Abstract
It is widely accepted that the consumption of ω-3 polyunsaturated fatty acids has beneficial effects on human health. In this work, ten brown macroalgae species collected along the Portuguese west coast were studied for their fatty acids composition by GC-MS after alkaline hydrolysis and derivatization. The results of this survey showed that different macroalgae from the same region display distinct fatty acids profile. Concerning ω-3 polyunsaturated fatty acids, eicosapentaenoic acid was found in all but one species. Additionally, some species contained docosahexaenoic acid. Linoleic acid, an essential fatty acid of the ω-6 series, was present in all studied macroalgae. Fucus spiralis L. exhibited the highest amounts of monounsaturated fatty acids and of polyunsaturated fatty acids of the ω-3 and ω-6 series. The ω-6/ω-3 ratio in half of the studied species was lower than 1. This information reinforces the potential application of some brown macroalgae as dietary sources of polyunsaturated fatty acids.
brown macroalgae; GC-MS; polyunsaturated fatty acids; w-6/w-3 ratio
Distinct fatty acid profile of ten brown macroalgae
Graça Silva; Renato B. Pereira; Patrícia Valentão; Paula B. Andrade; Carla Sousa
REQUIMTE/Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, Portugal
Correspondence Correspondence: Paula B. Andrade REQUIMTE/Laboratório de Farmacognosia Departamento de Química, Faculdade de Farmácia Universidade do Porto, Portugal pandrade@ff.up.pt Tel.: +351 220 428 6543
ABSTRACT
It is widely accepted that the consumption of ω-3 polyunsaturated fatty acids has beneficial effects on human health. In this work, ten brown macroalgae species collected along the Portuguese west coast were studied for their fatty acids composition by GC-MS after alkaline hydrolysis and derivatization. The results of this survey showed that different macroalgae from the same region display distinct fatty acids profile. Concerning ω-3 polyunsaturated fatty acids, eicosapentaenoic acid was found in all but one species. Additionally, some species contained docosahexaenoic acid. Linoleic acid, an essential fatty acid of the ω-6 series, was present in all studied macroalgae. Fucus spiralis L. exhibited the highest amounts of monounsaturated fatty acids and of polyunsaturated fatty acids of the ω-3 and ω-6 series. The ω-6/ω-3 ratio in half of the studied species was lower than 1. This information reinforces the potential application of some brown macroalgae as dietary sources of polyunsaturated fatty acids.
Keywords: brown macroalgae GC-MS polyunsaturated fatty acids ω-6/ω-3 ratio
Introduction
Seaweeds, including brown algae, produce polyunsaturated fatty acids (PUFA), especially long chain fatty acids of the ω-3 series (Colombo et al., 2006). These acids are of high importance for the nourishment of man (Li et al., 2002). The ingestion of ω-3 PUFA promotes the decrease of cardiovascular and inflammatory diseases (e.g. arthritis associated with inflammation) and additionally reduces the risk of cancer (Calder, 2006). The beneficial effects on preventing the mortality from heart diseases may be associated with the reduced concentration of triacylglycerol and the inhibition of platelet aggregation, together with a direct antiarrhythmic effect (Leaf & Kang, 1996; Harris et al., 1997). Some authors suggest that the reduction of dietary ω-6 PUFA and the increase in ω-3 by adults and new-borns can contribute not only to reduce cardiovascular disease, but also to improve mental health (Simopoulos et al., 2000). As so, in recent years the interest of fatty acids present in seaweed experienced a significant growth.
