Abstract
The flotation process currently considered for apatite concentration from the Santa Quitéria phosphate deposit (Brazil), involves bulk flotation of apatite and calcite with anionic collector at pH=10 followed by calcite flotation at pH=5.5, adjusted with H3PO4. Although this concept is efficient from the point of view of separation between apatite and calcite, the intensive use of inorganic acids causes ion accumulation in the process water, especially Ca2+ and PO4 2-, which leads to problems in the flotation stage as well as in the environment. CETEM has been studying a process for the separation of carbonate minerals and apatite that involves the use of carbonic gas injected into the bubble generation system of flotation machines instead of inorganic acids. The aim of this study was to evaluate the effect of Ca2+ ion concentration on the water during the calcite flotation stage of the Santa Quiteria phosphate ore, between 6 mg/L (standard test) and 670 mg/L (maximun concentration) on the flotation performance in terms of P2O5 grade and its loss and the CaO/P2O5 ratio (RCP). The results indicated a reduction in the selectivity on the flotation of calcite from apatite for Ca2+ concentrations from 6 mg/L to around 285 mg/L. Despite this, the results obtained in this study indicated that the process based on the application of CO2 for the separation of calcite and apatite may be a technical alternative that causes less impact in flotation performance for the phosphate concentration ores with carbonated gangue.
keywords:
process water; calcium ions; calcite; apatite; carbonic gas
1. Introduction
The agribusiness sector is of significant importance for the Brazilian economy. Between 2000 and 2015, fertilizer use in the country went up by 87%. However, the national production of fertilizers is historically lower and did not grow as much as the national demand. As a result, dependence on imports has been increasing year after year and, in 2015, about 65% of the total fertilizer consumption was supplied by imports (Cruz et al., 2017CRUZ, A. C.; PEREIRA, F. D. S.; FIGUEIREDO, V. S. Fertilizantes organominerais de resíduos do agronegócio: avaliação do potencial econômico brasileiro. BNDES Setorial, v. 1, n. 45, mar. 2017, p. 137-187. Available at: https://web.bndes.gov.br/bib/jspui/bitstream/1408/11814/1/BS%2045%20Fertilizantes%20organominerais%20de%20res%C3%ADduos%20%5B...%5D_P_BD.pdf. Accessed: 1 maio 2019.
https://web.bndes.gov.br/bib/jspui/bitst...
). Phosphate rock is a nonrenewable resource, which is the only economically feasible source of phosphate fertilizers. Around 60% of the world’s marketable phosphate are concentrated by the flotation process (Abouzeid, 2008ABOUZEID, A. Z. M. Physical and thermal treatment of phosphate ores - An overview. International Journal of Mineral Processing, v. 85, n. 4, p. 59-84, 2008.; Sis and Chander, 2003SIS, H.; CHANDER, S. Reagents used in the flotation of phosphate ores: a critical review. Minerals Engineering, v. 16, p. 577-585, 2003.). In Brazil, 13 phosphate mines were responsible for the phosphate production in 2014. However, most of the national production - 82% - is located in the states of Minas Gerais and Goiás.
Brazil has some ongoing phosphate mining projects that can increase the national production of phosphate rock concentrate. One of them is the Santa Quitéria uranium-phosphate ore deposit located in the northeast of Brazil. Besides the fertilizer production, another interesting question about this project is that the uranium contained in the apatite mineral supplies the Angra 3 nuclear power plant. The phosphate deposit is hosted in precambrian metamorphic rocks composed of fluorapatite, calcite, quartz and graphite, which represent around 90% of the total composition. Several studies have been carried out aiming to concentrate the apatite from the Santa Quitéria ore, mainly focused on the aspects of calcite and apatite separation by flotation (Aquino and Furtado, 1985AQUINO, J. A.; FURTADO, J. R.V. Flotação reversa aplicada ao minério fósforo-uranífero de Itataia (CE). In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 11., 1985, Natal. Anais [...]. Natal-RN, 1985. p. 31-48.; Albuquerque, 2010ALBUQUERQUE R. O.; PERES A. E. C.; AQUINO J. A.; PEREIRA C. A. Flotation routes for a phosphate ore bearing silicate-carbonate gangue. Revista de La Faculdad de Ingeniería, Atacama, v. 27, p. 26-32, 2012.; Louzada et al., 2010LOUZADA. J. C. G.; AQUINO. J. A.; OLIVEIRA, J. F. Selective flotation of calcite from apatite by using phosphoric acid and citric acid as depressants. In: ZANGH, P.; ZWAGER, K.; LEAL FILHO, L.; EL-SHALL, H. (ed.). Beneficiation of phosphates: technology advance and adoption. Littleton, Colorado: Society for Mining, Metallurgy, and Exploration, 2010. p. 305-310.; Paiva et al. 2010PAIVA, P. R. P.; MONTE, M. B. M.; GASPAR, J. C. Concentração por flotação da apatita proveniente de rochas de filiação carbonatítica. REM: Revista Escola de Minas. v. 64, n.1, p. 111-116, 2011., Matiolo et al., 2016MATIOLO E.; GONZAGA L. M.; GUEDES A. L. An alternative flotation process for apatite concentration of the Santa Quitéria (Brazil) carbonaceous uranium-phosphate ore. In: ZANGH, P.; MILLER, J.; WINGATE, E.; LEAL FILHO, L. (ed.). In: Beneficiation of phosphates: comprehensive extraction, technology innovations and advanced reagents. Englewood, Colorado: Society for Mining, Metallurgy, and Exploration, 2016. p. 81-89.; Santos et al., 2015SANTOS, E. P.; DUTRA, A.J. B.; OLIVEIRA, J. F. The effect of jojoba oil on the surfasse properties of calcite and apatite aiming at their selective flotation. International Journal of Mineral Processing, v. 143, p. 34-38, 2015.). The flowsheet currently considered for industrial scale was designed by the Center for Development of Nuclear Technology, Brazil (CDTN). The flotation process involves bulk flotation of apatite and calcite with anionic collector (fatty acid soap) at pH=10 followed by calcite flotation at pH=5.5 using H3PO4 as an apatite depressant. Calcite is collected in the froth phase and apatite concentrate is the sink product (Aquino and Furtado, 1985AQUINO, J. A.; FURTADO, J. R.V. Flotação reversa aplicada ao minério fósforo-uranífero de Itataia (CE). In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 11., 1985, Natal. Anais [...]. Natal-RN, 1985. p. 31-48.).
