Abstracts
The aim of this work was to study the clinical disorders and risk factors of canine ehrlichiosis in Ilhéus and Itabuna, Bahia, and compare different diagnostic methods. Blood samples were collected from 200 dogs. Each dog was clinically examined. A questionnaire was used to evaluate the risk factors. The blood samples were analyzed using the Dot-ELISA test; hematometry, platelet counts and searches for morulae on blood smears were performed. Nested PCR was carried out on 50 serologically positive samples and 50 negative samples. Three positive PCRs were sequenced. Thirty-six percent were serologically positivity and 5.5% from blood smears. The animals were anemic and thrombocytopenic. Presence of ticks and living in areas on the urban periphery were considered to be risk factors (p < 0.05). Nested PCR identified 11 positive dogs of which nine were serologically positive and two were negative. The DNA sequencing was consistent with the presence of Ehrlichia canis.
Ehrlichia canis; epidemiology; ELISA; Nested PCR
Objetivou-se com este trabalho estudar as alterações clínicas, fatores de risco da ehrlichiose canina nos municípios de Ilhéus e Itabuna, Bahia, e comparar diferentes métodos de diagnóstico. Amostras de sangue foram coletadas de 200 cães e cada animal foi examinado clinicamente. Foi preenchido um questionário para avaliar os fatores de risco. As amostras de sangue foram analisadas pelo teste Dot-ELISA e foram realizadas hematimetria, contagem de plaquetas e procura de mórulas em esfregaço de sangue. Nested-PCR foi realizada em 50 amostras positivas e 50 negativas na sorologia. Três amostras PCRs positivas foram seqüenciadas. Foi encontrado 36,0% de positividade na sorologia e 5,5% nos esfregaços sanguíneos. Os animais apresentavam anemia e trombocitopenia. Ter carrapatos e residir em áreas suburbanas foram considerados fatores de risco (p < 0,05). A Nested-PCR identificou 11 cães positivos, sendo 9 com sorologia positiva e 2 negativos. O sequenciamento de DNA foi compatível com a presença de Ehrlichia canis.
Ehrlichia canis; epidemiologia; ELISA; Nested PCR
FULL ARTICLE
Risk factors and clinical disorders of canine ehrlichiosis in the South of Bahia, Brazil
Fatores de risco e alterações clínicas da erhlichiose canina no sul da Bahia, Brasil
Renata Santiago Alberto CarlosI; Fábio Santos CarvalhoII; Amauri Arias WenceslauI; Nadia Regina Pereira AlmosnyIII; George Rêgo AlbuquerqueI
IDepartamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz - UESC
IIPrograma de Pós-Graduação em Ciência Animal, Universidade Estadual de Santa Cruz - UESC
IIIDepartamento de Patologia Clínica Veterinária, Universidade Federal Fluminense - UFF
Corresponding author Corresponding author: George Rêgo Albuquerque Departamento de Ciências Agrárias e Ambientais, Universidade Estadual de Santa Cruz - UESC CEP 45662-900, Ilhéus - BA, Brazil e-mail: gralbu@uesc.br
ABSTRACT
The aim of this work was to study the clinical disorders and risk factors of canine ehrlichiosis in Ilhéus and Itabuna, Bahia, and compare different diagnostic methods. Blood samples were collected from 200 dogs. Each dog was clinically examined. A questionnaire was used to evaluate the risk factors. The blood samples were analyzed using the Dot-ELISA test; hematometry, platelet counts and searches for morulae on blood smears were performed. Nested PCR was carried out on 50 serologically positive samples and 50 negative samples. Three positive PCRs were sequenced. Thirty-six percent were serologically positivity and 5.5% from blood smears. The animals were anemic and thrombocytopenic. Presence of ticks and living in areas on the urban periphery were considered to be risk factors (p < 0.05). Nested PCR identified 11 positive dogs of which nine were serologically positive and two were negative. The DNA sequencing was consistent with the presence of Ehrlichia canis.
Keywords:Ehrlichia canis, epidemiology, ELISA, Nested PCR.
