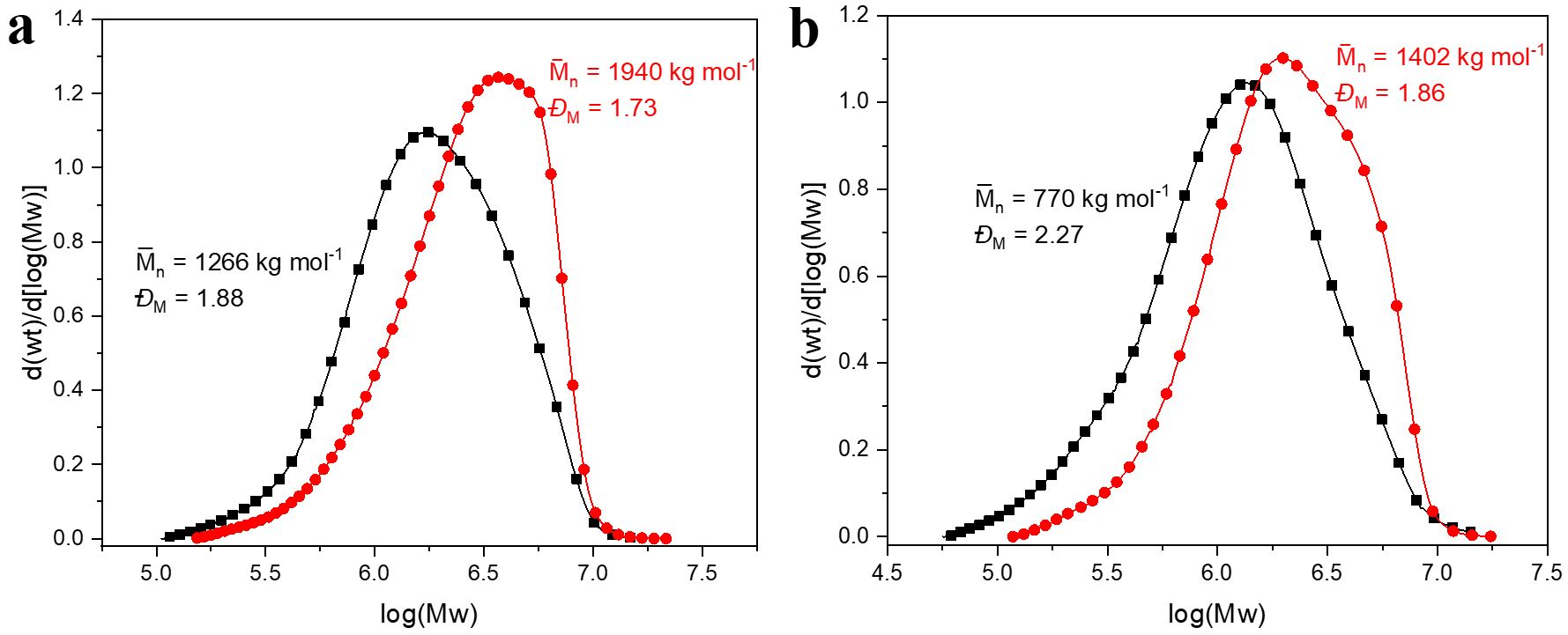
Figure 4 shows the molar mass distribution of the polystyrenes obtained at different synthesis temperatures. It is noticed that higher average molar masses and narrow dispersions were obtained as temperature is decreased (from = 1266 kg mol–1 and ĐM = 1.88 at 85 °C to = 1940 kg mol–1 and ĐM = 1.73 at 55° C using BMI⋅Fe2Cl7 catalyst; see Figure 3a). This behavior is typical of cationic polymerization, since they are better controlled at low temperatures, which favors the propagation reactions over the termination reactions, leading to the formation of polymers with higher average molar masses[3333 Aoshima, S., & Kanaoka, S. (2009). A renaissance in living cationic polymerization. Chemical Reviews, 109(11), 5245-5287. http://dx.doi.org/10.1021/cr900225g. PMid:19803510.
http://dx.doi.org/10.1021/cr900225g...
].
Molar mass distribution curves of polystyrene as a function of the synthesis temperature line and symbol black (85 °C) and red (55 °C) using catalysts (a) BMI⋅Fe2Cl7 and (B) bMIE⋅2Fe2Cl7.
The observed values for the molar-mass dispersity close to 2 is characteristic of cationic chain polymerizations where termination and transfer reactions are highly expected to take place, mainly when the reaction achieves elevated conversions. Based on this reaction behavior, it is strongly expected to produce polystyrenes exhibiting broad molar mass distributions with ĐM relatively higher than 2, reflecting the effect of the reaction operation conditions. As an additional effect, the increase of the local viscosity may also contribute to the occurrence of transfer reactions, accounting for the reduction of the reaction rates, as a result of the decreased migration of the monomer molecules to the active species.
The molar-mass dispersity, in all syntheses, remained higher than 1.8 throughout polymerization. ĐM values obtained here were relatively larger than those reported by Touchard et al.[99 Touchard, V., Graillat, C., Boisson, C., D’Agosto, F., & Spitz, R. (2004). Use of a Lewis acid surfactant combined catalyst in cationic polymerization in miniemulsion: Apparent and hidden initiators. Macromolecules, 37(9), 3136-3142. http://dx.doi.org/10.1021/ma0355352.
http://dx.doi.org/10.1021/ma0355352...
], Satoh et al.[33 Satoh, K., Kamigaito, M., & Sawamoto, M. (2000). Lanthanide triflates-mediated emulsion cationic polymerization of p-alkoxystyrenes in aqueous media. Macromolecules, 33(13), 4660-4666. http://dx.doi.org/10.1021/ma0000069.
http://dx.doi.org/10.1021/ma0000069...
], Kostjuk & Ganachaud[66 Kostjuk, S. V., & Ganachaud, F. (2006). Cationic polymerization of styrene in solution and aqueous suspension using B(C6F5)3 as a water-tolerant Lewis acid. Macromolecules, 39(9), 3110-3113. http://dx.doi.org/10.1021/ma052650z.
http://dx.doi.org/10.1021/ma052650z...
] and Biedrón & Kubisa[3939 Biedroń, T., & Kubisa, P. (2004). Cationic polymerization of styrene in a neutral ionic liquid. Journal of Polymer Science. Part A, Polymer Chemistry, 42(13), 3230-3235. http://dx.doi.org/10.1002/pola.20158.
http://dx.doi.org/10.1002/pola.20158...
