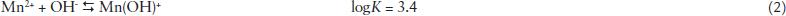Abstract
Silicates (rhodonite, tephroite, spessartine) and the carbonate (rhodochrosite) of manganese are of economic interest in silicate-carbonated manganese ores. The recovery of both mineral classes by flotation constitutes a challenge; rhodochrosite is a slightly soluble mineral that can release Mn2+ ions in pulp. In this work, the effects of inorganic and organic depressants on the cationic flotation at pH 10 with ether amine acetate and the surface charges of rhodonite and rhodochrosite have been investigated. For rhodonite, the influence of Mn2+ species on its recovery and surface charge at the conditions of maximum yield with amine has also been investigated. The organic depressants, especially the corn starch, were more effective depressants for both minerals. The poor recovery of rhodonite conditioned with MnCl2 is probably related to the colloidal Mn(OH)2 deposition on mineral surface. The increase in the rhodochrosite recovery with increasing water glass content is probably related to its negative value of species adsorbed on the mineral surface, since the rhodochrosite zeta potential, conditioned with this reagent, becomes more negative compared with the mineral without reagent, which attracts the ether ammonium.
Keywords:
Rhodonite; rhodochrosite; cationic flotation; inorganic and organic depressants; manganese ions
1. Introduction
The beneficiation flowsheets of high grade oxidized manganese ores normally consist of crushing followed by screening. The screening oversize constitutes the lump ore products, and the undersize of the last screen is classified by hydraulic classifiers. Based on the Mn content and size distribution, in some industrial plants, the underflow from the classifier step (fraction size +0.15 mm-sinter feed) is a final product or can be concentrated by gravimetric methods, high intensity magnetic separation, and flotation (Sampaio and Penna, 2001SAMPAIO, J. A., PENNA, M.T.M. CVRD/Mina do Azul. In: SAMPAIO, J.A., LUZ, A. B., LINS, F. F. (Ed.). Usinas de beneficiamento de minérios do Brasil. 2001. p. 93-102., Aplan, 1985APLAN, F. F. Manganese. In: Norman L. W. (Ed.). Mineral Processing Handbook. Society of Mining Engineering. , 1985. n. 2, p. 27-6, 27-10.).
The flotation of oxide and carbonate manganese ores is carried out by fatty acid soap at alkaline pH (Lima et al., 2008LIMA, R.M.F., VASCONCELOS, J.A., SILVA, G.R. Flotação aniônica de minério de manganês. REM - Revista da Escola de Minas, v. 61, p. 337-342, 2008., Acevedo, 1977ACEVEDO, G.S. Flotation of silicate-carbonated manganese ores using oleic acid and depressors. In: CASTRO, S., ALVAREZ, J. (Ed.). Avances en flotación. Universidad de Concepción, v. 3, p.50-60, 1977., Aplan, 1985APLAN, F. F. Manganese. In: Norman L. W. (Ed.). Mineral Processing Handbook. Society of Mining Engineering. , 1985. n. 2, p. 27-6, 27-10.). Oxide ores with silicate gangue can be successfully concentrated by reverse flotation with amine and the depression of manganese oxides at alkaline pH. Silicate manganese ore can be floated by cationic collectors (e.g. amines) at acidic pH or by anionic collectors (e.g. oleate or hydroxamate) after manganese silicate activation with polyvalent ions. Hydrolyzed metals are formed and precipitated at pH values above the isoelectric point (IEP) of manganese minerals (Andrade, 1978ANDRADE, V. L. L. Estudos de concentração do gondito. Belo Horizonte: Curso de Pós-Graduação em Engenharia Metalúrgica e de Minas, 1978, p. 70. (Dissertação de Mestrado).; Ciminelli, 1980CIMINELLI, V. S. T., Estudos de mecanismos de adsorção de reagents de flotação em minerais de Gondito. Belo Horizonte: Programa de Pós-Graduação em Engenharia Metalúrgica e de Minas, Universidade Federal de Minas Gerais, 1980, 74 p., Fuerstenau and Palmer, 1976FUERSTENAU, M. C., PALMER, B. R. Anionic flotation of oxides and silicates. In: FUERSTENAU, M. C. (Ed.). Flotation. New York: AIME, 1976, v. 1, p. 148-196. (A. M. Gaudin Memorial).).
In the flotation of manganese ores, which contain silicate manganese, carbonate manganese, dolomite and other carbonates (slightly soluble minerals), the recovery and selectivity can be very difficult due to the presence of polyvalent ions, hydroxyl complexes and hydroxide species in pulp, especially when recycled water is used in the flotation circuit. This paper presents the results of micro-flotation tests and zeta potential determination for rhodonite and rhodochrosite using a cationic collector, which is usually applied in iron ore flotation, with inorganic and organic depressants. The influence of Mn species on rhodonite recovery and surface charge at pH 10 were also investigated.