RESUMO
Objetivou-se com este trabalho estudar as alterações clínicas, fatores de risco da ehrlichiose canina nos municípios de Ilhéus e Itabuna, Bahia, e comparar diferentes métodos de diagnóstico. Amostras de sangue foram coletadas de 200 cães e cada animal foi examinado clinicamente. Foi preenchido um questionário para avaliar os fatores de risco. As amostras de sangue foram analisadas pelo teste Dot-ELISA e foram realizadas hematimetria, contagem de plaquetas e procura de mórulas em esfregaço de sangue. Nested-PCR foi realizada em 50 amostras positivas e 50 negativas na sorologia. Três amostras PCRs positivas foram seqüenciadas. Foi encontrado 36,0% de positividade na sorologia e 5,5% nos esfregaços sanguíneos. Os animais apresentavam anemia e trombocitopenia. Ter carrapatos e residir em áreas suburbanas foram considerados fatores de risco (p < 0,05). A Nested-PCR identificou 11 cães positivos, sendo 9 com sorologia positiva e 2 negativos. O sequenciamento de DNA foi compatível com a presença de Ehrlichia canis.
Palavras-chave: Ehrlichia canis, epidemiologia, ELISA, Nested PCR.
Introduction
Ehrlichia canis is the agent for canine monocytic ehrlichiosis (CME), which is transmitted by ticks, especially Rhipicephalus sanguineus. The first clinical manifestations occurred after an incubation period that varies from 8 to 20 days, with three distinct infection phases: acute, subclinical and chronic (HARRUS et al., 1997). Clinical signs such as fever, anorexia, vomiting, weight loss, hepatomegaly, splenomegaly, lymphadenopathy, epistaxis and hemorrhage are observed (HARRUS et al., 1997; MOREIRA et al., 2003). Thrombocytopenia and anemia are the most common blood abnormalities in dogs that have been naturally or experimentally infected (M'GHIRBI et al., 2009).
Epidemiological studies in Brazil have revealed that the seroprevalence of CME ranges from 1.7 to 65.6% among dogs in urban or rural environments (LABARTHE et al., 2003; COSTA JUNIOR et al., 2007). Early diagnosis of ehrlichiosis is important because this is an emerging zoonotic disease (PEREZ et al., 2006). The diagnostic techniques available include blood smears to view inclusions of corpuscles or morulae (BORIN et al., 2009), serum tests, especially Dot-ELISA and the indirect immunofluorescence assay (IFA) (TRAPP et al., 2006), and molecular techniques such as nested PCR (SOUZA et al., 2010).
The aim of the present study was to describe the clinical disorders and risk factors of canine ehrlichiosis in Ilhéus and Itabuna, Bahia, and compare the different diagnostic methods.
Materials and Methods
1. Location
This study was conducted in Ilhéus (Latitude 14º 47' S and Longitude 39º 02' W) and Itabuna (latitude 14º 47' S and longitude 39º 16' W), in the Southern region of the State of Bahia, from August 2004 to April 2005. During the study period, the average temperature was 23.4 ºC, with relative humidity of 84.8% and total rainfall of 1,320 mm.
2. Population studied, samples and procedures
Blood samples were collected from 200 dogs (100 dogs per city) at the Veterinary Teaching Hospital of the State University of Santa Cruz (UESC) (Ilhéus n = 35, Itabuna n = 20), at participating local veterinary clinics (Ilhéus n = 20, Itabuna n = 45), and selected households in both cities (Ilhéus n = 45, Itabuna n = 35). The animals were selected for the researchers' convenience, without specific inclusion criteria, exclusion criteria or randomization. At the local veterinary clinics, dogs were enrolled in this study when they were presented due to illness or the need for a surgical procedure or annual checkup. The participating households were invited to join in by staff at the clinics and were selected based on the owners' willingness to participate in this study. Only one dog per household was enrolled.
Any clinical abnormalities identified from each dog's history or during the physical examination were recorded. The epidemiological questionnaire was filled out by the owner, and the variables studied were gender (male, female), age (1-4 years, >4 years), contact with other dogs (yes, no), presence of ticks (yes, no), breed (purebred, mixed-breed), wellness care (yes, no), petechiae (presence, absence), apathy (presence, absence), fever (presence, absence), dehydration (presence, absence), lymphadenopathy (presence, absence), weight loss (presence, absence) and household location (urban, urban periphery). The dogs living on the urban periphery were in households in peripheral neighborhoods, i.e. outside the urban centers of the cities. Thrombocytopenia (presence, absence) and anemia (presence, absence) were included in the analysis based on the hematological tests.