] who polymerized p-methoxystyrene by miniemulsion, p-alkoxystyrenes by emulsion, and styrene by suspension and solution polymerization, respectively, and obtained ĐM of less than 1.5. However, the molar mass obtained in these studies ( ≤ 4.5 kg mol-1) were much lower than those reported here. There are reports in the literature of styrene-derived polymers with higher number-average molar mass, 40[1111 Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a. PMid:15917929.
http://dx.doi.org/10.1039/b501489a...
], 117[1212 Vasilenko, I. V., Yeong, H. Y., Delgado, M., Ouardad, S., Peruch, F., Voit, B., Ganachaud, F., & Kostjuk, S. V. (2015). A catalyst platform for unique cationic (co)polymerization in aqueous emulsion. Angewandte Chemie International Edition, 54(43), 12728-12732. http://dx.doi.org/10.1002/anie.201501157. PMid:26013180.
http://dx.doi.org/10.1002/anie.201501157...
], and 550 kg mol-1[4040 Patrocinio, V. M. B., Agner, T., Dutra, G. V. S., Machado, F., Neto, B. A. D., Araújo, P. H. H., & Sayer, C. (2019). High molecular weight polystyrene obtained by cationic emulsion polymerization catalyzed by imidazolium-based ionic liquid. Macromolecular Reaction Engineering, 13(2), 1800061-1800067. http://dx.doi.org/10.1002/mren.201800061.
http://dx.doi.org/10.1002/mren.201800061...
], in which, the distributions are relatively high, from 2.0 to 3.8, because it is very difficult to control these polymerization mechanism as large polymer chains are formed.
These results are very promising because the other authors who synthesized polystyrene or its derivatives via cationic polymerization in water-based systems (miniemulsion, emulsion or suspension) generally obtained low molar mass and used high synthesis times and excessive catalyst concentration and/or activators[33 Satoh, K., Kamigaito, M., & Sawamoto, M. (2000). Lanthanide triflates-mediated emulsion cationic polymerization of p-alkoxystyrenes in aqueous media. Macromolecules, 33(13), 4660-4666. http://dx.doi.org/10.1021/ma0000069.
http://dx.doi.org/10.1021/ma0000069...
,66 Kostjuk, S. V., & Ganachaud, F. (2006). Cationic polymerization of styrene in solution and aqueous suspension using B(C6F5)3 as a water-tolerant Lewis acid. Macromolecules, 39(9), 3110-3113. http://dx.doi.org/10.1021/ma052650z.
http://dx.doi.org/10.1021/ma052650z...
,77 Vasilenko, I. V., Ganachaud, F., & Kostjuk, S. V. (2016). New insights into the cationic polymerization in emulsion catalyzed by water-dispersible Lewis acid surfactant complexes: a case study with p-methoxystyrene. Macromolecules, 49(9), 3264-3273. http://dx.doi.org/10.1021/acs.macromol.6b00379.
http://dx.doi.org/10.1021/acs.macromol.6...
,1111 Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a. PMid:15917929.
http://dx.doi.org/10.1039/b501489a...
]. For example, Satoh et al.[33 Satoh, K., Kamigaito, M., & Sawamoto, M. (2000). Lanthanide triflates-mediated emulsion cationic polymerization of p-alkoxystyrenes in aqueous media. Macromolecules, 33(13), 4660-4666. http://dx.doi.org/10.1021/ma0000069.
http://dx.doi.org/10.1021/ma0000069...
] performed cationic emulsion polymerization of p-methoxystyrene (pMOS) using pMOS–HCl adduct/lanthanide triflates initiation system. The authors used [pMOS] = 3.0 M; [pMOS–HCl] = 60 mM; [Yb(OTf)3] = 300 mM and obtained a conversion of 78% after 50 h of synthesis at 30 °C and achieved = 2.38 kg mol-1 and ĐM = 1.38. Cauvin et al.[1111 Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a. PMid:15917929.
http://dx.doi.org/10.1039/b501489a...
] used [pMOS] = 1.5 M; [pentachlorophenol (PCP)] = 30 mM; [Yb(OTf)3] = 150 mM and obtained conversion of 67% in miniemulsion after 400 h at 60 °C and reached = 39.4 kg mol-1 and ĐM = 3.8. More recently, Zhang et al.[1010 Zhang, J., Wu, Y., Li, X., Yang, D., Zhang, M., Wang, H., Shang, Y., Ren, P., Mu, X., Li, S., & Guo, W. (2019). Characteristics and mechanism of styrene cationic polymerization in aqueous media initiated by cumyl alcohol/B(C6F5)3. Macromolecular Chemistry and Physics, 220(4), 1800419-1800427. http://dx.doi.org/10.1002/macp.201800419.
http://dx.doi.org/10.1002/macp.201800419...
] obtained conversion of 51% and = 3.2 kg mol-1 in cationic suspension polymerization of styrene initiated by cumyl alcohol (CumOH)/B(C6F5)3, using the following experimental conditions: [St] = 1.75 M; [CumOH] = 0.05 M; [B(C6F5)3] = 0.05 M at 20 °C and 50 h of synthesis. Therefore, our process enables cost savings, leading to reagent savings, low catalyst concentration, while also avoiding the use of rare earth catalysts such as ytterbium triftalates, and relatively shorter synthesis time.
In order to verify the living nature of these polymerizations, a monomer feeding was performed in the miniemulsion previously prepared with BMI⋅Fe2Cl7 at 85 °C (Xp = 83.0% Table S1, Supporting Information, = 989 kg mol-1 and ĐM = 1.65). In this experiment 1% (w/w) styrene was added to the total, and the reaction was kept under magnetic stirring at 800 rpm at 70 °C for 4 h. The molar mass distribution curves are shown in Figure 5.
Molar mass distribution curves of polystyrene from the monomer feeding experiment (A) before feeding and after feeding: (B) clot and (C) stable latex phase.
This styrene feeding caused a system destabilization, being observed the formation of two phases, a dark clot in less quantity (Xp = 84.7%) and a stable latex phase (Xp = 82.5%). The molar mass distribution curve of the clot exhibited a broad signal, with marked displacement towards higher molar masses, showing = 1163 kg mol-1 and ĐM = 1.82. According to Banerjee et al.[4141 Banerjee, S., Paira, T. K., Kotal, A., & Mandal, T. K. (2010). Room temperature living cationic polymerization of styrene with HX-styrenic monomer adduct/FeCl3 systems in the presence of tetrabutylammonium halide and tetraalkylphosphonium bromide salts. Polymer, 51(6), 1258-1269. http://dx.doi.org/10.1016/j.polymer.2010.01.051.
http://dx.doi.org/10.1016/j.polymer.2010...