2. Materials and Methods
2.1 Mineral samples and reagents
Table 1 depicts the chemical compositions of the mineral samples (natural and synthetic). In the micro-flotation tests and zeta potential measurements were used: Ether amine acetate with 50% of neutralization degree (Clariant A. S.) as collector; water glass (Na2SiO2), Na-fluorosilicate, and MnCl2.2H2O of analytical purity (VETEC A.S.), commercial corn starch (Unilever A.S) and quebracho (Unitan A.S.) as depressants; NaOH and HCl of analytical purity (VETEC A.S.) as pH control, NaCl analytical purity (VETEC A.S.) as ionic strength control.
Chemical composition of rhodonite and synthetic rhodochrosite (Andrade et al., 2011ANDRADE, E. M., LEÃO, V. A., LIMA, R. M. F., Influência da adição de fluorsilicato de sódio na flotabilidade de minerais de manganês e quartzo com oleato de sódio. REM - Revista Escola de Minas, v. 64, n. 2, p. 219-225, 2011., Andrade et al., 2012ANDRADE, E. M., COSTA, B. L. C. M., ALCÂNTARA, G. A. G., LIMA, R. M. F. Flotation of manganese minerals and quartz by sodium oleate and water glass. Latin American Applied Research, v. 42, p. 39-43, 2012.)
Fig. 1 shows the diagram of mononuclear manganese species at 25ºC in water, which was calculated based on the solubility and equilibrium constants of the following reactions (Snoeyki and Jenkins, 1980SNOEYINK, V. L., JENKINS, D. In: ____. Water chemistry. John Wiley & Sons, 1980. 477 p.):
2.3–Experimental methods
The micro-flotation tests (three replicates) were performed using a modified Hallimond cell equipped with a magnetic stirrer. In all tests, 1 g of rhodonite with a fraction size of −74+37 µm and not classified for manganese carbonate (synthetic rhodochrosite) was used. The rhodonite tests were first performed using 5 mg/L of amine (conditioning time of 5 min) at pH values ranging from 2–12 in order to verify the optimal flotation conditions (5 mg/L and pH 10) determined previously for this mineral (Alcântara et al., 2013ALCÂNTERA, G. A. G., DUARTE, R. S., MENDONÇA, G. A., LIMA, R. M. F. Estudos de flotabilidade de minerais de manganês e quartzo com amina e quebracho In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA (ENTMME), 25 MEETING OF THE SOUTHERN HEMISPHERE ON MINERAL TECHNOLOGY (MSHMT), 8. Proceedings. Goiânia-Brazil, 2013.). These values were fixed in the tests performed with rhodochrosite. Furthermore, depression studies were performed for each mineral after conditioning with different depressant concentrations for six minutes followed by five minutes of conditioning with the previously determined optimal amine concentration. After conditioning, the mineral sample was floated for one minute using commercial nitrogen (flow rate of 75 mL/min.).
A zeta-meter (Malvern Zetasizer Nano Z–ZEN 2600) was used to determine the zeta potentials of the mineral samples. This equipment automatically determines the electrophoretic mobility of the particles and transforms it into a zeta potential (ζ) using Smoluchowiski’s equation. Firstly, the zeta potential of rhodonite was determined in the pH range of 4 to 12 without reagent and conditioning with 5 mg/L of amine. For rhodochrosite and for both minerals conditioned with depressants, zeta potential was determined only at pH 10.
The experimental procedure for the zeta potential measurements was based on Nascimento et al. (2013)NASCIMENTO, D. R., PEREIRA, R. D., LIMA, R. M. F. Influence of sodium silicate on floatability and charge of hematite and quartz with sodium oleate. Latin American Applied Research, v. 43, p.189-191, 2013.. Briefly, a 0.01% w/w suspension was prepared by the addition of 0.025 g of the mineral (d90 of 10 µm) into a 250 mL solution of NaCl2 10−3 M. The suspension was transferred to a 50 mL beaker, and the pH was adjusted with hydrochloric acid (HCl) and sodium hydroxide (NaOH) under constant agitation in a magnetic shaker. The suspension pH was measured, and an aliquot was slowly poured into the folded capillary cell to measure the zeta potential using the zeta-meter. For each pH value, the zeta potential was determined as the average of the values obtained in four replicate measurements. The pH value of the suspension under constant stirring was measured after each zeta potential measurement was finished. Thus, the pH for each measurement was the value obtained at the end of each zeta potential test.