There are 1500-2000 species of brown algae worldwide (van den Hoek et al., 1995). The genus Cladostephus, Sargassum, Padina, Fucus, Cystoseira, Halopteris, Saccorhiza and Stypocaulon are representative examples found in the Portuguese Atlantic coast. In previous studies ten brown macroalgae species, namely Cladostephus spongiosus (Hudson) C. Agardh, Cystoseira nodicaulis (Withering) M. Roberts, Cystoseira tamariscifolia (Hudson) Papenfuss, Cystoseira usneoides (Linnaeus) M. Roberts, Fucus spiralis (Linnaeus), Halopteris filicina (Grateloup) Kützing, Padina pavonica (Linnaeus) Thivy, Saccorhiza polyschides (Lightfoot)Batters, Sargassum vulgare (J. V. Lamouroux) C. Agardh and Stypocaulon scoparium (Linnaeus) Kützing, were studied by our group for their proline, phloroglucinol, mannitol, sterols and phlorotannins contents (Andrade et al., 2013).This study also allowed the identification of eight fatty acids in the ethanolic extract used for metabolic profiling (Andrade et al., 2013). The major fatty acids of P. pavonica and S. scoparium have also been reported (Kanias et al, 1992). However, as far as we know, a deeper stydy of the fatty acids composition of most of the algae found in this reagion was never attempted. C. spongiosus and S. vulgare from a different origin, the meditrerranean Portuguese coast (Pereira et al., 2012), and two other species, P. pavonica from the Persian Gulf (Tabarsa et al., 2012) and S. polyschides from the 'Ría de Arousa', Coruña, Spain (Sánchez-Machado et al., 2004), have been studied for their fatty acids composition.
The ten species considered herein were thoroughly studied by our group for their sterols and phlorotannins contents, and anti-inflammatory and antimicrobial effects have been demonstrated for the phlorotannins rich extracts (Lopes et al., 2011; 2012).
In this work, fatty acids profile was performed by gas chromatography-mass spectrometry (GC-MS) after alkaline hydrolysis and derivatization to the respective fatty acids methyl esters (FAME).
Materials and methods
Standards and reagents
All reagents and solvents were of analytical grade. Authentic standards for GC-MS analysis were obtained from Supelco (Bellefonte, PA, USA).
Sampling
Three-four individuals of each species were randomly collected in Peniche, west coast of Portugal (39°22'3"N; 9°22'26"W). Samples were collected in September 2008, with the exceptions of S. polyschides, collected in June 2008, and C. usneoides and C. nodicaulis, collected in November 2009. Identity was ascertained by Teresa Mouga, PhD (GIRM). Voucher specimens were deposited at Laboratório de Farmacognosia, Faculdade de Farmácia, Universidade do Porto (C. spong-010908; C. nodic-011109; C. tamar-010908; C. usneoi-011109; F. spir-010908; H. filic-010908; P. pavon-010908; S. polys-010608; S. vulg-010908; S. scopar-010908). After collection, samples were protected from heat, air and light exposure, washed with NaCl 3.5% (m/v) to remove epiphytes, and immediately frozen. Then samples were lyophilised in a Labconco 4.5 Freezone apparatus (Kansas City, MO, USA). The dried material was powdered (particle size <910 µm) and kept in the dark, in a desiccator, until fatty acids extraction.
Fatty acids extraction and derivatization
Fatty acids extraction was performed by the Folch method as previously described (Ribeiro et al., 2009), with slight modifications. Briefly, 0.25 g of the dried algae plus 200 µL of 1 g/L methanolic solution of methyl jasmonate (internal standard) were extracted with 150 mL of chloroform:methanol (2:1), with magnetic stirring at 500 rpm, for 10 min, at 40 °C. The extraction procedure was repeated five times and the resulting extracts were pooled and concentrated to dryness under reduced pressure (40 °C).
The residue was dissolved in 1 mL of methanol and then hydrolysed with 1 mL of KOH methanolic solution (11 g/L), at 90 °C, for 10 min. The free fatty acids originally present and those resulting from the alkaline hydrolysis were derivatized with 1 mL of BF3 methanolic solution (10%), at 90 °C, for 10 min. FAME were purified with 2×6 mL of isooctane and anhydrous sodium sulphate was added to assure the total absence of water. The resulting extract was evaporated to dryness under a stream of nitrogen and dissolved in 100 µL of isooctane. Each algae species was assayed in triplicate.