] the increase in molar mass distribution after monomer feeding is due to the slow initiation and slow exchange between reversibly terminated and propagating species. Regarding the obtained stable latex phase (Figure 5c), a change in the distribution curves was observed, leading to a reduction in the average molar mass and a narrow distribution, indicating that the polymeric chains present in dispersion were more monodisperse. This behavior and experimental data from Figure 1c may suggest a certain livingness of the reactive species, however the styrene polymerization with the IL catalyst evaluated here cannot be considered as a living polymerization in its strict sense, due to destabilization of cationic propagating species at the reaction temperature.
The glass transition temperature (Tg) values of the purified and unpurified polymers were determined through the DSC curves and are presented in Table 2. In this work, Tg values of unpurified polymers were determined in the range from 87 °C to 95 °C, depending on the type of catalyst used. Samples with higher molar mass presented higher Tg values, due to reduced mobility of polymeric chains. The blank reaction (performed in the absence of catalyst) showed a Tg value close to the unpurified polymer, around 96 °C. On the other hand, the Tg values of purified polymers were determined in the range from 102 °C to 108 °C, depending on the amount of hexadecane present in the polymeric materials.
Average molar masses, molar-mass dispersity and glass transition temperature (Tg) of the polymers synthesized at 85 °C using ILs catalysts and blank polymerization.
Tg values obtained for the purified polymers are similar to the polystyrene samples reported in the literature (107 ± 2 °C)[4242 Medeiros, A. M. M. S., Machado, F., Rubim, J. C., & McKenna, T. F. L. (2017). Bio-based copolymers obtained through miniemulsion copolymerization of methyl esters of acrylated fatty acids and styrene. Journal of Polymer Science. Part A, Polymer Chemistry, 55(8), 1422-1432. http://dx.doi.org/10.1002/pola.28511.
http://dx.doi.org/10.1002/pola.28511...
,4343 Rieger, J. (1996). The glass transition temperature of polystyrene. Journal of Thermal Analysis, 46(3), 965-972. http://dx.doi.org/10.1007/BF01983614.
http://dx.doi.org/10.1007/BF01983614...
] that presented high average molar mass. The increase in Tg values above 15 °C after purification of the polymers is due to the hexadecane removal occurred during the drying process with dichloromethane and cannot be associated to very small amount of ILs catalysts used in the polymerizations. The evidences supporting this hypothesis are: i) very low molar concentration of catalysts, around 0.4%; ii) blank reaction (without the addition of ILs) with low Tg, around 96 °C; and iii) reduction of the hexadecane content in the polymeric structure calculated by relative integration method of the spectrum of 1H-NMR[4444 Szkudlarek, M., Beginn, U., Keul, H., & Möller, M. (2017). Synthesis of terpolymers with homogeneous composition by free radical copolymerization of maleic anhydride, perfluorooctyl and butyl or dodecyl methacrylates: application of the continuous flow monomer addition technique. Polymers, 9(11), 610. http://dx.doi.org/10.3390/polym9110610. PMid:30965911.
http://dx.doi.org/10.3390/polym9110610...
] (Figure S9 of the Supporting Information).
The co-stabilizer, hexadecane, used to prevent Ostwald ripening, may act as a plasticizer due to its extremely low volatility, remaining in the polymeric structure, causing a reduction in intermolecular forces between chains, increasing molecular mobility and consequently reducing Tg. Recently, polystyrene with Tg = 90.1 °C was obtained by miniemulsion polymerization containing 4 wt.% of hexadecane[4545 Metanawin, S., Panutumron, P., Thongsale, A., & Metanawin, T. (2018). The functionalization of hybrid titanium dioxide by miniemulsion polymerization technique. Materials Today: Proceedings, 5(3, Part 2), 9651-9657. http://dx.doi.org/10.1016/j.matpr.2018.01.133.
https://doi.org/10.1016/j.matpr.2018.01....
]. Christie et al.[4646 Christie, D., Zhang, C., Fu, J., Koel, B., & Priestley, R. D. (2016). Glass transition temperature of colloidal polystyrene dispersed in various liquids. Journal of Polymer Science. Part B, Polymer Physics, 54(17), 1776-1783. http://dx.doi.org/10.1002/polb.24082.
http://dx.doi.org/10.1002/polb.24082...
] observed the same effect using glycerol suspended polystyrene films and Shen et al.[4747 Shen, Y., Du, C., Zhou, J., & Ma, F. (2018). The facile modification of polyacrylate emulsion via hexadecane to enhance controlled-release profiles of coated urea. Scientific Reports, 8(1), 12279. http://dx.doi.org/10.1038/s41598-018-30585-5. PMid:30116004.
http://dx.doi.org/10.1038/s41598-018-305...
] reported that hexadecane dispersed in the polyacrylate matrix caused a significant reduction in Tg, decreasing from 6.38 °C (emulsion) to 3.92 °C (miniemulsion).
In addition, the plasticizing effect of residual catalyst can be ruled out, since the molar ratio used is very low in relation to the polymeric fraction obtained and in a previous study low Tg values (up to 88 °C with = 183 kg mol-1) were obtained after the polystyrene purification[3131 Rodrigues, T. S., Machado, F., Lalli, P. M., Eberlin, M. N., & Neto, B. A. D. (2015). Styrene polymerization efficiently catalyzed by iron-containing imidazolium-based ionic liquids: reaction mechanism and enhanced ionic liquid effect. Catalysis Communications, 63, 66-73. http://dx.doi.org/10.1016/j.catcom.2014.11.002.
http://dx.doi.org/10.1016/j.catcom.2014....
]. The formation of branched polystyrene can also be phased out, because these polymers have Tg and molar masses much lower than those obtained here[3939 Biedroń, T., & Kubisa, P. (2004). Cationic polymerization of styrene in a neutral ionic liquid. Journal of Polymer Science. Part A, Polymer Chemistry, 42(13), 3230-3235. http://dx.doi.org/10.1002/pola.20158.
http://dx.doi.org/10.1002/pola.20158...
40 Patrocinio, V. M. B., Agner, T., Dutra, G. V. S., Machado, F., Neto, B. A. D., Araújo, P. H. H., & Sayer, C. (2019). High molecular weight polystyrene obtained by cationic emulsion polymerization catalyzed by imidazolium-based ionic liquid. Macromolecular Reaction Engineering, 13(2), 1800061-1800067. http://dx.doi.org/10.1002/mren.201800061.
http://dx.doi.org/10.1002/mren.201800061...