3. Results and Discussion
3.1 Micro-flotation tests
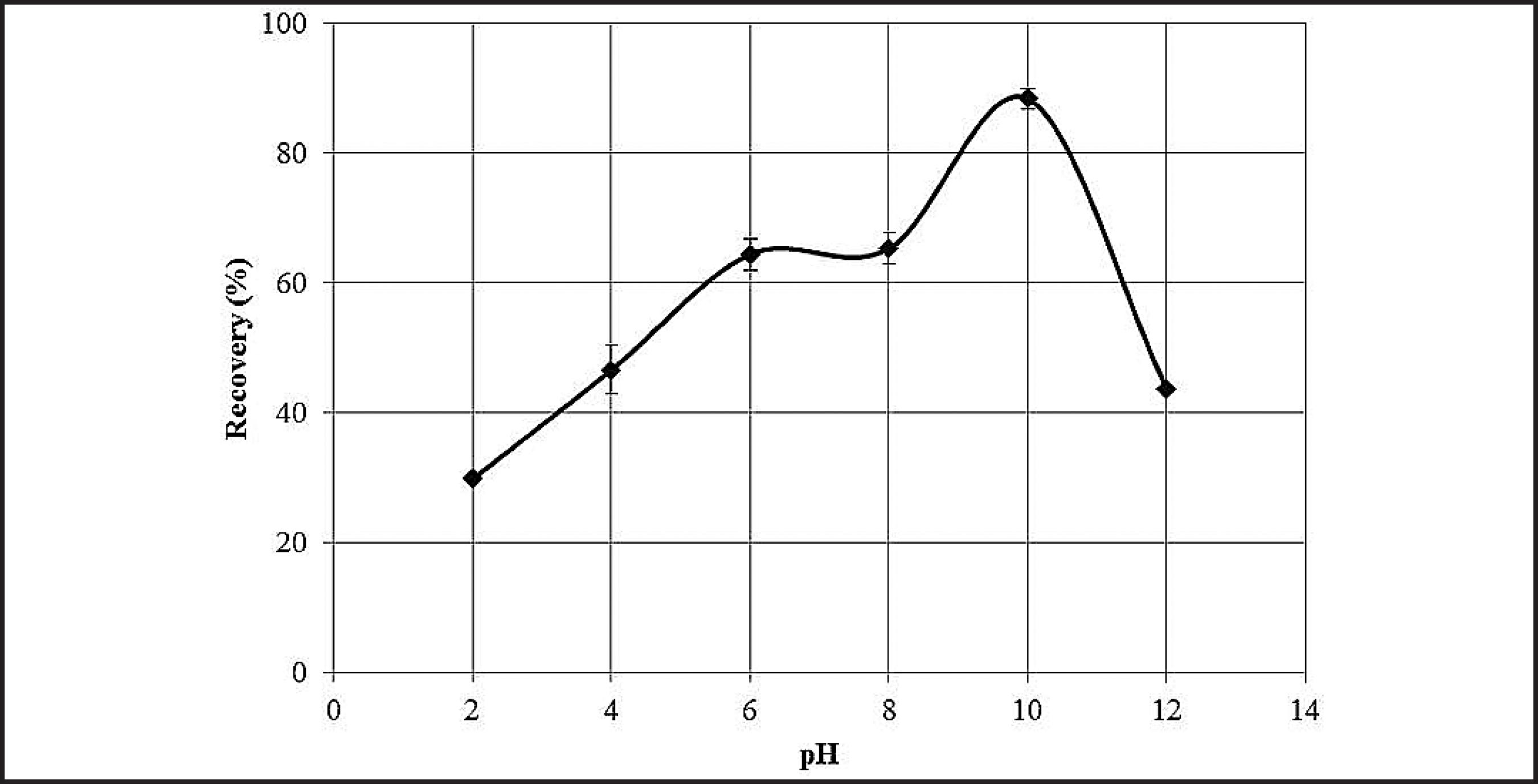
As can be observed in Fig. 2, the maximum recovery of rhodonite with 5 mg/L of amine was obtained at pH 10, confirming the value determined by Alcântara et al. (2013)ALCÂNTERA, G. A. G., DUARTE, R. S., MENDONÇA, G. A., LIMA, R. M. F. Estudos de flotabilidade de minerais de manganês e quartzo com amina e quebracho In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA (ENTMME), 25 MEETING OF THE SOUTHERN HEMISPHERE ON MINERAL TECHNOLOGY (MSHMT), 8. Proceedings. Goiânia-Brazil, 2013.. Thus, the amine concentration and pH were fixed at 5 mg/L and 10, respectively, for all micro-flotation tests.