GC-MS analysis
Samples extracts (1 µL) were analysed using a Varian CP-3800 gas chromatograph (USA) equipped with a Varian Saturn 4000 mass selective detector (USA) and a Saturn GC/MS workstation software version 6.8. A VF-5 ms (30 m×0.25 mm×0.25 µm) column (Varian) was used. The injector port was heated to 250 °C. Injections were performed in split mode, with a ratio of 1/40. The carrier gas was Helium C-60 (Gasin, Portugal), at a constant flow of 1 mL/min. The oven temperature was set at 40 °C for 1 min, then increased 5 °C/min to 250 °C, 3 °C/min to 300 °C and held for 15 min. All mass spectra were acquired in electron impact (EI) mode. Ionization was maintained off during the first 4 min, to avoid solvent overloading. The Ion Trap detector was set as follows: transfer line, manifold and trap temperatures were respectively 280, 50 and 180 °C. The mass ranged from 50 to 600 m/z, with a scan rate of 6 scan/s. The emission current was 50 µA, and the electron multiplier was set in relative mode to auto tune procedure. The maximum ionization time was 25,000 µs, with an ionization storage level of 35 m/z. The analysis was performed in Full Scan mode.
Identification of compounds was achieved by comparison of their retention indices and mass spectra with those from pure standards injected under the same conditions, and from NIST 05 MS Library Database. The amount of FAME present in the samples was achieved from the calibration curve of the respective standard, obtained with a concentration series of 4.00, 2.00, 1.00, 0.50 and 0.25 mg/L prepared in isooctane.
Statistical analysis
All the analytical determinations were performed in triplicate and the mean values were reported. The amounts of fatty acids for each macroalgal were compared using GraphPad Prism 5 software, by analysis of variance (ANOVA), with Bonferroni post hoc test. Significant differences were considered for p<0.05.
Results and Discussion
The fatty acids composition of marine and terrestrial organisms significantly differs, the former being richer in long chain PUFA. As so, considering their potential beneficial health effects, marine organisms, especially brown macroalgae, can represent an interesting source of dietary fatty acids (Colombo et al., 2006; Kumari et al., 2010).
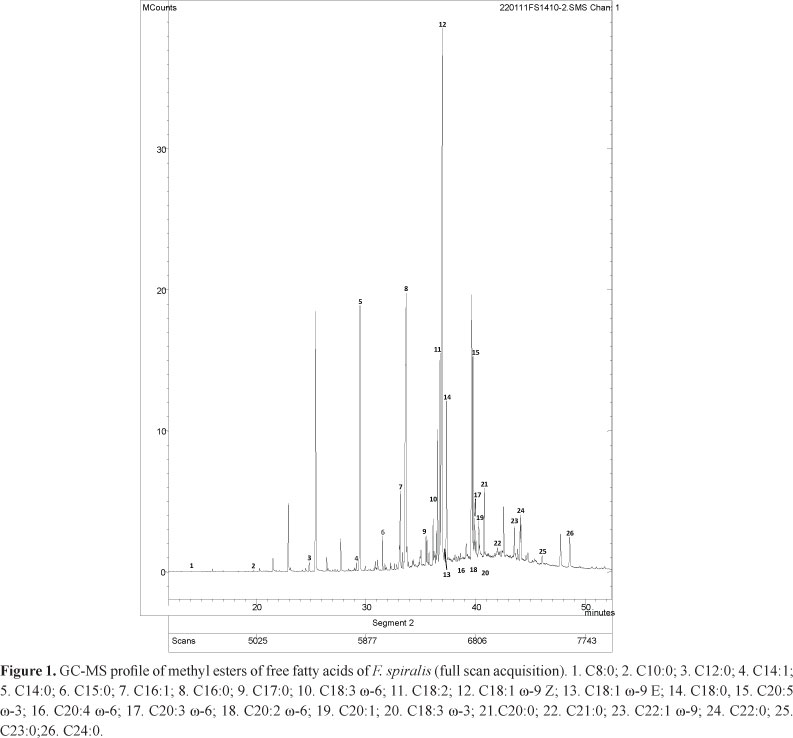
In this work, the fatty acids of ten brown macroalgae, which are usually found as esters in different lipid groups, were determined. The obtained fatty acids profiles revealed to be qualitatively and quantitatively different. Figure 1 shows the GC-MS chromatogram of F. spiralis, the species exhibiting the greatest diversity of compounds. Fatty acids containing between 8 and 24 carbon atoms were identified, although the ones with 12-22 carbon atoms were more common. This composition agrees with the profile commonly seen in brown algae (Kumari et al., 2010; Tabarsa et al., 2012). Among the ten algae analyzed herein, and in what concerns to fatty acids composition, all of them were already screened by our research group, but only eight fatty acids containing between 14 and 20 carbons were determined (Andrade et al., 2013). In that work, only C14:0, 16:0, C18:3, C18:1, C18:0, C20:4 and C20:5 fatty acids were reported in these samples, C16:0 being the only one found in these ten brown macroalgae. Compound C18:1 was described in C. spongiosus, P. pavonica, S. vulgare and F. spiralis, C18:0 in C. tamariscifolia, C. usneoides, H. filicina, S. polyschides, P. pavonica, S. vulgare, F. spiralis and S. scoparium, while C14:0, C20:4 and C20:5 were only found in F. spiralis species (Andrade et al., 2013). In the work of Kanias and collaborators seven fatty acids containing between 14 and 18 carbons were determined for P. pavonica and S. scoparium (Kanias et al., 1992).