41 Banerjee, S., Paira, T. K., Kotal, A., & Mandal, T. K. (2010). Room temperature living cationic polymerization of styrene with HX-styrenic monomer adduct/FeCl3 systems in the presence of tetrabutylammonium halide and tetraalkylphosphonium bromide salts. Polymer, 51(6), 1258-1269. http://dx.doi.org/10.1016/j.polymer.2010.01.051.
http://dx.doi.org/10.1016/j.polymer.2010...
-4242 Medeiros, A. M. M. S., Machado, F., Rubim, J. C., & McKenna, T. F. L. (2017). Bio-based copolymers obtained through miniemulsion copolymerization of methyl esters of acrylated fatty acids and styrene. Journal of Polymer Science. Part A, Polymer Chemistry, 55(8), 1422-1432. http://dx.doi.org/10.1002/pola.28511.
http://dx.doi.org/10.1002/pola.28511...
] [4848 Gaynor, S. G., Edelman, S., & Matyjaszewski, K. (1996). Synthesis of branched and hyperbranched polystyrenes. Macromolecules, 29(3), 1079-1081. http://dx.doi.org/10.1021/ma9513877.
http://dx.doi.org/10.1021/ma9513877...
49 Huang, W., Gu, W., Yang, H., Xue, X., Jiang, B., Zhang, D., Fang, J., Chen, J., Yang, Y., & Guo, J. (2017). Preparation and properties of branched polystyrene through radical suspension polymerization. Polymers, 9(1), 14. http://dx.doi.org/10.3390/polym9010014. PMid:30970692.
http://dx.doi.org/10.3390/polym9010014...
50 Li, B., Liu, W., & Wu, Y. (2012). Synthesis of long-chain branched isotactic-rich polystyrene via cationic polymerization. Polymer, 53(15), 3194-3202. http://dx.doi.org/10.1016/j.polymer.2012.04.030.
http://dx.doi.org/10.1016/j.polymer.2012...
-5151 Rozentsvet, V. A., Kozlov, V. G., Sablina, N. A., Stotskaya, O. A., Peruch, F., & Kostjuk, S. V. (2017). New insight into the polymerization mechanism of 1,3-dienes cationic polymerization. IV. Mechanism of unsaturation loss in the polymerization of isoprene. Polymer Chemistry, 8(5), 926-935. http://dx.doi.org/10.1039/C6PY01736C.
http://dx.doi.org/10.1039/C6PY01736C...
] and the formation of branching favors a significant increase of and molar-mass dispersity with an increase in conversion[4848 Gaynor, S. G., Edelman, S., & Matyjaszewski, K. (1996). Synthesis of branched and hyperbranched polystyrenes. Macromolecules, 29(3), 1079-1081. http://dx.doi.org/10.1021/ma9513877.
http://dx.doi.org/10.1021/ma9513877...
,5151 Rozentsvet, V. A., Kozlov, V. G., Sablina, N. A., Stotskaya, O. A., Peruch, F., & Kostjuk, S. V. (2017). New insight into the polymerization mechanism of 1,3-dienes cationic polymerization. IV. Mechanism of unsaturation loss in the polymerization of isoprene. Polymer Chemistry, 8(5), 926-935. http://dx.doi.org/10.1039/C6PY01736C.
http://dx.doi.org/10.1039/C6PY01736C...
], which did not occur in our work (Table S2, Supporting Information).
In addition, the polystyrene tacticity after purification was determined by integrating the signals between 144-147 ppm of the 13C-NMR spectra (Figure S10, Supporting Information). On the basis of isotactic (mm), atactic (mr) and syndiotactic (rr) triads assignments, syndiotactic polystyrenes were obtained, consisting essentially of around 10% isotactic, 20% atactic and 70% syndiotactic configurations.
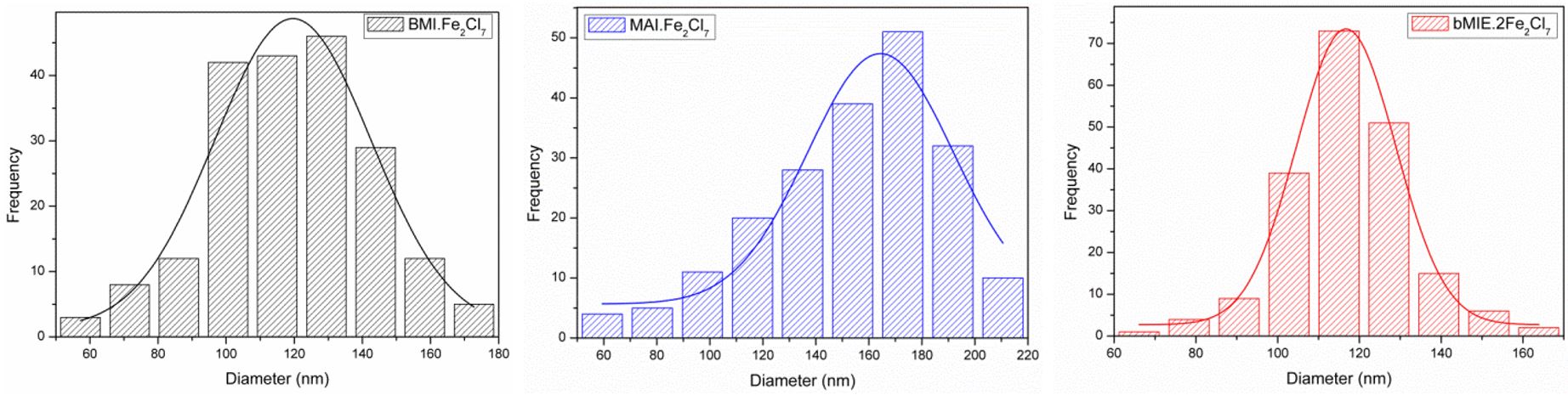
The micrographs (Figure 6) and the size distribution histograms (Figure 7) show the formation of particles of nanometer size, relatively uniform in size and shape, being spherical and having an average size of 119.6 ± 23.3 nm (BMI⋅Fe2Cl7), 153.5 ± 37.3 nm (MAI⋅Fe2Cl7) and 116.7 ± 15.2 nm (bMIE⋅2Fe2Cl7). The average size estimated by TEM is slightly smaller than the hydrodynamic size obtained by DLS, approximately 30 nm smaller (Entries 2, 5 and 8 of Table 1). This difference is explained by the fact that the techniques are performed under completely different conditions. The DLS measures the Brownian motion of aqueous dispersion and relates them to the diameter of the particles, which may be affected by the swelling of the polymeric shell, causing an increase in the average particle size. While TEM analysis the sample is dried on a film and exposed to electron beam, probably promoting shrinking phenomena[5252 Artusio, F., Bazzano, M., Pisano, R., Coulon, P. E., Rizza, G., Schiller, T., & Sangermano, M. (2018). Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer, 139, 155-162. http://dx.doi.org/10.1016/j.polymer.2018.02.019.
http://dx.doi.org/10.1016/j.polymer.2018...