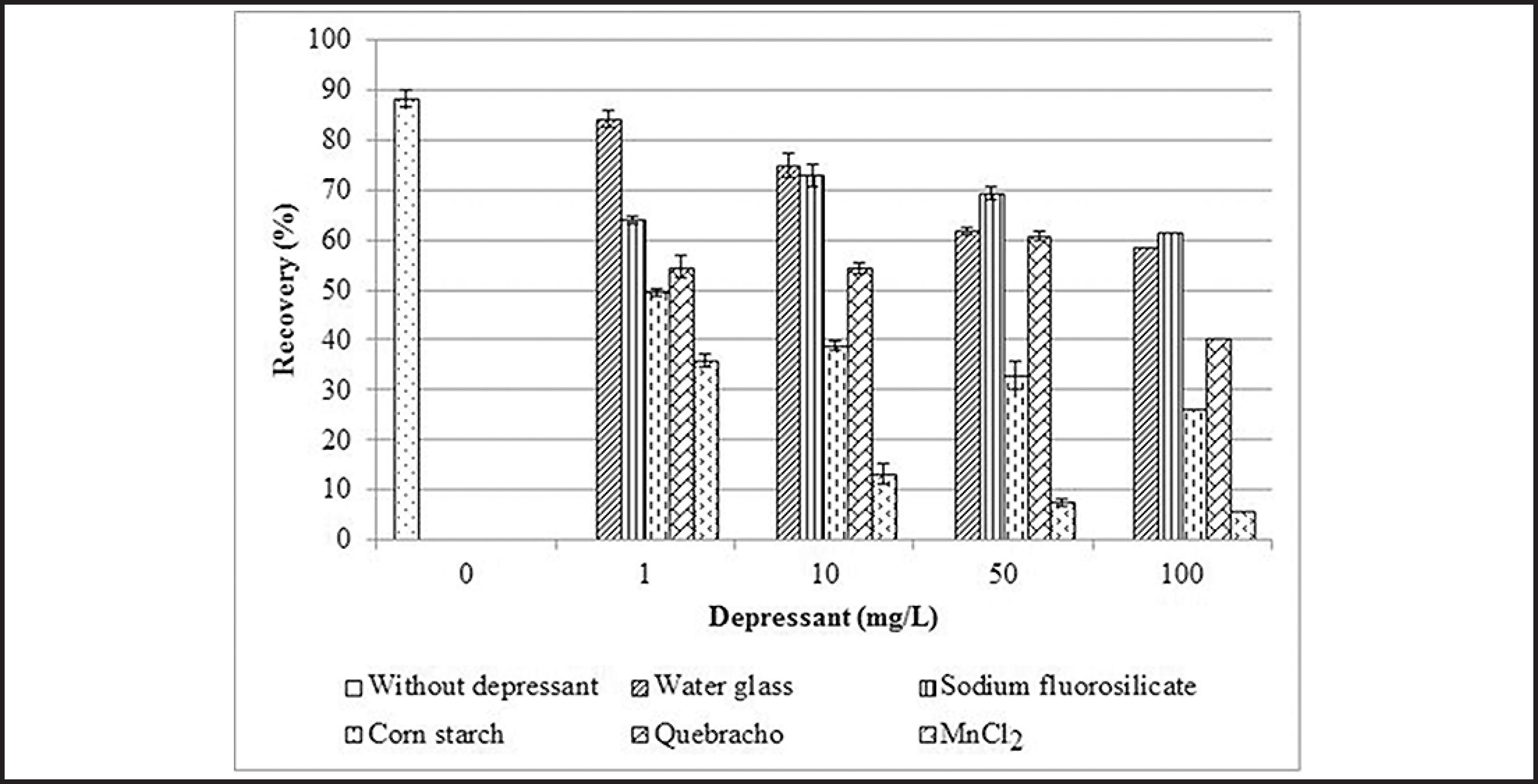
Figs. 3 and 4 depict the effects of different depressants on the recoveries of rhodonite and rhodochrosite, respectively. In general, rhodonite recovery decreased with increasing depressant dosage (Fig. 3). The organic depressants were more efficient to depress this mineral than the inorganic ones.
Recovery of rhodochrosite vs. depressant dosage at pH 10 and 5 mg/L of ether amine acetate.
