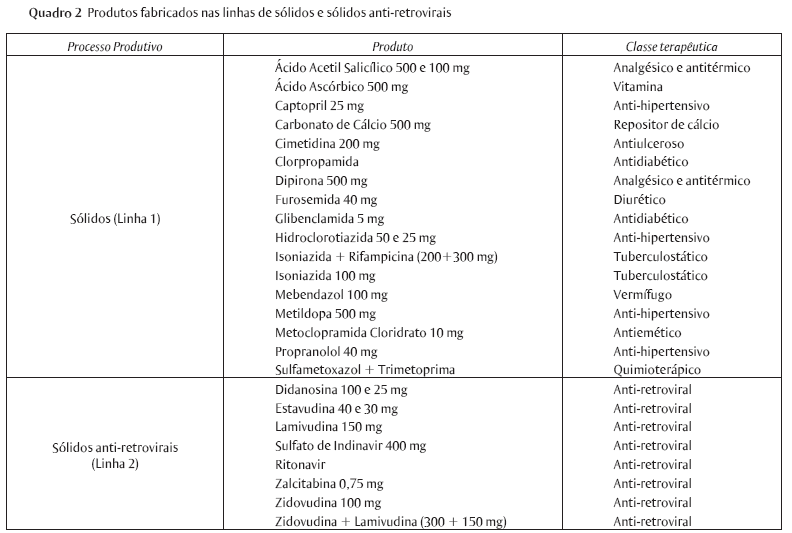
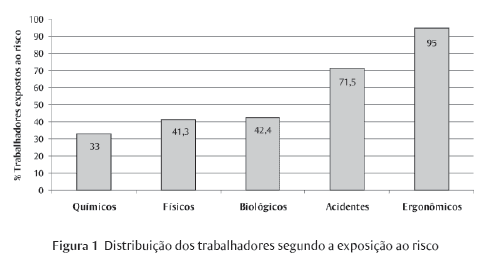
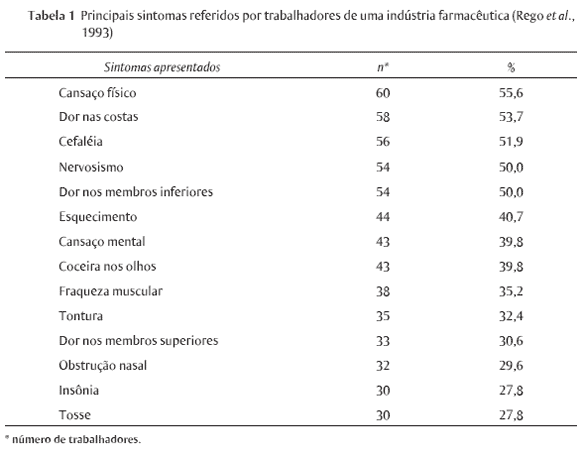
The production of medicine is a complex industrial process that demands high investments not only in research and development, but also in production and quality control, in acquiring chemicals, in storage and distribution, and in keeping staff qualified and updated. Paradoxically, in spite of being a segment that deals with ultra modern technology, and the support provided by the good manufacturing practices (GMP) requirements foreseen by the sanitary legislation, the drug industry runs several environmental risks, including their consumers' and workers' health hazards as well as the environment around their plants. This study aims at presenting the results of a research on occupational hazards developed by the company's Internal Commission for the Prevention of Accidents (CIPA) in a governmental pharmaceutical industry located in the northeast of Brazil. I hope it will contribute to stimulate other advanced studies related to workers' health and illness in the pharmaceutical industry.
occupational risks; production of medicine; good manufacturing practices (GMP); pharmaceutical industry