Blood samples were collected into tubes with and without EDTA. Blood samples were examined for E. canis antibodies using a commercial kit for dot-ELISA (Snap 3DX®, IDEXX Laboratories), in accordance with the manufacturer's instructions. Blood smears were prepared, fixed in methanol for 5 minutes and stained with 10% Giemsa. Complete blood counts were performed. Dogs with counts of less than 200,000 platelets.mL-1 were considered to be thrombocytopenic (HARRUS et al., 1997). Animals with packed cell volume (PCV) below 37% were considered anemic.
Fifty seropositive and 50 seronegative dogs were tested by means of nested PCR for molecular detection of E. canis. Only 100 PCRs were possible because of losses of several samples at the beginning of the study. DNA was extracted from 100 mL of EDTA blood using a commercial kit (ChargeSwitchTM gDNA 50-100 mL Blood Kit, InvitrogenTM, USA), in accordance with the manufacturer's instructions, thus resulting in a final DNA concentration of at least 3 mg of total extracted volume. Molecular-grade water was extracted to confirm that there was no cross-contamination between samples during DNA extraction. The genetic presence of any species of the Ehrlichia genus was investigated using the ECC forward primer (5'-AGAACGAACGCTGGCGGCAAGC-3'') and ECB reverse primer (5'-CGTATTACCGCGGCTGCTGGCA-3'). Samples that were positive for Ehrlichia sp. were then tested with the ECAN5 forward primer (5'-CAATTATTTATAGCCTCTGGCTATAGGA-3') and HE3 reverse primer (5'- TATAGGTACCGTCA TTATCTTCCCTAT-3') to obtain a specific amplification of E. canis. The PCR mixture consisted of 5 mL of purified DNA, 0.4 mM of ECB/ECC, 200 mM of each dNTP, 5.0 mM of MgCl2, 2.5 U of Taq DNA polymerase (InvitrogenTM) and 1.6x PCR buffer (InvitrogenTM), to make up a total volume of 25 mL of reaction mixture. The amplification program used to identify the genetic sequence of the genus Ehrlichia spp. consisted of a first step of denaturation for 3 minutes at 94 ºC, followed by 35 cycles of denaturation at 94 ºC for 1 minute, annealing at 68 ºC for 2 minutes and extension at 72 ºC for 2 minutes. For the nested PCR, 3 mL of amplicons from the first PCR and 0.2 mM of ECAN5/HE3 were used to identify the specific genetic sequence for E. canis, in an amplification program consisting of denaturation for 3 minutes at 94 ºC, followed by 35 denaturation cycles at 94 ºC for 1 minute, annealing at 58 ºC for 2 minutes and extension at 72 ºC for 1.5 minute. The PCR products were analyzed using 1.5% agarose gel electrophoresis, stained with ethidium bromide and photographed. The positive control used was a blood sample from a dog that was positive for E. canis, and which had previously been confirmed by sequencing. For the negative control, ultra-pure water was used in both PCRs.
The sequencing of the samples was carried out using the automatic sequencer ABI-PRISM 3100 Genetic Analyzer (Applied Biosystems). The DNA template (45 ng) was marked with 3.2 pmol of the primer ECAN5 and 2 mL of the reagent BigDye Terminator v3.1 Cycle Sequencing RR-100 (Applied Biosystems), in a final volume of 10 mL. The BLAST software ( http://www.ncbi.nlm.nih.gov/blast ) was used for comparison and analysis of the sequences obtained.
3. Statistical analysis
Normal data distribution was confirmed by means of the Kolmogorov-Smirnov test in the SPSS software, and statistical differences in platelet counts and PCV between E. canis seropositive and seronegative dogs were analyzed by means of the t-test, to 5% (RODRIGUEZ-VIVAS et al., 2005).
The association between presence of antibodies against E. canis and the study variables was evaluated by means of the chi-square (χ2) test and Fisher's exact test. The Epi-Info software, version 3.5.1, was used to evaluate the dispersion frequency. The variables that presented P < 0.2 in the univariate analysis were selected for multivariate analysis by means of logistic regression, also using the Epi-Info software, version 3.5.1.