The recoveries of rhodonite conditioned with MnCl2 were smaller than those for all tested depressants (Fig. 3). In accordance with the Log[Mn] vs. pH diagram (Fig. 1), the Mn species present in solution at pH 10 are Mn2+ (10−5.8 M = 0.0870 mg/L) and MnOH+ (10−5.9 M = 0.0692 mg/L). Table 2 shows that Mn(OH)2 precipitates at all tested MnCl2 dosages. The depression of rhodonite is mainly ascribed to the deposition of this colloidal species on the mineral surface.
Due to the solubility of MnCO3 and the complexion of MnCO3, the species present in water are as follows: Mn2+, HCO3-, CO3−2, MnOH+, OH-, H+, MnHCO3+, MnCO30 (Snoeyki and Jenkins, 1980). The low recovery (21%) of rhodochrosite with 5 mg/L of ether amine acetate (Fig. 4) is ascribed to these ionic species in solution.
Duarte (2012)DUARTE, R. S. Flotação catiônica de finos de minério de manganês sílico-carbonatado. Ouro Preto: Programa de Pós-graduação em Engenharia Mineral, Escola de Minas, Universidade Federal de Ouro Preto, 2012, 101 p. performed bench flotation tests at pH 10 with a manganese ore sample constituted by manganese silicates (rhodonite, tephroite and spessartine) and manganese carbonate (rhodochrosite). The collector used was ether amine acetate, inorganic (water glass, fluorosilicate) and organic (corn starch, quebracho) depressants. The author verified only the mechanical drag of the fines particles (mass recovery of 9%) present in pulp and there was no selectivity in the separation of manganese minerals from gangue minerals (dolomite, magnesite, huntite, muscovite, biotite, phlogopite, quartz, feldspar, magnetite, rutile, ilmenite, pyrite and others). Filtration of the separation products was not possible due the blinding of filter medium. As can be seen in Fig. 3, the recoveries of rhodonite dropped drastically with the increase of MnCl2 dosages, which is related to the precipitation of the colloidal dominant specie (Mn(OH)2)(s) at pH 10 (Fig. 1 and Table 2) on the mineral surface. The same effect could have occurred with all mineral particles present in manganese ore. Furthermore, the presence of several ionic species and hydroxides in pulp come from the dissolution of the carbonates and oxidized sulfides.
3.2 Zeta potential measurements
The rhodonite zeta potential without reagent was negative at all tested pH values (4 to 12; Fig. 5). This result is consistent with the results in literature, as the isoelectric point of this mineral is pH 2.8 (Fuerstenau and Palmer, 1976FUERSTENAU, M. C., PALMER, B. R. Anionic flotation of oxides and silicates. In: FUERSTENAU, M. C. (Ed.). Flotation. New York: AIME, 1976, v. 1, p. 148-196. (A. M. Gaudin Memorial).; Andrade et al., 2011ALCÂNTERA, G. A. G., DUARTE, R. S., MENDONÇA, G. A., LIMA, R. M. F. Estudos de flotabilidade de minerais de manganês e quartzo com amina e quebracho In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA (ENTMME), 25 MEETING OF THE SOUTHERN HEMISPHERE ON MINERAL TECHNOLOGY (MSHMT), 8. Proceedings. Goiânia-Brazil, 2013.). After conditioning with ether amine acetate, the zeta potential values became positive, with the exception of at pH 12. This behavior is in accordance with the increased recovery of rhodonite from pH 2 to pH 10 (Fig. 3), which can be ascribed to the electrostatic attraction between positive ether ammonium ions and the negative mineral surface. The maximum recovery at pH 10 (Fig. 2) is ascribed to the adsorption of ether ammonium ion-molecular species in solution, which decreases the electrostatic repulsion between the polar heads of positive ions adsorbed on the mineral surface. The smaller recovery observed at pH 12 (Fig. 2) is related to the low concentration of ether amine positive species in solution (Fuerstenaul et al., 1985FUERSTENAU, M.C., PALMER, R. et alii. Chemistry of Flotation. 1985. 177 p.). This was confirmed by the negative zeta potential at this pH before (higher negative value) and after conditioning with ether amine (smaller negative value; Fig. 5).
Zeta potential of rhodonite without reagent and conditioned with 10−3 M of ether amine acetate.