The amounts of fatty acids (evaluated as the sum of all fatty acids identified by GC-MS) ranged from 74 to 1897 mg/kg, dry weight (H. filicina and C. nodicaulis, respectively) (Table 1). The levels of fatty acids in algae depend on the algae species, geographical origin and season, among other factors. In fact, the algae species greatly affects, as it can be seen by comparing the results of H. filicina and F. spiralis collected in the same region and in the same season. H. filicina significantly differs from the other species as it shows a much smaller fatty acids total content, although it contains important ones. Overall, F. spiralis stands out for its high quantity and diversity of compounds.
Saturated fatty acids (SFA) with 16 carbons were the most abundant ones in P. pavonica, C. tamariscifolia, H. filicina, S. polyschides, S. scoparium and S. vulgare, as those with 18 carbons were in C. nodicaulis, C. usneoides, C. spongiosus and F. spiralis (Table 1). C. nodicaulis contains the highest amount of SFA (1620 mg/kg, dry weight) in opposition to H. filicina, only presenting 62 mg/kg SFA. The short chain fatty acids (less than 14 carbon atoms) are minor compounds. It can be noted that not all fatty acids having an odd number of carbons are present in all algae and in general the amount thereof is relatively low when compared with those with an even number of carbons.
Monounsaturated fatty acids (MUFA) predominating in the studied brown macroalgae contain 16 or 18 carbons (Table 1). Oleic acid (C18:1, ω-9Z) was in general the most abundant MUFA, representing 2.3-12.1% of total FAME. As reported before, this acid commonly is one of the major MUFA in other brown algae species (Khotimchenko et al., 2002; Li et al, 2002; Dawczynski et al., 2007). S. scoparium has similar amounts of the two isomers of C18:1 ω-9, while in C. spongiosus the trans isomer (elaidic acid) is dominant. F. spiralis, C. nodicaulis and S. polyschides are the only studied algae containing the short chain MUFA C14:1,ω-5Z. The species that appears as the richest source of PUFA is F. spiralis, containing about 430 mg/kg (dry weight). This amount of PUFA is significantly different from that found in the other species (1.9 to 99.8 mg/kg) (Table 1). In eight of the ten studied species (F. spiralis, C. tamariscifolia, C. usneoides, H. filicina, S. polyschides, S. scoparium, S. vulgare and C. spongiosus) eicosapentaenoic acid (EPA, C20:5 ω-3) was the major PUFA (Table 1). In the remaining species (P. pavonica and C. nodicaulis) linoleic acid (C18:2 ω-6), an essential fatty acid of the ω-6 series, was the main PUFA (Table 1). Docosahexaenoic acid (DHA, C22:6 ω-3) was determined in C. usneoides, S. scoparium and S. vulgare. Considering the essential fatty acid α-linolenic acid (ALA, C18:3 ω-3), it was only quantified in F. spiralis (Table 1). Nevertheless, it can be assumed that, with the exception of P. pavonica, all species are a good source of ω-3 fatty acids since they contain EPA and some also contain DHA (Table 1).