Results
Out of all the samples tested, 72 (36%) were positive for E. canis according to ELISA Snap 3DX®: 43 in Ilhéus and 29 in Itabuna (CARLOS et al., 2007). In relation to the place where the samples were collected, the rates of positive findings were 52.7% (29) at the Veterinary Hospital, 26.15% (17) for the dogs at private veterinary clinics and 32.5% (26) among samples collected from households. Evaluating according to municipality, the rates of positive findings in Ilhéus were 65.7% (23) for the samples collected at UESC, 35% (7) in private veterinary clinics and 28.9% (13) from households. In Itabuna, the rates of positive findings were 30% (6) at UESC, 22.2% (10) in veterinary clinics and 37.1% (13) from households. A statistical difference was observed in relation to the samples collected in Ilhéus, at UESC (p = 0.02). Only 11 dogs (5.5%) presented typical morulae in monocytes (Table 1).
The clinical examination detected fever (n = 24, 12.0%), apathy (n = 13, 6.5%), petechiae (n = 3, 1.5%), dehydration (n = 13, 6.5%), lymphadenopathy (n = 49, 24.5%), and weight loss (n = 10, 5.0%). Among the 72 seropositive animals, 10 presented fever (13.8%), 9 apathy (12.5%), 3 petechiae (4.2%), 7 dehydration (9.7%) and 28 lymphadenopathy (38.9%). All the animals with petechiae were seropositive.
The average number of platelets was 249,000 (range: 23,000 - 628,000) platelets.mL-1 of blood. The seropositive dogs presented an average count of 180,000 platelets.mL-1 of blood (range: 23,000 - 590,000). The seronegative dogs presented an average of 288,000 platelets.mL-1 of blood (range: 76,000 - 628,000) (p < 0.0001). Regarding hematocrit values, the total average was 40.5% (range: 8.0 - 60.0). The seropositive animals presented an average of 35.2% (range: 8.0 - 56.0) whereas the seronegative animals presented an average of 43.5% (range: 22.0 - 60.0) (p < 0.0001).
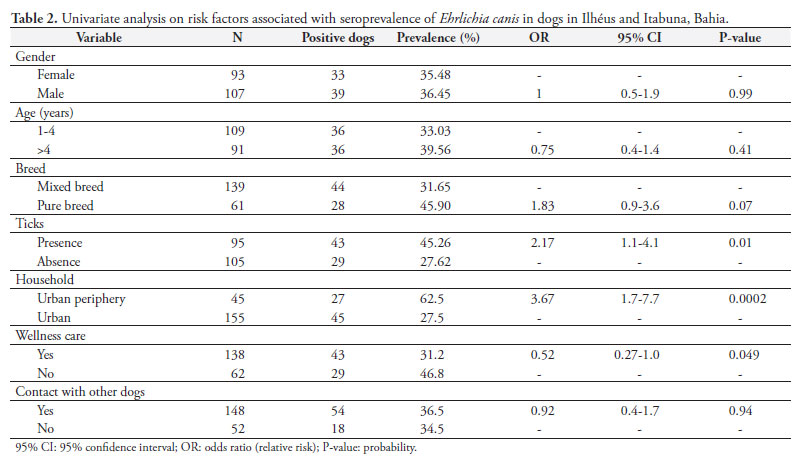
The univariate analysis used to identify risk factors for E. canis seropositivity are presented in Table 2. Logistic regression revealed a positive relationship between the presence of ticks and dogs living on the urban periphery (Table 3). Regarding clinical abnormalities, based on univariate analysis, seropositive animals more frequently were anemic (p < 0.01) and thrombocytopenic (p < 0.01) and presented lymphadenopathy (p < 0.01), in comparison with seronegative dogs. There were no differences in temperature between the groups (Table 4). Logistic regression revealed positive relationships with anemia, thrombocytopenia and lymphadenopathy (Table 5).
Among the 100 blood samples tested for E. canis DNA by means of nested PCR, 11 were considered positive. Out of these, nine were from seropositive dogs and two from seronegative dogs. All the sequenced fragments had approximately 364 base pairs (bp). These sequences had 97-99% identicality with the corresponding sequences of E. canis strains that have been deposited at GenBank (access codes: GU386289.1; GU386285.1).