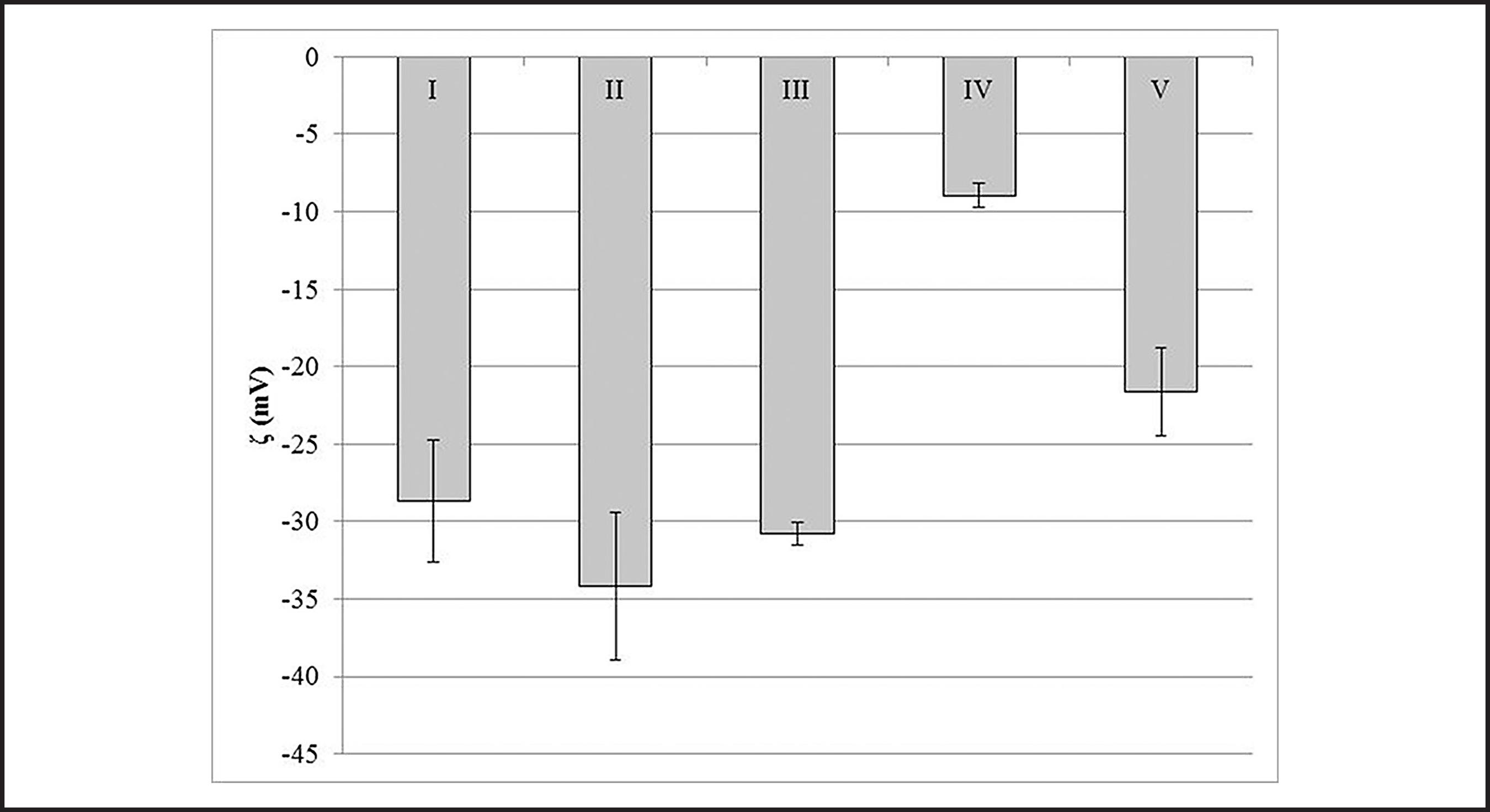
The zeta potential of rhodonite became more negative in the presence of water glass and quebracho compared to the measurement carried out without reagent. The opposite occurred for the measurement performed with sodium fluorosilicate, corn starch, and MnCl2 (Fig. 6). The zeta potential of rhodochrosite at pH 10 was ~−29 mV (Fig. 7I). This value is consistent with the point of zero charge values (5.5 to 6.85) reported by Charlet et al. (1990)CHARLET, L., WERSIN, P., ATUMM, W. Surface Charge of MnCO3 and FeCO3. Geochemica et Chosmochimic Acta, v. 54, Issue 8, p. 2329-2336, 1990.. With the exception of MnCl2, the tested reagents had the same effects on the zeta potential of rhodochrosite as they did on the zeta potential of rhodonite.
Zeta potential of rhodonite at pH 10 and constant ionic strength (10−3 M NaCl) in the following conditions: (I) without reagent; (II) water glass (10−3 M); (III) sodium fluorosilicate (10−3 M); (IV) Mn (10−3 M); (V) corn starch (10 mg/L); and (VI) quebracho (10 mg/L).