As referred above, ω-3 PUFA are considered important for the prevention of cardiovascular diseases (Leaf & Kang, 1996; von Schacky, 2003). Algae are the primary producers of DHA and EPA in the ecosystem and some species of freshwater and marine algae are widely used in aquaculture to produce PUFA enriched fish (Guschina & Harwood, 2006). It is thought that these PUFA may have a beneficial effect superior to that of ALA, the predominant ω-3 fatty acid in most oils obtained from terrestrial plants (von Schacky, 2003). DHA is the principal ω-3 in tissues and is particularly abundant in neural and retinal tissue. Epidemiological and preclinical studies suggest that DHA may protect against Alzheimer disease and other types of dementia (Arterburn et al., 2006). From a biochemical and nutritional point of view, the direct intake of DHA is very important since the conversion of ALA to EPA and then to DHA is poor and supplementation with this fatty acid is probably the most effective way to raise its content in plasma, tissues, or human milk (Arterburn et al., 2006).
Current guidelines recommend the increase of ω-3 PUFA in the diet (von Schacky, 2003). For this purpose it is recommended a daily intake of 0.65 g of EPA and DHA (Simopoulos et al., 2000). C. spongiosus, F. spiralis, S. polyschides, S. vulgare and S. scoparium have a ω-6/ω-3 ratio lower than 1. Although F. spiralis contains the highest ω-3 fatty acids content (Table 1), S. vulgare is the species with the highest amount of ω-3 fatty acids compared to that of ω-6.
Many brown macroalgae are described to contain more unsaturated fatty acids than saturated ones, especially C18 and C20 PUFA (Kumari et al., 2010; 2011; Pereira et al., 2012). However, as already reported by other authors, brown macroalgae collected on the Atlantic coast of the Iberian Peninsula and in other areas with warm waters exhibited more SFA than it is usually found for brown macroalgae grown in colder waters (Sánchez-Machado et al., 2004; Colombo et al., 2006). The collection season and specific conditions of the habitat can also account for the differences observed in fatty acids profile (Khotimchenko et al., 2002). F. spiralis is distinct from the other studied species for having similar amounts of saturated and unsaturated fatty acids (considering unsaturated fatty acids as the sum of MUFA + PUFA). C. tamariscifolia and S. polyschides also have relatively low saturated/unsaturated fatty acids ratios (1.9 and 1.7, respectively).
In what concerns to studies on the same species but from distinct origin, P. pavonica collected in the Persian Gulf showed a fatty acids profile significantly different from the one found in this study (Tabarsa et al., 2012). The major compound was palmitic acid followed by oleic acid. In opposition to the results obtained in this work, PUFA of the ω-3 and ω-6 series were present in significant amounts (Tabarsa et al., 2012). The sample from the Persian Gulf was collected in April and differences in season and climate can significantly affect the fatty acids within a species. In the work of Sánchez-Machado et al. (2004) canned S. polyschides exhibited palmitic acid as the major compound, followed by oleic acid, as it was observed with the S. polyschides studied herein. The sample used in the study of Sánchez-Machado and that analysed in the present work were collected in the Atlantic coast of the Iberian peninsula. More evident differences can be observed between S. vulgare and C. spongiosus samples collected in the mediterranean coast of Portugal (Pereira et al., 2012) and the ones studied herein (obtained in September in the Atlantic coast of Portugal). For the samples obtained in May in the Mediterranean coast, the amount of saturated fatty acids was lower than that of unsaturated ones; however, the ratio ω-6 / ω-3 was much higher than 1, in opposition to the results obtained for the samples analysed in the present work.
Conclusion
The ten macroalgae species studied exhibited different profiles and contained unsaturated fatty acids in important amounts. Considering PUFA of the ω-3 series, EPA was determined in almost all species and some of them also contained measurable amounts of DHA. The ratio ω-6/ω-3 demonstrated a high proportion of ω-3 fatty acids. This ratio was lower than 1 in half of the studied species. Taking into account the obtained results, some brown macroalgae species can be regarded as good sources of PUFA, either for direct human nutrition, in food supplements or in feeding materials.Comparing our results with the ones found in literature for the same species, it can be seen that the location and the season in which the algae were collected greatly affects the qualitative and quantitative fatty acids composition of brown algae.