]. In addition, the average particle size computed based on TEM images refers to the number-average, whereas DLS determines the intensity average particle size that provides a higher weight to the bigger particles.
TEM micrographs of unpurified polystyrene samples synthesized with catalysts (a) BMI⋅Fe2Cl7; (b) MAI⋅Fe2Cl7 and (c) bMIE⋅2Fe2Cl7 at 85 °C; (d) presence of bMIE⋅2Fe2Cl7 catalyst and (e) crystalline plane observed in the sample with BMI⋅Fe2Cl7.
Size distribution histograms of polystyrene synthesized with ILs catalysts at 85 °C obtained from TEM analysis.
It is also observed, in all micrographs, the presence of some smaller nanoparticles, with undefined shapes and sizes below 10 nm, being also present inside the spherical polystyrene particles (Figure 6d). The presence of crystalline planes (Figure 6e) indicates that ILs catalysts are contained in the polymeric matrix. This corroborates that polymerization occurs within the monomer droplets and with the reduction in Tg previously reported.
4. Conclusions
The use of iron-containing ILs catalysts has been shown to be very effective in styrene miniemulsion polymerization, achieving high conversions even at low catalyst concentrations. The largest conversions were obtained using the bMIE⋅2Fe2Cl7 catalyst and the largest molar masses were achieved in BMI⋅Fe2Cl7 and MAI⋅Fe2Cl7. The size of the polymeric nanoparticles remained practically unchanged during the reactions and high number-average molar masses were obtained (around 1300 kg mol-1) with molar-mass dispersity of 2.0, indicating that the polymerization mechanism occurred preferentially within the monomer droplets. The cationic polymerization behavior was confirmed by the reduction of the average molar masses with increased concentration of the catalytic species, Fe2Cl7, and with increasing temperature, by the first-order kinetics until a limit conversion, successful monomer feeding evaluation, obtaining polystyrene at low temperatures and inability to polymerize methyl methacrylate and other monomers not able to be polymerized through a cationic mechanism. Finally, these results are very promising and future work may focus on the application of these catalysts in cationic polymerization in miniemulsion of other vinyl monomers or via ring opening, as well as studying the encapsulation of different materials inside polystyrene nanoparticles.
Supplementary Material
Supplementary Material accompanies this paper.
Figure S1. 1H-NMR spectrum of BMI⋅Cl (CDCl3, 600 MHz).
Figure S2. 1H-NMR spectrum of MAI⋅Cl (D2O, 600 MHz).
Figure S3. 13C-NMR spectrum of MAI⋅Cl (D2O, 600 MHz).
Figure S4. 1H-NMR spectrum of bMIE⋅2Cl (D2O, 600 MHz).
Figure S5. 13C-NMR spectrum of bMIE⋅2Cl (D2O, 600 MHz).
Figure S6. Photograph of the synthesized polymers using the catalysts BMI⋅Fe2Cl7, MAI⋅Fe2Cl7 and bMIE⋅2Fe2Cl7 (a) before and (b) after purification.
Figure S7. 1H-NMR spectrum of unpurified polystyrene sample synthesized using BMI⋅Fe2Cl7 catalyst at 85 °C (CDCl3, 600 MHz).
Table S1. Conversion, average diameter of the monomer droplets (initial Dp) and polymeric particles (final Dp) and polydispersity index for all polymerizations after 8 h of reaction.
Figure S8. 1H-NMR spectrum of the vinyl pivalate polymerization test in the presence of BMI⋅Fe2Cl7 (CDCl3, 600 MHz).
Table S2. Average molar masses and molar-mass dispersity as a function of conversion (Xp) of the polymers synthesized at 85 °C using ILs catalysts.
Figure S9. 1H-NMR spectra of purified polystyrene samples synthesized using (a) BMI⋅Fe2Cl7; (b) MAI⋅Fe2Cl7 and (c) bMIE⋅2Fe2Cl7 at 85 °C (CDCl3, 600 MHz).
Figure S10. 13C-NMR spectra of purified polystyrene samples synthesized using (a) BMI⋅Fe2Cl7; (b) MAI⋅Fe2Cl7 and (c) bMIE⋅2Fe2Cl7 at 85 °C (CDCl3, 600 MHz).
This material is available as part of the online article from http://www.scielo.br/po
-
How to cite: Dutra, G. V. S., Silvério Neto, W., Araújo, P. H. H., Sayer, C., Silveira Neto, B. A., & Machado, F. (2021). Cationic polymerization of styrene using iron-containing ionic liquid catalysts in an aqueous dispersed medium. Polímeros: Ciência Tecnologia, 31(1), e2021005. https://doi.org/10.1590/0104-1428.04620
-
5. Acknowledgements
-
This work has been supported by CNPq, CAPES – Finance code 001, FINEP, FAPDF and PROCAD – Process nº 88881.068432/2014-01. The authors thank the Laboratório Multiusuário de Microscopia de Alta Resolução (LabMic) for the TEM micrographs.
-
1
Selected paper presented at the 15th Brazilian Polymer Conference – (15thCBPol) held in Bento Gonçalves, Brazil, on 27–31 October, 2019.
6. References
-
1Distler, D., Neto, W. S., & Machado, F. (2017). Emulsion polymerization. In S. Hashmi (Eds.), Reference module in materials science and materials engineering (pp. 1-14). New York: Elsevier. http://dx.doi.org/10.1016/B978-0-12-803581-8.03746-2
» http://dx.doi.org/10.1016/B978-0-12-803581-8.03746-2 -
2Bompart, M., Vergnaud, J., Strub, H., & Carpentier, J. F. (2011). Indium(III) halides as exceptionally active, water-tolerant catalysts for cationic polymerization of styrenics. Polymer Chemistry, 2(8), 1638-1640. http://dx.doi.org/10.1039/c1py00145k
» http://dx.doi.org/10.1039/c1py00145k -
3Satoh, K., Kamigaito, M., & Sawamoto, M. (2000). Lanthanide triflates-mediated emulsion cationic polymerization of p-alkoxystyrenes in aqueous media. Macromolecules, 33(13), 4660-4666. http://dx.doi.org/10.1021/ma0000069
» http://dx.doi.org/10.1021/ma0000069 -
4Maitre, C., Ganachaud, F., Ferreira, O., Lutz, J. F., Paintoux, Y., & Hémery, P. (2000). Anionic polymerization of phenyl glycidyl ether in miniemulsion. Macromolecules, 33(21), 7730-7736. http://dx.doi.org/10.1021/ma0007132
» http://dx.doi.org/10.1021/ma0007132 -
5Kostjuk, S. V., & Ganachaud, F. (2010). Cationic polymerization of vinyl monomers in aqueous media: from monofunctional oligomers to long-lived polymer chains. Accounts of Chemical Research, 43(3), 357-367. http://dx.doi.org/10.1021/ar900198q PMid:19957949.