The most widely developed flotation process for the treatment of phosphate ores containing carbonate as gangue minerals uses inorganic acids as apatite depressants. Studies have been performed on the flotation process and the fundamental flotation behavior of carbonaceous phosphate ores (Houot, 1982HOUOT, R. Beneficiation of phosphatic ores through flotation: review of industrial applications and potential developments. International Journal of Mineral Processing, v. 9, p. 53-384, 1982.; Zhong et al., 1991ZHONG, K.; VASUDEVAN, T. V.; SOMASUNDARAN, P. Beneficiation of a high dolomitic phosphate ore: a bench scale optimization study. Minerals Engineering, v. 4, n. 4-5, p.563-571, 1991.; Elgillani and Abouzeid, 1993ELGILLANI, D. A.; ABOUZEID, A.Z. M. Flotation of carbonates from phosphate ores in acidic media. International Journal of Mineral Processing, v. 38, p. 235-256, 1993.; El-Shall et al., 2003EL-SHALL, H.; ZHANG, P.; ABDEL KHALEK, N.; EL-MOFTY, S. Beneficiation technology of phosphates: challenges and solutions. Minerals & Metallurgical Processing, v. 21, p. 17-26, 2003.; El-Midany, 2004EL-MIDANY, A.A. Separating dolomite from phosphate rock by reactive flotation: fundamentals and application. 2004. Thesis (PhD) - University of Florida, Gainesville, 2004. ; Abouzeid, 2009ABOUZEID, A. Z. M.; NEGM, A. T.; ELGILLANIA, D. A. Upgrading of calcareous phosphate ores by flotation: effect of ore characteristics. International Journal of Mineral Processing, v. 90, n.1-4, p. 81-89, 2009.). Abdel-Khalek (2000)ABDEL-KHALEK, N. A. Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonate gangues. Technical note. Minerals Engineering, v. 13, n. 7, p. 789-793, 2000. performed studies on different flotation strategies to separate both calcite and silica from a sedimentary phosphate ore from Egypt. Among three different processes for carbonate flotation, the results revealed a better selectivity when phosphoric acid was used as a depressant for phosphate. Al- Fariss et al. (2013)AL-FARISS T. F.; EL-ALEEM F. A. A.; EL-NAGDY, K. A. Beneficiation of Saudi phosphate ores by column flotation technology. Journal of King Saud University - Engineering Sciences, v. 25, p. 113-117, 2013. carried out studies with the AI-Jalamid phosphate rock from Saudi Arabia by column flotation. The optimum conditions achieved were 2.0 kg/ton of Na2SO4 (depressant), 1.82 kg/ton of oleic acid dissolved in kerosene (1:2.5) as a collector, at pH 6.0 - 6.5 (pH adjusted using sodium hydroxide and sulfuric acid). The best experimental beneficiation results were P2O5 grade above 35%, recovery above 95% and a lower CaO:P2O5 ratio of 1.53 from a feed containing 25% P2O5 and CaO:P2O5 2.1.
In order to understand the complex flotation behavior of the carbonate-phosphate system, Elgillani and Abouzeid (1993)ELGILLANI, D. A.; ABOUZEID, A.Z. M. Flotation of carbonates from phosphate ores in acidic media. International Journal of Mineral Processing, v. 38, p. 235-256, 1993. and Abouzeid et al., (2009)ABOUZEID, A. Z. M.; NEGM, A. T.; ELGILLANIA, D. A. Upgrading of calcareous phosphate ores by flotation: effect of ore characteristics. International Journal of Mineral Processing, v. 90, n.1-4, p. 81-89, 2009. concluded that in acidic condition, the depression of phosphate occurs due to the adsorption (or formation) of aqueous CaHPO4 on its surface. Another important issue raised in this study is that free Ca2+ in solution may affect the formation of aqueous CaHPO4. Based on thermodynamic considerations, it may be predicted that selective carbonate flotation from phosphates in acid media can be enhanced by minimizing Ca2+ in the solution and increasing HPO4 2- in the system. Liu at el. (2017LIU, X.; RUAN, Y.; LI, C.; CHENG, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. International Journal of Mineral Processing, v. 167, p. 95-102, 2017.) also developed studies to demonstrate the selective depressive effect of H3PO4.