Discussion
The results obtained from this study showed that dogs from the cities of Ilhéus and Itabuna, in the State of Bahia, Brazil, are exposed to E. canis. The serum prevalence in this study was similar to that found by Souza et al. (2010) using IFA, which found a prevalence of 35.6% in Salvador, Bahia. Using the Dot-ELISA test on 2,553 dogs from several Brazilian States, Labarthe et al. (2003) found that 505 (19.8%) were positive, with highest prevalence in the States of the Northeast (43%) and lowest in the Southern region (1.7%). Among the 2,553 dogs, 117 of the samples were collected in Salvador, and 42 (35.9%) were positive, which was also similar to the present study. In the same region of Bahia, with 153 different dogs, Carvalho et al. (2008) found using PCR that 12 (7.8%) were positive. In the present study, there was a statistical difference in relation to the dog samples from UESC in Ilhéus, because the majority of these animals came from the urban periphery, which was considered to be a risk factor for infection.
The serum prevalence of E. canis varies according to climatic conditions and certain epidemiological factors, such as the presence and distribution of the vector, the animal's behavior, the age and type of the population under study, and the habitat (RODRIGUEZ-VIVAS et al., 2005). The dogs that lived on the urban periphery presented a higher seropositivity rate for E. canis (ORS= 3.7) than shown by those in the urban area. The same association was observed by Trapp et al. (2006). These results suggest that dogs that live on the urban periphery are more exposed to vector ticks, which may be related to the fact that urban dogs are more frequently sprayed with acaricides than are dogs on the urban periphery or rural dogs.
The weather is an important factor that influences the population dynamics of ticks. The average temperature in the region of Ilhéus and Itabuna provides excellent conditions for R. sanguineus ticks, the natural vector for E. canis, to develop. Ticks were found on 59.7% of the positive animals, whereas on 59.4% of the negative animals, this vector was not found. Presence of the tick as a risk factor for ehrlichiosis was also detected by Trapp et al. (2006) and Costa Junior et al. (2007).
A higher rate of seronegative animals (p = 0.049) was also observed among the dogs that were not provided with wellness care, thereby showing that visits to veterinary clinics were a protection factor. This is explained by the care that owners provide for their animals and the consequent lower infestation rate by the vector.
The main clinical signs observed during clinical examinations were similar to those described by Moreira et al. (2003) and Borin et al. (2009). Ehrlichia canis may cause variable signs, which make the diagnosis more challenging. The hematological abnormalities identified in the present study (thrombocytopenia and anemia) were similar to those previously described by Rodriguez-Vivas et al. (2005), Borin et al. (2009) and M'Ghirbi et al. (2009). The effects of the mononuclear phagocytic system, cell lysis mediated by the complement system and suppression of erythropoiesis at the bone marrow may be the mechanisms responsible for the anemia relating to the disease (MOREIRA et al., 2003). Ehrlichia canis induces thrombocytopenia through destruction and consumption of platelets, increased hepatic or splenic platelets sequestration, decreased platelet production due to bone marrow hypoplasia (WOODY; HOSKINS, 1991) and production of antiplatelet antibodies (GAUNT et al., 2010).
Diagnosing of ehrlichiosis through blood smear analysis is difficult because intracytoplasmic morulae are only occasionally seen during the acute phase of the disease (RODRIGUEZ-VIVAS et al., 2005). In the present study, we observed that 5.5% of the blood smears presented the typical morulae of E. canis, and this was similar to the observations of Rodriguez-Vivas et al. (2005), who found that 44.1% of the dogs were seropositive but that only six dogs (5%) presented morulae detected in blood smears.
Serological tests (dot-ELISA and IFA) are the method most commonly used for veterinary diagnosis. However, the fact that an animal is seropositive does not mean that it is sick. Since the presence of antibodies reveals exposure to the agent, PCR may help in reaching a diagnostic conclusion. Souza et al. (2010) found that 35.6% (168/472) of the dogs examined were seropositive, but that only 58 (34.5%) of these animals were PCR-positive.
In the present s udy, nested PCR was used on 100 samples (50 seropositive and 50 seronegative for E. canis), and nine dogs were found to be PCR-positive among the seropositive dogs. It was found that two dogs were seronegative for E. canis, but PCR-positive. Similar results were obtained by Diniz et al. (2007). Hyperacute cases may be PCR-positive with no antibody response, in which the molecular test detects the presence of the agent before the dog's immunological response occurs. Nested PCR is the technique most used for diagnosing E. canis, but false-positive results may occur if primers that generate unspecific fragments are used. In the present study, such results did not occur because the primers used were ECAN5 and HE-3. In sequencing the E. canis DNA fragments using the ECAN5 primer, the homology ranged from 97 to 99%. This low homology may have occurred because of the few samples sequenced (three), as well as incomplete DNA purification prior to sequencing.