Acknowledgements
The authors thank Fundação para a Ciência e a Tecnologia for grant no. PEst-C/EQB/LA0006/2011. R. Pereira is grateful for the FCT research scholarship.
Authors'contributions
GS contributed in collecting algae sample and identification, confection of herbarium, running the laboratory work, analysis of the data and drafted the paper. RBP contributed to laboratorial work. PV contributed in herbarium confection, chromatographic analysis and to critical reading of the manuscript. PBA and CS designed the study, supervised the laboratory work and contributed to critical reading of the manuscript. All the authors have read the final manuscript and approved the submission.
References
Received 13 Mar 2013
Accepted 26 Jun 2013
- Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P 2013. Valuable compounds in macroalgae extract. Food Chem 138: 1819-1828.
- Arterburn LM, Hall EB, Oken H 2006. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83: 1467S-1476S.
- Calder PC 2006. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83: 1505S-1519S.
- Colombo ML, Risé P, Giavarini F, de Angelis L, GalliC, Bolis CL 2006. Marine macroalgae as source of polyunsaturated fatty acids. Plant Foods Hum Nutr 61: 67-72.
- Dawczynski C, Schubert R, Jahreis G 2007. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103: 891-899.
- Guschina IA, Harwood JL 2006. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45: 160-166.
- Harris WS, Lu G, Rambjør GS, Walen AI, Ontko JA, Cheng Q, Windsor SL 1997. Influence of n-3 fatty acid supplementation on the endogenous activities of plasma lipases. Am J Clin Nutr 66: 254-260.
- Kanias GGD, Skaltsa H, Tsitsa E, Loukis A, Bitis J 1992. Study of the correlation between trace elements, sterols and fatty acids in brown algae from the Saronikos gulf of Greece. Fresen J Anal Chem 344: 334-339
- Khotimchenko SV, Vaskovsky EV, Titlyanova VT 2002. Fatty acids of marine algae from Pacific coast of North California. Bot Mar 45: 17-22.
- Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B 2010. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem 120: 749-757.
- Kumari P, Reddy CRK, Jha B 2011. Comparative evaluation and selection of a method for lipid and fatty acid extraction from macroalgae. Anal Biochem 415: 134-144.
- Leaf A, Kang JX 1996. Prevention of cardiac sudden death by n-3 fatty acids: a review of the evidence. J Intern Med 240: 5-12.
- Li X, Fan X, Han L, Lou Q 2002. Fatty acids of some algae from the Bohai Sea. Phytochemistry 59: 157-161.
- Lopes G, Sousa C, Bernardo J, Andrade PB, Valentão P, Ferreres F, Mouga T 2011. Sterol profiles in 18 macro algae of the Portuguese coast. J Phycol 47: 1210-1218.
- Lopes G, Sousa C, Silva LR, Pinto E, Andrade PB, Bernardo J, Mouga T, Valentão P 2012. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoSOne7: e31145, doi:10.1371/journal.pone.0031145
- Pereira H, Barreira L, Figueiredo F, Custódio L, Vizetto-Duarte C, Polo C, Eva Resek, Engelen A, Varela J 2012. Polyunsaturated fatty acids of marine macroalgae: potential for nutritional and pharmaceutical applications. Mar Drugs 10: 1920-1935
- Ribeiro B, de Pinho PG, Andrade PB, Baptista P, Valentão P 2009. Fatty acid composition of wild edible mushrooms species: A comparative study. Microchem J 93: 29-35.
- Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P 2004. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem 85: 439-444.
- Simopoulos PA, Leaf A, Salem Jr N 2000. Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 63: 119-120.
- Tabarsa M, Rezaei M, Ramezanpour Z, Waaland JR, Rabiei R 2012. Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds. J Phycol 48: 285-292.
- van den Hoek C, Mann DG, Jahns HM 1995. Algae: An Introduction to Phycology. Cambridge: Cambridge University Press. p. 165-218.
- von Schacky C 2003. The role of omega-3 fatty acids in cardiovascular disease. Cur Atherosclerosis Rep 5: 139-145.
 Correspondence:
Correspondence: Publication Dates
-
Publication in this collection
02 Aug 2013 -
Date of issue
Aug 2013
History
-
Received
13 Mar 2013 -
Accepted
26 June 2013