Although this concept is efficient from the point of view of separation between apatite and carbonates, the intensive use of inorganic acids (>15 kg/t) causes accumulation of ions in the process water, especially Ca2+ and PO4 2-, which leads to problems in the flotation stage and in the environment. Tavares (Personal Comunication. Rio de Janeiro, 2017) reported to CETEM that experimental tests with the Santa Quiteria ore have been developed at pilot scale using the flowsheet proposed by CDTN and achieved the expected process results. However, when the process water is recycled containing high levels of calcium and phosphates ions, it seriously affects apatite recovery.
The composition of the flotation water depends on the ore being processed, the reagent suite, the water source and the way the water system site is managed. For example, salts in solution that cause water hardness are particularly liable to react with fatty acid reagents and form insoluble complexes. Therefore, in an apatite flotation process (using H3PO4 as a depressant) containing high levels of dissolved salts, it is recirculated and the apatite recovery process can be seriously affected. The effects of dissolved species on flotation performance of some minerals have been studied by some authors (Aquino, 1985AQUINO, J. A.; FURTADO, J. R.V. Flotação reversa aplicada ao minério fósforo-uranífero de Itataia (CE). In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 11., 1985, Natal. Anais [...]. Natal-RN, 1985. p. 31-48.; Guimarães and Peres, 1999GUIMARÃES, R. C.; PERES, A. E. C. Interferingions in the flotation of a phosphate ore in a batch column. Minerals Engineering, v. 12, n. 7, p. 757-768, 1999.; Santos, 2010SANTOS, M. A.; SANTANA, R. C.; CAPPONI, F.; ATAIDE, C. H.; BARROZO, M. A. S. Effect of ionic species on the performance of apatite flotation. Separation and Purification Technology, v. 76, p. 15-20, 2010.). Studies published decades ago pointed out as a common experience, low recoveries were achieved when performing flotation with recirculated process water. This would suggest the desirability of applying water treatment methods to improve the quality of the recycled water or search for an alternative flotation process towards a lesser impact on flotation performance.
Mehrotra and Sivaramakrishnan (1986)MEHROTRA, V. P.; SIVARAMAKRISHNAN, K. N. Beneficiation of high carbonate phosphate rock. US Patent 4.568.454. 1986. highlighted the problems associated with the use of inorganic acids as phosphate depressants, including the high costs and contamination of the water supply, thus preventing water recirculation in flotation processes. As an alternative, they patented a process to beneficiate high carbonate phosphate rocks by conditioning the pulp with CO2 and an anionic collector. Carbonate minerals are concentrated in the froth and CO2 acts as an apatite depressant. Along the same lines, Brazil has developed studies on the flotation process for the siliceous carbonate phosphate ore from the Araxá carbonitite (the Barreiro Mine, Brazil) based on calcite/dolomite flotation by using CO2 as a pH modifier and bubble formation (Takata and Shimabukuro 2006TAKATA, L. A.; SHIMABUKURO, N. T. Processo para obtenção de concentrados de apatita. BR n. PI 0504210-0 A. 2006.). Rezende et al. (2011)REZENDE, S. E.; MARTINS, J. S.; TAKATA, L. A.; MATIOLO, E. Processo para obtenção de concentrados de apatita por flotação. BR n. PI 0902233-3. 2011. patented a flotation process for the Catalão Mine that involved the rougher stage of a bulk concentrate containing apatite and carbonate minerals followed by a cleaner stage, where CO2 was added to the pulp through a bubble generation system. As a result, the apatite concentrate was obtained in the froth phase and the carbonate minerals remained in the sink fraction.
As an alternative for the Santa Quiteria ore, CETEM has been studying a process for the separation of calcite and apatite instead of inorganic acids (Matiolo et al., 2016MATIOLO E.; GONZAGA L. M.; GUEDES A. L. An alternative flotation process for apatite concentration of the Santa Quitéria (Brazil) carbonaceous uranium-phosphate ore. In: ZANGH, P.; MILLER, J.; WINGATE, E.; LEAL FILHO, L. (ed.). In: Beneficiation of phosphates: comprehensive extraction, technology innovations and advanced reagents. Englewood, Colorado: Society for Mining, Metallurgy, and Exploration, 2016. p. 81-89.). The flotation process is based on calcite flotation using carbonic gas in association with coconut soap as a calcite collector, followed by apatite flotation with sulphosuccinate as a collector and corn starch as a depressant in bench scale. The results achieved in fine fraction (D50 = 28µm) were similar to those obtained in the coarse fraction (D50 = 100µm), reaching 33% P2O5 and 3.7% SiO2 with 66% mass and 77% P2O5 recoveries.
Due to the lack of information on the influence of Ca2+ ions on the calcite flotation, this study seeks to evaluate the effect of calcium ions on calcite flotation from apatite using carbonic gas in a sample from the Santa Quiteria deposit. Primary experiments were carried out in a batch flotation and the flotation performance was assessed in terms of grade/recovery and loss of P2O5, the ratio CaO/P2O5 (RCP) and the mineralogical characterization.