» http://dx.doi.org/10.1021/ar900198q -
6Kostjuk, S. V., & Ganachaud, F. (2006). Cationic polymerization of styrene in solution and aqueous suspension using B(C6F5)3 as a water-tolerant Lewis acid. Macromolecules, 39(9), 3110-3113. http://dx.doi.org/10.1021/ma052650z
» http://dx.doi.org/10.1021/ma052650z -
7Vasilenko, I. V., Ganachaud, F., & Kostjuk, S. V. (2016). New insights into the cationic polymerization in emulsion catalyzed by water-dispersible Lewis acid surfactant complexes: a case study with p-methoxystyrene. Macromolecules, 49(9), 3264-3273. http://dx.doi.org/10.1021/acs.macromol.6b00379
» http://dx.doi.org/10.1021/acs.macromol.6b00379 -
8Cauvin, S., Sadoun, A., Dos Santos, R., Belleney, J., Ganachaud, F., & Hemery, P. (2002). Cationic polymerization of p-methoxystyrene in miniemulsion. Macromolecules, 35(21), 7919-7927. http://dx.doi.org/10.1021/ma0202890
» http://dx.doi.org/10.1021/ma0202890 -
9Touchard, V., Graillat, C., Boisson, C., D’Agosto, F., & Spitz, R. (2004). Use of a Lewis acid surfactant combined catalyst in cationic polymerization in miniemulsion: Apparent and hidden initiators. Macromolecules, 37(9), 3136-3142. http://dx.doi.org/10.1021/ma0355352
» http://dx.doi.org/10.1021/ma0355352 -
10Zhang, J., Wu, Y., Li, X., Yang, D., Zhang, M., Wang, H., Shang, Y., Ren, P., Mu, X., Li, S., & Guo, W. (2019). Characteristics and mechanism of styrene cationic polymerization in aqueous media initiated by cumyl alcohol/B(C6F5)3 Macromolecular Chemistry and Physics, 220(4), 1800419-1800427. http://dx.doi.org/10.1002/macp.201800419
» http://dx.doi.org/10.1002/macp.201800419 -
11Cauvin, S., Ganachaud, F., Moreau, M., & Hémery, P. (2005). High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chemical Communications, (21), 2713-2715. http://dx.doi.org/10.1039/b501489a PMid:15917929.
» http://dx.doi.org/10.1039/b501489a -
12Vasilenko, I. V., Yeong, H. Y., Delgado, M., Ouardad, S., Peruch, F., Voit, B., Ganachaud, F., & Kostjuk, S. V. (2015). A catalyst platform for unique cationic (co)polymerization in aqueous emulsion. Angewandte Chemie International Edition, 54(43), 12728-12732. http://dx.doi.org/10.1002/anie.201501157 PMid:26013180.
» http://dx.doi.org/10.1002/anie.201501157 -
13Radchenko, A. V., Kostjuk, S. V., Vasilenko, I. V., Ganachaud, F., & Kaputsky, F. N. (2007). Controlled/living cationic polymerization of styrene with BF3OEt2 as a coinitiator in the presence of water: improvements and limitations. European Polymer Journal, 43(6), 2576-2583. http://dx.doi.org/10.1016/j.eurpolymj.2007.03.026
» http://dx.doi.org/10.1016/j.eurpolymj.2007.03.026 -
14Zhang, X., Guo, W., Wu, Y., Gong, L., Li, W., Li, X., Li, S., Shang, Y., Yang, D., & Wang, H. (2016). Cationic polymerization of p-methylstyrene in selected ionic liquids and polymerization mechanism. Polymer Chemistry, 7(32), 5099-5112. http://dx.doi.org/10.1039/C6PY00796A
» http://dx.doi.org/10.1039/C6PY00796A -
15Yoshimitsu, H., Kanazawa, A., Kanaoka, S., & Aoshima, S. (2016). Cationic polymerization of vinyl ethers with alkyl or ionic side groups in ionic liquids. Journal of Polymer Science. Part A, Polymer Chemistry, 54(12), 1774-1784. http://dx.doi.org/10.1002/pola.28039
» http://dx.doi.org/10.1002/pola.28039 -
16Wu, Y., Han, L., Zhang, X., Mao, J., Gong, L., Guo, W., Gu, K., & Li, S. (2015). Cationic polymerization of isobutyl vinyl ether in an imidazole-based ionic liquid: characteristics and mechanism. Polymer Chemistry, 6(13), 2560-2568. http://dx.doi.org/10.1039/C4PY01784F
» http://dx.doi.org/10.1039/C4PY01784F -
17Li, X., Wu, Y., Zhang, J., Li, S., Zhang, M., Yang, D., Wang, H., Shang, Y., Guo, W., & Yan, P. (2019). Synthesis of highly reactive polyisobutylenes via cationic polymerization in ionic liquids: characteristics and mechanism. Polymer Chemistry, 10(2), 201-208. http://dx.doi.org/10.1039/C8PY01141A
» http://dx.doi.org/10.1039/C8PY01141A -
18Berezianko, I. A., Vasilenko, I. V., & Kostjuk, S. V. (2018). Acidic imidazole-based ionic liquids in the presence of diisopropyl ether as catalysts for the synthesis of highly reactive polyisobutylene: effect of ionic liquid nature, catalyst aging, and sonication. Polymer, 145, 382-390. http://dx.doi.org/10.1016/j.polymer.2018.04.059
» http://dx.doi.org/10.1016/j.polymer.2018.04.059 -
19Berezianko, I. A., Vasilenko, I. V., & Kostjuk, S. V. (2019). Cationic polymerization of isobutylene co-initiated by chloroferrate imidazole-based ionic liquid: the advantageous effect of initiator and aromatic compounds. European Polymer Journal, 121, 109307. http://dx.doi.org/10.1016/j.eurpolymj.2019.109307
» http://dx.doi.org/10.1016/j.eurpolymj.2019.109307 -
20Agner, T., Zimermann, A., Machado, F., Silveira Neto, B. A., Araújo, P. H. H., & Sayer, C. (2020). Thermal performance of nanoencapsulated phase change material in high molecular weight polystyrene. Polímeros: Ciência e Tecnologia, 30(2), e2020013. http://dx.doi.org/10.1590/0104-1428.01320
» http://dx.doi.org/10.1590/0104-1428.01320 -
21Dutra, G. V. S., Teixeira, T. S., Medeiros, G. A., Abdelnur, P. V., Hermes de Araújo, P. H., Sayer, C., Neto, B. A. D., & Machado, F. (2020). On the role of metal-containing imidazolium-based ionic liquid catalysts in the formation of tailored polystyrene. Industrial & Engineering Chemistry Research, 59(50), 21685-21699. http://dx.doi.org/10.1021/acs.iecr.0c04327
» http://dx.doi.org/10.1021/acs.iecr.0c04327 -
22Alves, R. C., Agner, T., Rodrigues, T. S., Machado, F., Neto, B. A. D., Costa, C., Araújo, P. H. H., & Sayer, C. (2018). Cationic miniemulsion polymerization of styrene mediated by imidazolium based ionic liquid. European Polymer Journal, 104, 51-56. http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035
» http://dx.doi.org/10.1016/j.eurpolymj.2018.04.035 -
23Ayat, M., Belbachir, M., & Rahmouni, A. (2017). Synthesis of block copolymers consists on vinylidene chloride and α- methylstyrene by cationic polymerization using an acid exchanged motmorillonite clay as heterogeneous catalyst (Algerian MMT). Journal of Molecular Structure, 1139, 381-389. http://dx.doi.org/10.1016/j.molstruc.2017.03.056
» http://dx.doi.org/10.1016/j.molstruc.2017.03.056 -
24Sang, W., & Yan, Q. (2018). Electro-controlled living cationic polymerization. Angewandte Chemie International Edition, 57(18), 4907-4911. http://dx.doi.org/10.1002/anie.201712270 PMid:29508512.