Zeta potential of rhodochrosite at pH 10 and constant ionic strength (10−3 M NaCl) in the following conditions: (I) without reagent; (II) water glass (10−3 M); (III) sodium fluorosilicate (10−3 M); (IV) corn starch (10 mg/L); and (V) quebracho.
In accordance with Marinakis and Shergold (1985)MARINAKIS, K.I., SHERGOLD, H.L. Influence of sodium silicate addition on the adsorption of oleic acid by fluorite, calcite and barite. International Journal of Mineral Processing, v. 14, p. 177-193, 1985., for 10−3 M of water glass at pH 10, the concentrations of the present species decrease in the following order: [SiO(OH)3-] (~10−3 M), [Si(OH)4] (~10−3.5 M), [SiO2(OH)22-] (~10−4.3 M) and [Si4O6(OH)62-] (~10−6 M). The increased negative zeta potentials of rhodonite and rhodochrosite conditioned with water glass (Figs. 6II and 7II) compared to the minerals without reagent at pH 10 (Figs. 6I and 7I) is likley related to the adsorption of anionic species, particularly [SiO(OH)3-], which is the dominant species at this pH.
The zeta potential of rhodonite without reagent (Fig. 6I) was more negative compared to that mineral conditioned with sodium fluorosilicate (Fig. 6III), which is likely related to the adsorption of Si(OH)4 on the mineral surface. In accordance with Song et al. (2002)SONG, S., VALDIVIESO, A. L., LU, S., OUYANG, J. Selective dispersion in a diaspore-rutile suspension by sodium fluorosilicate. Powder Technology, v. 123, p. 178-184, 2002., Si(OH)4 species are dominant at pH values ranging from 9 to 11.
The lower efficiency of water glass for the depression of rhodonite compared to corn starch (Fig. 3) can be attributed to the more negative charge of the mineral after conditioning with water glass (Fig. 6II) compared to corn starch (Fig. 6V); the adsorption of amine on the quartz surface occurs by physical processes (Fuerstenau et al., 1985FUERSTENAU, M.C., PALMER, R. et alii. Chemistry of Flotation. 1985. 177 p.; Lima (1997)LIMA, R. M. Adsorção de amido e amina sobre as superfícies da hematita e do quartzo e sua influência na flotação. Belo Horizonte: Curso de Pós-Graduação em Engenharia Metalúrgica e de Minas, Universidade Federal de Minas Gerais, 1997. 238 p. DSc. (Tese de Doutorado). and Vidyadhar et al., 2002)VIDYADHAR, A., RAO, H. K., CHERNYSHOVA, I. V., PRADIP, FORSSBERG, K. S. E. Mechanisms of amine-quartz interaction in the absence and presence of alcohols studied by Spectroscopic Methods. Journal of Colloid and Interface Science, v. 256, p. 59-72, 2002., primarily electrostatic attraction (Fuerstenau et al., 1985FUERSTENAU, M.C., PALMER, R. et alii. Chemistry of Flotation. 1985. 177 p.).
Although the concentrations of Mn2+ and MnOH+ at pH 10 are 10−5.8 and 10−5.9 M, respectively (Fig. 1), the smaller negative zeta potential modulus of rhodonite conditioned with Mn2+ (10−3 M; Fig. 6IV) compared to the mineral without reagent (Fig. 6I) is likely ascribed to the adsorption of Mn2+ and MnOH+ on the mineral surface by electrostatic attraction.
The recovery of rhodochrosite increases with increasing sodium fluorosilicate and quebracho concentrations (Fig. 4). This is likely related to the adsorption of negatively-charged SiF62- (fluorosilicate) and negative quebracho, which attract the positive ether ammonium positive ions.
4. Conclusions
Based on the results of micro-flotation tests and zeta potential measurements carried out with manganese minerals (rhodonite and rhodochrosite) with amine and different depressants (inorganic and organic), the recovery of rhodochrosite with amine was very low, likely due to the several ionic species present in solution. The organic depressants were more effective for the depression of rhodonite at pH 10. The strong depression of rhodonite with MnCl2 is mainly related to the deposition of colloidal Mn(OH)2 on the mineral surface. The increased rhodochrosite recovery with increasing water glass and quebracho concentrations can be ascribed to the anionic species of these reagents adsorbed on the mineral surfaces, which attracted the positive ether ammonium ions.
References
- ACEVEDO, G.S. Flotation of silicate-carbonated manganese ores using oleic acid and depressors. In: CASTRO, S., ALVAREZ, J. (Ed.). Avances en flotación. Universidad de Concepción, v. 3, p.50-60, 1977.