2. Experiment
2.1 Ore sample
A uranium-phosphate sample from the Santa Quiteria deposit was utilized in this study. The sample preparation involved crushing, grinding, sieve classifying in coarse fraction (> 106 µm) and fine fraction (< 106 µm > 15 µm), desliming and flotation. The experiments were carried out using the sample from the coarse fraction of the circuit. The chemical analysis of the coarse stream was 20.3 % P2O5 grade, 47.7% CaO, 7.1% SiO2, 0.5% MgO and RCP 2.3. The particle size was D80=106 µm and D44=38 µm. The main mineral phases in the sample were apatite - around 50%, calcite - 38% grade and quartz - 6%. These three minerals represent around 95% of the total sample composition. More details on the flowsheet were developed and the ore sample characterization can be found in Matiolo et al. (2016)MATIOLO E.; GONZAGA L. M.; GUEDES A. L. An alternative flotation process for apatite concentration of the Santa Quitéria (Brazil) carbonaceous uranium-phosphate ore. In: ZANGH, P.; MILLER, J.; WINGATE, E.; LEAL FILHO, L. (ed.). In: Beneficiation of phosphates: comprehensive extraction, technology innovations and advanced reagents. Englewood, Colorado: Society for Mining, Metallurgy, and Exploration, 2016. p. 81-89..
2.2 Calcite flotation
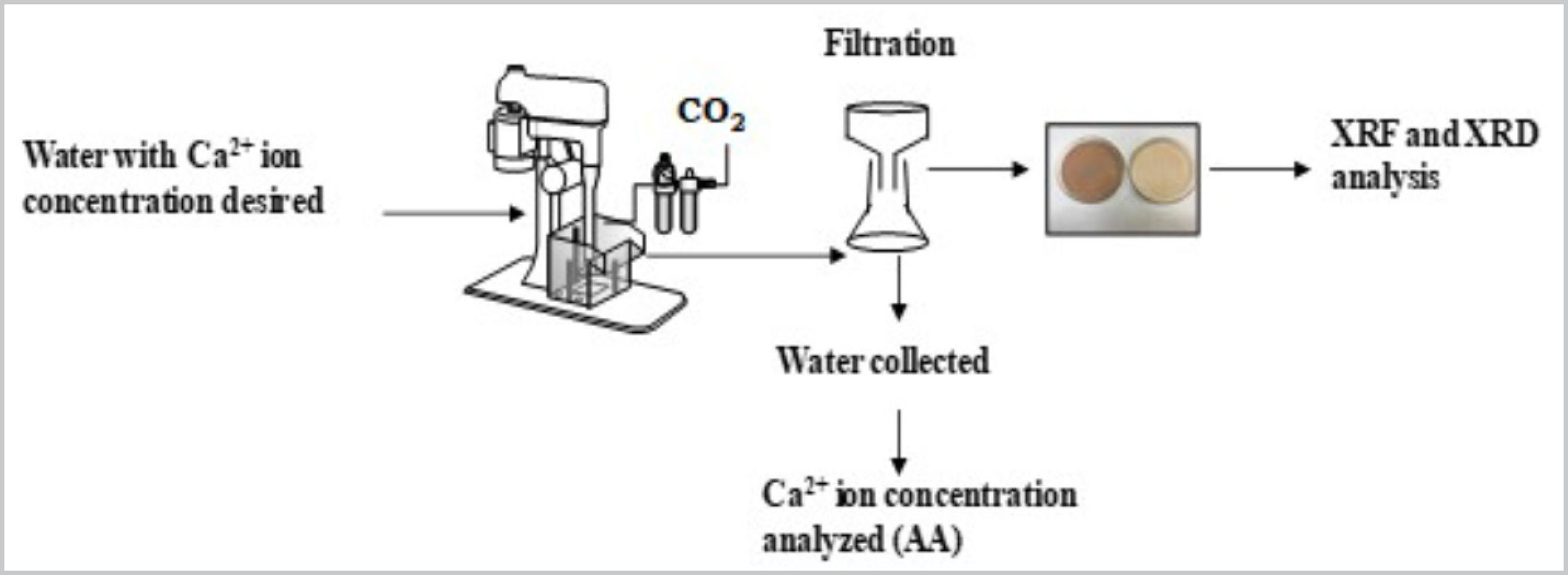
Calcite flotation tests were carried out in a Denver D12 model, bench scale device (Fig.1). In order to assess the calcium concentration, two different strategies were used:
-
Effect of the addition of calcium [Ca2+] between 6 mg/L (standard test) and 670 mg/L (maximun concentration) on the flotation performance in terms of P2O5 grade and loss and the CaO/P2O5 ratio (RCP).
-
Effect of recirculating process water in calcite flotation in terms of P2O5 grade and loss and the CaO/P2O5 ratio (RCP) as well as evaluate the calcium ions concentration in the process water according to recirculating water in the operating cycles.
Chemical analyses were carried out by the X-Ray Fluorescence technique and the mineralogical characterization was performed through X-Ray Diffraction associated with Rietveld refinement to identify and quantify the mineral species. The calcium ions concentration was analyzed through atomic absorption spectrometry (AA).