» http://dx.doi.org/10.1002/anie.201712270 -
25Rodrigues, T. S., Medeiros, G. A., Silva, F. M., & Neto, B. A. D. (2011). Polimerização do estireno com líquidos iônicos que possuem ácidos de Lewis incorporados na estrutura. In 34° Reunião Anual da Sociedade Brasileira de Química (p. 1). Florianópolis: Sociedade Brasileira de Química.
-
26Bolner, F. M., Jensen, A. T., Sayer, C., Araújo, P. H. H., Machado, F., & Neto, B. A. D. (2017). Polimerização de estireno com catalisador a base de líquido iônico modificado. In 14° Congresso Brasileiro de Polímeros (pp. 1-5). Águas de Lindóia: Associação Brasileira de Polímeros.
-
27Ramos, J., & Forcada, J. (2010). The role of cationic monomers in emulsion polymerization. European Polymer Journal, 46(5), 1106-1110. http://dx.doi.org/10.1016/j.eurpolymj.2010.01.012
» http://dx.doi.org/10.1016/j.eurpolymj.2010.01.012 -
28Dupont, J., Consorti, C. S., Suarez, P. A. Z., Souza, R. F., Fulmer, S. L., Richardson, D. P., Smith, T. E., & Wolff, S. (2002). Preparation of 1-butyl-3-methyl imidazolium-based room temperature ionic liquids. Organic Syntheses, 79, 236-243. http://dx.doi.org/10.15227/orgsyn.079.0236
» http://dx.doi.org/10.15227/orgsyn.079.0236 -
29Ramos, L. M., Guido, B. C., Nobrega, C. C., Corrêa, J. R., Silva, R. G., Oliveira, H. C. B., Gomes, A. F., Gozzo, F. C., & Neto, B. A. D. (2013). The Biginelli reaction with an imidazolium–tagged recyclable iron catalyst: kinetics, mechanism, and antitumoral activity. Chemistry (Weinheim an der Bergstrasse, Germany), 19(13), 4156-4168. http://dx.doi.org/10.1002/chem.201204314 PMid:23460474.
» http://dx.doi.org/10.1002/chem.201204314 -
30Ahrens, S., Zeller, A., Taige, M., & Strassner, T. (2006). Extension of the alkane bridge in bisNHC−palladium−chloride complexes. Synthesis, structure, and catalytic activity. Organometallics, 25(22), 5409-5415. http://dx.doi.org/10.1021/om060577a
» http://dx.doi.org/10.1021/om060577a -
31Rodrigues, T. S., Machado, F., Lalli, P. M., Eberlin, M. N., & Neto, B. A. D. (2015). Styrene polymerization efficiently catalyzed by iron-containing imidazolium-based ionic liquids: reaction mechanism and enhanced ionic liquid effect. Catalysis Communications, 63, 66-73. http://dx.doi.org/10.1016/j.catcom.2014.11.002
» http://dx.doi.org/10.1016/j.catcom.2014.11.002 -
32Machado, F., Lima, E. L., & Pinto, J. C. (2007). Uma revisão sobre os processos de polimerização em suspensão. Polímeros: Ciência e Tecnologia, 17(2), 166-179. http://dx.doi.org/10.1590/S0104-14282007000200016
» http://dx.doi.org/10.1590/S0104-14282007000200016 -
33Aoshima, S., & Kanaoka, S. (2009). A renaissance in living cationic polymerization. Chemical Reviews, 109(11), 5245-5287. http://dx.doi.org/10.1021/cr900225g PMid:19803510.