- ALCÂNTERA, G. A. G., DUARTE, R. S., MENDONÇA, G. A., LIMA, R. M. F. Estudos de flotabilidade de minerais de manganês e quartzo com amina e quebracho In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E METALURGIA EXTRATIVA (ENTMME), 25 MEETING OF THE SOUTHERN HEMISPHERE ON MINERAL TECHNOLOGY (MSHMT), 8. Proceedings Goiânia-Brazil, 2013.
- ANDRADE, V. L. L. Estudos de concentração do gondito Belo Horizonte: Curso de Pós-Graduação em Engenharia Metalúrgica e de Minas, 1978, p. 70. (Dissertação de Mestrado).
- ANDRADE, E. M., LEÃO, V. A., LIMA, R. M. F., Influência da adição de fluorsilicato de sódio na flotabilidade de minerais de manganês e quartzo com oleato de sódio. REM - Revista Escola de Minas, v. 64, n. 2, p. 219-225, 2011.
- ANDRADE, E. M., COSTA, B. L. C. M., ALCÂNTARA, G. A. G., LIMA, R. M. F. Flotation of manganese minerals and quartz by sodium oleate and water glass. Latin American Applied Research, v. 42, p. 39-43, 2012.
- APLAN, F. F. Manganese. In: Norman L. W. (Ed.). Mineral Processing Handbook Society of Mining Engineering. , 1985. n. 2, p. 27-6, 27-10.
- DUARTE, R. S. Flotação catiônica de finos de minério de manganês sílico-carbonatado Ouro Preto: Programa de Pós-graduação em Engenharia Mineral, Escola de Minas, Universidade Federal de Ouro Preto, 2012, 101 p.
- CHARLET, L., WERSIN, P., ATUMM, W. Surface Charge of MnCO3 and FeCO3. Geochemica et Chosmochimic Acta, v. 54, Issue 8, p. 2329-2336, 1990.
- CIMINELLI, V. S. T., Estudos de mecanismos de adsorção de reagents de flotação em minerais de Gondito Belo Horizonte: Programa de Pós-Graduação em Engenharia Metalúrgica e de Minas, Universidade Federal de Minas Gerais, 1980, 74 p.
- FUERSTENAU, M. C., PALMER, B. R. Anionic flotation of oxides and silicates. In: FUERSTENAU, M. C. (Ed.). Flotation New York: AIME, 1976, v. 1, p. 148-196. (A. M. Gaudin Memorial).
- FUERSTENAU, M.C., PALMER, R. et alii. Chemistry of Flotation. 1985. 177 p.
- LIMA, R. M. Adsorção de amido e amina sobre as superfícies da hematita e do quartzo e sua influência na flotação Belo Horizonte: Curso de Pós-Graduação em Engenharia Metalúrgica e de Minas, Universidade Federal de Minas Gerais, 1997. 238 p. DSc. (Tese de Doutorado).
- LIMA, R.M.F., VASCONCELOS, J.A., SILVA, G.R. Flotação aniônica de minério de manganês. REM - Revista da Escola de Minas, v. 61, p. 337-342, 2008.
- MARINAKIS, K.I., SHERGOLD, H.L. Influence of sodium silicate addition on the adsorption of oleic acid by fluorite, calcite and barite. International Journal of Mineral Processing, v. 14, p. 177-193, 1985.
- NASCIMENTO, D. R., PEREIRA, R. D., LIMA, R. M. F. Influence of sodium silicate on floatability and charge of hematite and quartz with sodium oleate. Latin American Applied Research, v. 43, p.189-191, 2013.
- SAMPAIO, J. A., PENNA, M.T.M. CVRD/Mina do Azul. In: SAMPAIO, J.A., LUZ, A. B., LINS, F. F. (Ed.). Usinas de beneficiamento de minérios do Brasil 2001. p. 93-102.
- SNOEYINK, V. L., JENKINS, D. In: ____. Water chemistry. John Wiley & Sons, 1980. 477 p.
- SONG, S., VALDIVIESO, A. L., LU, S., OUYANG, J. Selective dispersion in a diaspore-rutile suspension by sodium fluorosilicate. Powder Technology, v. 123, p. 178-184, 2002.
- VIDYADHAR, A., RAO, H. K., CHERNYSHOVA, I. V., PRADIP, FORSSBERG, K. S. E. Mechanisms of amine-quartz interaction in the absence and presence of alcohols studied by Spectroscopic Methods. Journal of Colloid and Interface Science, v. 256, p. 59-72, 2002.
Publication Dates
-
Publication in this collection
Oct-Dec 2015
History
-
Received
06 Feb 2015 -
Accepted
02 Sept 2015