2.3 Effect of Ca 2+ concentration in calcite flotation
A set of experiments was proposed to evaluate the effect of different calcium ion concentrations. The tests were carried out using a cell with 1.8L. For the first test, tap water from CETEM`s supply network was used (analysed 6mg/L of Ca2+ ions concentration). For subsequent tests, the addition of Ca2+ ions in the process water for each desired concentration was performed through the solubilization of Ca(OH)2. Therefore, the concentrations studied were 95, 246, 419 and 670 mg/L of Ca2+ ions. The calcite flotation tests were carried out with 500g of sample each. The pulp was conditioned with optimum conditions achieved by Matiolo et al. (2016)MATIOLO E.; GONZAGA L. M.; GUEDES A. L. An alternative flotation process for apatite concentration of the Santa Quitéria (Brazil) carbonaceous uranium-phosphate ore. In: ZANGH, P.; MILLER, J.; WINGATE, E.; LEAL FILHO, L. (ed.). In: Beneficiation of phosphates: comprehensive extraction, technology innovations and advanced reagents. Englewood, Colorado: Society for Mining, Metallurgy, and Exploration, 2016. p. 81-89.. Thus, the coconut fatty acid soap collector was fixed at 500 g/t and the conditioning time was 5 minutes (natural pH,8) at a rotation of 800 rpm with 50% solids percentage, already adjusted with the water at the pre-established Ca2+ ion concentration for each test. After conditioning, the pulp was fed to the flotation cell (35% solids) and flotation started with carbonic gas injection at a 2-3 L/min rate through the bubble, generating a system for the flotation machine and as a pH regulator (5.8) and for bubble generation. Rougher flotation was carried out until froth exhaustion and the froth obtained in the rougher fed a cleaner stage that was carried out with carbonic gas rate at 2 L/min and flotation was accomplished until froth exhaustion. In both stages the pulp volume was kept constant with the water at the pre-established Ca2+ ion concentration. The cleaner froth corresponded to the final calcite concentrate (float) and the rougher and cleaner sink fractions were considered tailings (Figure 2). The yielded products (sink and float) were filtered, dried at 100°C and weighed for chemical and mineralogical characterization (XRF and XRD). The calcium ions concentration in the residual water from each calcite flotation test was determited by AA.
Flowsheet illustrating the test on the effect of the addition of calcium ion concentration in calcite flotation.
2.4 Effect of recirculating process water in calcite flotation
These tests sought to evaluate the effect of recirculating process water on calcite flotation performance using carbonic gas by bench scale simulation of the process of water recirculation in industrial plants. The tests were carried out using a different cell size (10L-5L-3L-8L) for each test. The experimental procedure maintained the process conditions described in the previous topic, such as rougher/cleaner stages, solid percentages, conditioning time and collector dosage. After the first cycle ended, with tap water supplied by CETEM, the float and sink products from the calcite flotation test were filtered and the residual water was used to run the second cycle with a new ore sample, and the procedure was repeated until completing the cycles as shown in Figure 3. The yielded products (sink and float) were filtered, dried at 100°C and weighed for chemical and mineralogical characterization (XRF and XRD). The calcium ion concentration in the residual water from each test was determined by AA.
Flowsheet illustrating the test to evaluate the effect of recirculating process water in calcite flotation.
3. Results and discussion
3.1 Effect of Ca 2+ concentration in calcite flotation
Table 1 shows the effect of the Ca2+ ions concentration on the grade/recovery and P2O5 losses from the float and sink products of the calcite flotation. The concentration of Ca2+ studied ranged from 6 mg/L to a maximum value of 670 mg/L. The P2O5 losses of calcite concentrate (float) ranged from 9% to values up to 12%, which may be considered a slight variation. On the other hand, it was observed that the P2O5 grade in the sink fraction decreased, going from 25% for the standard test with 6 mg/L (Ca2+) to 22% for the tests run with 670 mg/L calcium concentration. In addition, an increase of the RCP in the sink fraction, from 1.9 up to 2.2 was observed, showing that calcite recovery was affected by the increase of the Ca2+ ion concentration in the process water. Moreover, in the float fraction, an increase in the P2O5 grade was observed as a function of a calcium concentration increase and RCP decrease to 5.1 considering the Ca2+ concentration (670 mg/L) from values of 7.6 for the lowest ions Ca2+ concentration. These results indicated a reduction in the selectivity of calcite flotation for Ca2+ concentrations studied, as a tendency in the increase of apatite flotation was evidenced by the increase of the P2O5 grade in the float fraction and the decrease of RCP values.
Effect of the Ca2+ ion concentration on the yielded products (sink and float) of the calcite flotation. Collector dosage = 500 g/t; pH 5.8.
The mineralogical compositions of calcite flotation (sink and float fractions) were identified by the Rietveld method (Fig. 4). The results for the apatite grade in the sink fraction varied from 63% - for the test with 6 mg/L Ca2+ concentration - to 57% and, in contrast, an increase in the calcite grade (22.1% - 28.5%) in the sink fraction was observed as it increased the calcium ion concentration in the process water. However, in the float fraction, the apatite grade increased from 14% to 23%, corresponding, respectively, to 6 and 670 mg/L of calcium concentration. In addition, the calcite grade decreased from 80.5% to 71.0% as the calcium ion concentration increased. As for the quartz grade, no variation was observed along the entire range of the calcium ion concentration studies, evidencing that such mineral was not affected by the variation of the ions concentration present in the process water.
Effect of Ca2+ concentration in calcite flotation on mineralogical characterization in the sink (a) and float (b) fractions. Collector dosage = 500 g/t; pH 5.8.