» http://dx.doi.org/10.1021/cr900225g -
34Islam, M. N., Haldorai, Y., Nguyen, V. H., & Shim, J.-J. (2014). Synthesis of poly(vinyl pivalate) by atom transfer radical polymerization in supercritical carbon dioxide. European Polymer Journal, 61, 93-104. http://dx.doi.org/10.1016/j.eurpolymj.2014.09.003
» http://dx.doi.org/10.1016/j.eurpolymj.2014.09.003 -
35Kostjuk, S. V., Dubovik, A. Y., Vasilenkol, I. V., Mardykin, V. P., Gaponik, L. V., Kaputsky, F. N., & Antipin, L. M. (2004). Novel initiating system based on AlCl3 etherate for quasiliving cationic polymerization of styrene. Polymer Bulletin, 52(3), 227-234. http://dx.doi.org/10.1007/s00289-004-0280-2
» http://dx.doi.org/10.1007/s00289-004-0280-2 -
36Banerjee, S., Paira, T. K., & Mandal, T. K. (2013). Control of molecular weight and tacticity in stereospecific living cationic polymerization of α-methylstyrene at 0 °C using FeCl3-based initiators: effect of tacticity on thermal properties. Macromolecular Chemistry and Physics, 214(12), 1332-1344. http://dx.doi.org/10.1002/macp.201300092
» http://dx.doi.org/10.1002/macp.201300092 -
37Paira, T. K., Banerjee, S., Raula, M., Kotal, A., Si, S., & Mandal, T. K. (2010). Peptide−polymer bioconjugates via atom transfer radical polymerization and their solution aggregation into hybrid micro/nanospheres for dye uptake. Macromolecules, 43(9), 4050-4061. http://dx.doi.org/10.1021/ma1001904
» http://dx.doi.org/10.1021/ma1001904 -
38Yamada, K., Minoda, M., & Miyamoto, T. (1999). Controlled synthesis of amphiphilic block copolymers with pendant N-acetyl-D-glucosamine residues by living cationic polymerization and their interaction with WGA lectin. Macromolecules, 32(11), 3553-3558. http://dx.doi.org/10.1021/ma9816315
» http://dx.doi.org/10.1021/ma9816315 -
39Biedroń, T., & Kubisa, P. (2004). Cationic polymerization of styrene in a neutral ionic liquid. Journal of Polymer Science. Part A, Polymer Chemistry, 42(13), 3230-3235. http://dx.doi.org/10.1002/pola.20158
» http://dx.doi.org/10.1002/pola.20158 -
40Patrocinio, V. M. B., Agner, T., Dutra, G. V. S., Machado, F., Neto, B. A. D., Araújo, P. H. H., & Sayer, C. (2019). High molecular weight polystyrene obtained by cationic emulsion polymerization catalyzed by imidazolium-based ionic liquid. Macromolecular Reaction Engineering, 13(2), 1800061-1800067. http://dx.doi.org/10.1002/mren.201800061
» http://dx.doi.org/10.1002/mren.201800061 -
41Banerjee, S., Paira, T. K., Kotal, A., & Mandal, T. K. (2010). Room temperature living cationic polymerization of styrene with HX-styrenic monomer adduct/FeCl3 systems in the presence of tetrabutylammonium halide and tetraalkylphosphonium bromide salts. Polymer, 51(6), 1258-1269. http://dx.doi.org/10.1016/j.polymer.2010.01.051
» http://dx.doi.org/10.1016/j.polymer.2010.01.051 -
42Medeiros, A. M. M. S., Machado, F., Rubim, J. C., & McKenna, T. F. L. (2017). Bio-based copolymers obtained through miniemulsion copolymerization of methyl esters of acrylated fatty acids and styrene. Journal of Polymer Science. Part A, Polymer Chemistry, 55(8), 1422-1432. http://dx.doi.org/10.1002/pola.28511
» http://dx.doi.org/10.1002/pola.28511 -
43Rieger, J. (1996). The glass transition temperature of polystyrene. Journal of Thermal Analysis, 46(3), 965-972. http://dx.doi.org/10.1007/BF01983614
» http://dx.doi.org/10.1007/BF01983614 -
44Szkudlarek, M., Beginn, U., Keul, H., & Möller, M. (2017). Synthesis of terpolymers with homogeneous composition by free radical copolymerization of maleic anhydride, perfluorooctyl and butyl or dodecyl methacrylates: application of the continuous flow monomer addition technique. Polymers, 9(11), 610. http://dx.doi.org/10.3390/polym9110610 PMid:30965911.
» http://dx.doi.org/10.3390/polym9110610 -
45Metanawin, S., Panutumron, P., Thongsale, A., & Metanawin, T. (2018). The functionalization of hybrid titanium dioxide by miniemulsion polymerization technique. Materials Today: Proceedings, 5(3, Part 2), 9651-9657. http://dx.doi.org/10.1016/j.matpr.2018.01.133.
» https://doi.org/10.1016/j.matpr.2018.01.133 -
46Christie, D., Zhang, C., Fu, J., Koel, B., & Priestley, R. D. (2016). Glass transition temperature of colloidal polystyrene dispersed in various liquids. Journal of Polymer Science. Part B, Polymer Physics, 54(17), 1776-1783. http://dx.doi.org/10.1002/polb.24082
» http://dx.doi.org/10.1002/polb.24082 -
47Shen, Y., Du, C., Zhou, J., & Ma, F. (2018). The facile modification of polyacrylate emulsion via hexadecane to enhance controlled-release profiles of coated urea. Scientific Reports, 8(1), 12279. http://dx.doi.org/10.1038/s41598-018-30585-5 PMid:30116004.
» http://dx.doi.org/10.1038/s41598-018-30585-5 -
48Gaynor, S. G., Edelman, S., & Matyjaszewski, K. (1996). Synthesis of branched and hyperbranched polystyrenes. Macromolecules, 29(3), 1079-1081. http://dx.doi.org/10.1021/ma9513877
» http://dx.doi.org/10.1021/ma9513877 -
49Huang, W., Gu, W., Yang, H., Xue, X., Jiang, B., Zhang, D., Fang, J., Chen, J., Yang, Y., & Guo, J. (2017). Preparation and properties of branched polystyrene through radical suspension polymerization. Polymers, 9(1), 14. http://dx.doi.org/10.3390/polym9010014 PMid:30970692.
» http://dx.doi.org/10.3390/polym9010014 -
50Li, B., Liu, W., & Wu, Y. (2012). Synthesis of long-chain branched isotactic-rich polystyrene via cationic polymerization. Polymer, 53(15), 3194-3202. http://dx.doi.org/10.1016/j.polymer.2012.04.030
» http://dx.doi.org/10.1016/j.polymer.2012.04.030 -
51Rozentsvet, V. A., Kozlov, V. G., Sablina, N. A., Stotskaya, O. A., Peruch, F., & Kostjuk, S. V. (2017). New insight into the polymerization mechanism of 1,3-dienes cationic polymerization. IV. Mechanism of unsaturation loss in the polymerization of isoprene. Polymer Chemistry, 8(5), 926-935. http://dx.doi.org/10.1039/C6PY01736C
» http://dx.doi.org/10.1039/C6PY01736C -
52Artusio, F., Bazzano, M., Pisano, R., Coulon, P. E., Rizza, G., Schiller, T., & Sangermano, M. (2018). Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer, 139, 155-162. http://dx.doi.org/10.1016/j.polymer.2018.02.019
» http://dx.doi.org/10.1016/j.polymer.2018.02.019
Publication Dates
-
Publication in this collection
21 May 2021 -
Date of issue
2021
History
-
Received
05 May 2020 -
Reviewed
14 Feb 2021 -
Accepted
18 Feb 2021