In the present study, subject enrollment done according to convenience, because of certain constraints, such as structural and financial limitations. Despite the lack of random sampling in this study, the results provide important information about disease prevalence and the risk factors and clinical abnormalities associated with E. canis infection in the Southern region of Bahia.
Received December 13, 2010
Accepted August 17, 2011
- BORIN, S.; CRIVELENTI, L. Z.; FERREIRA, F. A. Aspectos epidemiológicos, clínicos e hematológicos de 251 cães portadores de mórula de Ehrlichia spp. naturalmente infectados. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v. 61, n. 3, p. 566-571, 2009.
- CARLOS, R. S. A. et al. Frequência de anticorpos anti-Erhlichia canis, Borrelia burgdorferi e antígenos de Dirofilaria immitis em cães na microrregião Ilhéus-Itabuna, Bahia, Brasil. Revista Brasileira de Parasitologia Veterinária,v. 16, n. 3, p. 117-120, 2007.
- CARVALHO, F. S. et al. Epidemiological and molecular study of Ehrlichia canis in dogs in Bahia, Brazil. Genetics and Molecular Research, v. 7, n. 3, p. 657-662, 2008. PMid:18752193. http://dx.doi.org/10.4238/vol7-3gmr468
- COSTA JUNIOR, L. M. et al. Sero-prevalence and risk indicators for canine ehrlichiosis in three rural areas of Brazil. Veterinary Journal, v. 174, n. 3, p. 673-676, 2007. PMid:17204439. http://dx.doi.org/10.1016/j.tvjl.2006.11.002
- DINIZ, P. P. V. P et al. Surveillance for zoonotic vector-borne infections using sick dogs from southeastern Brazil. Vector-Borne and Zoonotic Diseases,v. 7, n. 4, p. 689-697, 2007.
- GAUNT, S. D. et al. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasites and Vectors, v. 3, n. 1, p. 33, 2010. http://dx.doi.org/10.1186/1756-3305-3-33
- HARRUS, S. et al. Canine monocytic ehrlichiosis: an update. Parasitology, v. 19, p. 431-444, 1997.
- LABARTHE, N. et al. Serologic prevalence of Dirofilaria immitis, Ehrlichia canis, and Borrelia burgdorferi infections in Brazil. Veterinary Therapeutics, v. 4, p. 67-75, 2003.
- M'GHIRBI, Y. et al. Clinical, serological and molecular evidence of ehrlichiosis and anaplasmosis in dogs in Tunisia. Parasitology Research, v. 104, n. 4, p. 767-774, 2009.
- MOREIRA, S. M. et al. Retrospective study (1998-2001) on canine ehrlichiosis in Belo Horizonte, MG, Brazil. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v. 55, n. 2, p. 141-147, 2003.
- PEREZ, M. et al. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Annals of New York Academic Science, v. 1078, p. 110-117, 2006. PMid:17114689. http://dx.doi.org/10.1196/annals.1374.016
- RODRIGUEZ-VIVAS, R. I.; ALBORNOZ, R. E. F.; BOLIO, G. M. E. Ehrlichia canis in dogs in Yucatan, México: seroprevalence, prevalence of infection and associated factors. Veterinary Parasitology, v. 127, n. 1, p. 75-79, 2005. PMid:15619376. http://dx.doi.org/10.1016/j.vetpar.2004.08.022
- SOUZA, B. M. P. S. et al. Prevalence of ehrlichial infection among dogs and ticks in Northeastern Brazil. Revista Brasileira de Parasitologia Veterinária, v. 19, n. 2, p. 89-93, 2010.
- TRAPP, S. M. et al. Seroepidemiology of canine babesiosis and ehrlichiosis in a hospital population. Veterinary Parasitology, v. 140, n. 3-4, p. 223-230, 2006. PMid:16647817. http://dx.doi.org/10.1016/j.vetpar.2006.03.030
- WOODY, B. J.; HOSKINS, J. D. Ehrlichial diseases of dogs. Veterinary Clinics of North America: Small Animal Practice, v. 21, n. 1, p. 75-98, 1991. PMid:2014630.
Corresponding author:
Publication Dates
-
Publication in this collection
14 Oct 2011 -
Date of issue
Sept 2011
History
-
Accepted
17 Aug 2011 -
Received
13 Dec 2010