3.2 Effect of recirculating process water in calcite flotation
The effect of recirculating process water in calcite flotation can be observed in Table 2. The concentration values for the Ca2+ ionic species are presented as well as the results of P2O5 grade/recovery and losses in the sink and float fraction. The Ca2+ ion concentration went up from 8 mg/L to 573 mg/L. The P2O5 losses on calcite concentrate (float) ranged from 18% to values up to 24%, in addition to the 11.1% increase of P2O5 grade in the second cycle compared to the first cycle, which was 8.5%. RCP decreased from 6.1 to 4.7. In the first test, the P2O5 grade in the sink fraction was 31% and the values remained constant in the order of 28% in the subsequent tests. However, the RCP varied significantly from 1.4 to 1.7. The results indicated that when the water is recycled in the first cycle, the Ca2+ concentration went up from 8 mg/L to 285 mg/L. The considerable decrease in calcite flotation performace in the presence of high calcium concentrations was due to the increase of P2O5 grade and the decrease of RCP values. Nevertheless, after the second cycle, the increase of the ion concentration did not affect the calcite flotation performance, since the P2O5 grade in the sink fraction remained constant at around 29% and showed P2O5 losses of approximately 21%.
Effect of recirculating process water in calcite flotation on the yielded products (sink and float) of the calcite flotation. Collector dosage = 500 g/t; pH 5.8.
The mineralogical compositions of the yielded products (sink and float) of the calcite flotation were identified by the Rietveld method (Fig. 5). The calcite grade in the float fraction was 78.3% for the lowest calcium ion concentration (8 mg/L) to 71.1% for the maximum calcium ions concentration (573 mg/L), whereas the apatite grade results ranged from 17.2% to 25.0%. In contrast, the apatite grade in the sink fraction was 78.4% for the lowest calcium ion concentration (8 mg/L) and up to 67.6% for the maximum calcium ion concentration (573 mg/L). An increase in the calcite grade is observed in the sink fraction according to the accumulation of calcium ions due to recirculating process water (5.3% to 15.7%). As noted in the previous test, no variation along the entire range of the calcium ion concentration studies for the quartz grade was observed, evidencing that this mineral is not affected by the variation of the concentration of ions present in the process water.
Effect of recirculating process water in calcite flotation on mineralogical characterization in the sink (a) and float (b) fractions. Collector dosage = 500 g/t; pH 5.8.
The study on the effect of recirculating process water showed that the accumulation of Ca2+ ions increased 30% the P2O5 losses on calcite flotation performance when using carbon dioxide in the range of Ca2+ concentrations from 8 mg/L to 285 mg/L. Although the P2O5 grade in the sink fraction and P2O5 losses were practically constant for the Ca2+ ions concentrations above 285 mg/L, the RCP went up from 1.5 to 1.7. Furthermore, the mineralogical composition results showed an increase of calcite grade in the sink fraction 5.3% for the standard test with 6 mg/L to 13.0% for the tests run with 285 mg/L calcium concentration and a decrease in the float fraction from 78.5% to 68.2% for the same range.
The same tendency was observed in the results for the tests carried out adding Ca2+ concentration in calcite flotation. For Ca2+ concentrations ranging from 6mg/L to 246 mg/L, a reduction in the selectivity of calcite flotation was observed, since a tendency in the increase of apatite flotation was evidenced by an increase of the P2O5 grade in the float fraction and a decrease of RCP values (6.1 to 4.7). The mineralogical composition results presented the increase of calcite grade in the sink fraction 22.1% to 24.7% and a decrease in the float fraction from 80.5% to 74.2 % for the same range. Nevertheless, for Ca2+ concentrations above 246 mg/L, the values of P2O5 in the sink fraction and P2O5 losses were practically constant. The mineralogical composition results also proved this tendency.
The results in the both studies for the tests performed with Ca2+ concentrations above 250 mg/L Ca2+ were practically constant. However, for the tests performed with Ca2+ concentrations from 6 mg/L to around 250 mg/L indicated a reduction in the calcite flotation. As a result, the quality of the phosphate concentrate was compromised. One of the alternatives to improve the concentrate quality (sink fraction) would be adding one more flotation stage of the sink fraction. Another point that can also be considered to understand the loss of the concentrate quality is the relationship between the collector and the free Ca2+ ions. According to Hanna and Somasundaran (1976)HANNA H. S.; SOMASUNDARAN, P. Flotation of salt-type minerals. In: FUERSTENAU, M. C. (ed.). Flotation: Gaudin Memorial Volume. New York: American Institute of Mining, Metallurgical, and Petroleum Engineers, 1976. p. 197-272., the collector reacts with the calcium present in the apatite and thus the Ca2+ addition consumes the available collector for the mineral, resulting in a significant reduction in the apatite recovery. Considering that the collector reacts with the calcium present in the calcite as well as with the free Ca2+ ions, the calcite flotation must be conducted with a higher collector dosage in order to reach the same flotation performance of contamination-free phosphate rock.
There are several studies in literature focused on the evaluation of the effects of the ions concentration on apatite/carbonates flotation, however, full understanding of the mechanism is still a challenge. Guimarães and Peres (1999)GUIMARÃES, R. C.; PERES, A. E. C. Interferingions in the flotation of a phosphate ore in a batch column. Minerals Engineering, v. 12, n. 7, p. 757-768, 1999. developed a batch flotation column to evaluate the effect of ions (added in the conditioning stage) on barite followed by apatite flotation of oxidized phosphate ore from Araxá - Brazil. Recovery decreased significantly as the ion concentration increased in both floation stages. In apatite flotation, the Ca2+ and Mg2+ ions reacted with the apatite collector (rice bran oil soap), forming insoluble soaps of rice oils, reducing the collector level in the system. Based on these results, limited ion concentrations - 20 mg/L Ca2+ and 30 mg/L Mg2+ - were proposed to ensure the quality of recirculating water without impairing the flotation process. For the same oxidise phosphate ore, Santos et al. (2010)SANTOS, M. A.; SANTANA, R. C.; CAPPONI, F.; ATAIDE, C. H.; BARROZO, M. A. S. Effect of ionic species on the performance of apatite flotation. Separation and Purification Technology, v. 76, p. 15-20, 2010. presented the effects of the Ca2+ ions concentration on apatite recovery. Their results showed that high concentrations of calcium (300 mg/L) were harmful to the process, causing a sharp decrease in apatite recovery to values below 10%. Aquino (1987b)AQUINO, J. A. Influência de alguns íons sobre a flotação de apatita do minério de Itataia. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 12.; ENCONTRO DO HEMISFÉRIO SUL SOBRE TECNOLOGIA MINERAL, 2., 1987, Rio de Janeiro. Anais [...]. Rio de Janeiro, 1987. p. 538-552. carried out studies with the Santa Quitéria ore evaluating the effects of the calcium and magnesium ions concentration on the bulk concentration of calcite and apatite. The results indicated that recovery decreased from 94.2% to 53.3% for P2O5 and 89.6% to 37.5% for CaCO3 for concentrations of 49 mg/L Ca2+ and 30 mg/L Mg2+.
Even though the accumulation of calcium ions affects the quality of phosphate concentrate using carbonic gas, according to Mehrotra and Sivaramakrishnan (1986)MEHROTRA, V. P.; SIVARAMAKRISHNAN, K. N. Beneficiation of high carbonate phosphate rock. US Patent 4.568.454. 1986. the process can be a technical alternative that causes less impact in process performance when comparing with the other studies abovementioned.
4. Conclusions
The results showed that Ca2+ ions accumulation reduces the selectivity of the calcite flotation process using carbonic gas, as a pH regulator and bubble generation and fatty acid soap as a collector. The reduction on the flotation performance is evidenced in the tests carried out with Ca2+ concentrations increased from 6 mg/L to around 250 mg/L. However, for the tests performed with Ca2+ concentrations above 250 mg/L Ca2+, the process results were practically constant. Even though the accumulation of calcium ions affects the quality of phosphate concentrate (sink fraction) when using carbonic gas, the process can be a technical alternative that causes less impact on flotation performance between carbonate minerals and apatite.
Acknowledgements
The authors would like to thank all the Brazilians institutes supporting research, namely CNPq by GRANT Number 426023/2016-1, CETEM/MCTIC and UFRGS. Special thanks is also extended to Industrias Nucleares do Brasil (INB) and Yara-Galvani for permission to publish this work.
References
- ABDEL-KHALEK, N. A. Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonate gangues. Technical note. Minerals Engineering, v. 13, n. 7, p. 789-793, 2000.
- ABOUZEID, A. Z. M. Physical and thermal treatment of phosphate ores - An overview. International Journal of Mineral Processing, v. 85, n. 4, p. 59-84, 2008.
- ABOUZEID, A. Z. M.; NEGM, A. T.; ELGILLANIA, D. A. Upgrading of calcareous phosphate ores by flotation: effect of ore characteristics. International Journal of Mineral Processing, v. 90, n.1-4, p. 81-89, 2009.
- AL-FARISS T. F.; EL-ALEEM F. A. A.; EL-NAGDY, K. A. Beneficiation of Saudi phosphate ores by column flotation technology. Journal of King Saud University - Engineering Sciences, v. 25, p. 113-117, 2013.
- ALBUQUERQUE R. O.; PERES A. E. C.; AQUINO J. A.; PEREIRA C. A. Flotation routes for a phosphate ore bearing silicate-carbonate gangue. Revista de La Faculdad de Ingeniería, Atacama, v. 27, p. 26-32, 2012.
- AQUINO, J. A. Influência de alguns íons sobre a flotação de apatita do minério de Itataia. In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 12.; ENCONTRO DO HEMISFÉRIO SUL SOBRE TECNOLOGIA MINERAL, 2., 1987, Rio de Janeiro. Anais [...] Rio de Janeiro, 1987. p. 538-552.
- AQUINO, J. A.; FURTADO, J. R.V. Flotação reversa aplicada ao minério fósforo-uranífero de Itataia (CE). In: ENCONTRO NACIONAL DE TRATAMENTO DE MINÉRIOS E HIDROMETALURGIA, 11., 1985, Natal. Anais [...] Natal-RN, 1985. p. 31-48.
- CRUZ, A. C.; PEREIRA, F. D. S.; FIGUEIREDO, V. S. Fertilizantes organominerais de resíduos do agronegócio: avaliação do potencial econômico brasileiro. BNDES Setorial, v. 1, n. 45, mar. 2017, p. 137-187. Available at: https://web.bndes.gov.br/bib/jspui/bitstream/1408/11814/1/BS%2045%20Fertilizantes%20organominerais%20de%20res%C3%ADduos%20%5B...%5D_P_BD.pdf Accessed: 1 maio 2019.
» https://web.bndes.gov.br/bib/jspui/bitstream/1408/11814/1/BS%2045%20Fertilizantes%20organominerais%20de%20res%C3%ADduos%20%5B...%5D_P_BD.pdf - ELGILLANI, D. A.; ABOUZEID, A.Z. M. Flotation of carbonates from phosphate ores in acidic media. International Journal of Mineral Processing, v. 38, p. 235-256, 1993.
- EL-MIDANY, A.A. Separating dolomite from phosphate rock by reactive flotation: fundamentals and application. 2004. Thesis (PhD) - University of Florida, Gainesville, 2004.
- EL-SHALL, H.; ZHANG, P.; ABDEL KHALEK, N.; EL-MOFTY, S. Beneficiation technology of phosphates: challenges and solutions. Minerals & Metallurgical Processing, v. 21, p. 17-26, 2003.
- GUIMARÃES, R. C.; PERES, A. E. C. Interferingions in the flotation of a phosphate ore in a batch column. Minerals Engineering, v. 12, n. 7, p. 757-768, 1999.
- HANNA H. S.; SOMASUNDARAN, P. Flotation of salt-type minerals. In: FUERSTENAU, M. C. (ed.). Flotation: Gaudin Memorial Volume. New York: American Institute of Mining, Metallurgical, and Petroleum Engineers, 1976. p. 197-272.
- HOUOT, R. Beneficiation of phosphatic ores through flotation: review of industrial applications and potential developments. International Journal of Mineral Processing, v. 9, p. 53-384, 1982.
- LIU, X.; RUAN, Y.; LI, C.; CHENG, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. International Journal of Mineral Processing, v. 167, p. 95-102, 2017.
- LOUZADA. J. C. G.; AQUINO. J. A.; OLIVEIRA, J. F. Selective flotation of calcite from apatite by using phosphoric acid and citric acid as depressants. In: ZANGH, P.; ZWAGER, K.; LEAL FILHO, L.; EL-SHALL, H. (ed.). Beneficiation of phosphates: technology advance and adoption. Littleton, Colorado: Society for Mining, Metallurgy, and Exploration, 2010. p. 305-310.
- MATIOLO E.; GONZAGA L. M.; GUEDES A. L. An alternative flotation process for apatite concentration of the Santa Quitéria (Brazil) carbonaceous uranium-phosphate ore. In: ZANGH, P.; MILLER, J.; WINGATE, E.; LEAL FILHO, L. (ed.). In: Beneficiation of phosphates: comprehensive extraction, technology innovations and advanced reagents. Englewood, Colorado: Society for Mining, Metallurgy, and Exploration, 2016. p. 81-89.
- MEHROTRA, V. P.; SIVARAMAKRISHNAN, K. N. Beneficiation of high carbonate phosphate rock US Patent 4.568.454. 1986.
- PAIVA, P. R. P.; MONTE, M. B. M.; GASPAR, J. C. Concentração por flotação da apatita proveniente de rochas de filiação carbonatítica. REM: Revista Escola de Minas v. 64, n.1, p. 111-116, 2011.
- REZENDE, S. E.; MARTINS, J. S.; TAKATA, L. A.; MATIOLO, E. Processo para obtenção de concentrados de apatita por flotação BR n. PI 0902233-3. 2011.
- SANTOS, E. P.; DUTRA, A.J. B.; OLIVEIRA, J. F. The effect of jojoba oil on the surfasse properties of calcite and apatite aiming at their selective flotation. International Journal of Mineral Processing, v. 143, p. 34-38, 2015.
- SANTOS, M. A.; SANTANA, R. C.; CAPPONI, F.; ATAIDE, C. H.; BARROZO, M. A. S. Effect of ionic species on the performance of apatite flotation. Separation and Purification Technology, v. 76, p. 15-20, 2010.
- SIS, H.; CHANDER, S. Reagents used in the flotation of phosphate ores: a critical review. Minerals Engineering, v. 16, p. 577-585, 2003.
- SOMASUNDARAN, P. On the problem of separation of calcite from calcareous apatite, beneficiation of leanphosphates with carbonate gangue. In: INTERNATIONAL MINERAL PROCESSING CONGRESS, 11th, 1975, Cagliari. Proceedings [...] Cagliari: Itália, 1975. V. 2, p.155-156.
- SOMASUNDARAN, P.; AMANKONAH J. O.; ANANTHAPADMANABHAN, K.P. Mineral-solution equilibria in sparingly soluble mineral systems. Colloids and Surfaces, v.15, p. 309-333, 1985.
- TAKATA, L. A.; SHIMABUKURO, N. T. Processo para obtenção de concentrados de apatita BR n. PI 0504210-0 A. 2006.
- ZHONG, K.; VASUDEVAN, T. V.; SOMASUNDARAN, P. Beneficiation of a high dolomitic phosphate ore: a bench scale optimization study. Minerals Engineering, v. 4, n. 4-5, p.563-571, 1991.
Publication Dates
-
Publication in this collection
17 Apr 2020 -
Date of issue
Apr-Jun 2020
History
-
Received
01 May 2019 -
Accepted
31 Dec 2019