Abstract
Abstract:Hypostomus commersoni Valenciennes 1836, Hypostomus cordovae (Günther 1880) and Hypostomus laplatae (Eigenmann 1907) have been little studied since their original descriptions. This study shows a comprehensive review of these species from the Lower La Plata Basin, including their taxonomic history, distribution, color patterns, morphology, and ecological and molecular phylogenetic data. Morphological and phylogenetic analyses based on D-loop sequences suggested that H. commersoni can be separated into two subclades, or subgroups. Based on these results and on the non-overlapping distribution range of the two subclades, we conclude that they represent two distinct species, thereby revalidating H. spiniger. The results also suggest that H. paranensis should be considered as species inquirenda and H. cordovae as valid species. This integrated approach provides key information for assessing the conservation status and biogeographic aspects of the genus Hypostomus in the Lower La Plata Basin.
Key words
Argentina; Brazil; freshwater fishes; molecular phylogenetics; Paraná Basin
INTRODUCTION
Within the family Loricariidae, Hypostomus Lacépède 1803 is the most diverse genus, with more than 146 valid species (EschmeyerESCHMEYER WN, FRICKE R and VAN DER LAAN R. 2017. Catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Electronic version accessed 17 Set 2017. [This version was edited by Bill Eschmeyer].
http://researcharchive.calacademy.org/re...
et al. 2017). The species are widely distributed in South America, from Costa Rica to the Salado River Basin in Argentina (RingueletRINGUELET RA. 1975. Zoogeografía y ecología de los peces de aguas continentales de la Argentina y consideraciones sobre las áreas ictiológicas de América del Sur. Ecosur 2: 1-122. 1975). Two recent studies show the phylogenetic relationships of several species of Hypostomus in the southern part of its distribution, which comprises the Lower Paraná and the Río de la Plata Basin (CardosoCARDOSO YP, ALMIRÓN A, CASCIOTTA J, AICHINO D, LIZARRALDE M and MONTOYA-BURGOS JI. 2012. Origin of species diversity in the catfish genus Hypostomus (Siluriformes: Loricariidae) inhabiting the Paraná River basin, with the description of a new species. Zootaxa 3453: 69-83. et al. 2012, 2016CARDOSO YP, BRANCOLINI F, PARACAMPO A, LIZARRALDE M, COVAIN R and MONTOYA-BURGOS JI. 2016. Hypostomus formosae, a new catfish species from the Paraguay River Basin with redescription of H. boulengeri (Siluriformes: Loricariidae). Ichthyol Explor Freshwaters 27: 9-23. ).
Ca et al. (2012) raised the question of the sources of diversity in Hypostomus in the La Plata Basin, showing that the richness of species has been shaped by both inter- and intra-basin pressures. However, no further information on the biology, validated distribution, ecology, taxonomic status, or abundance of most of the species inhabiting the basin is available. More than 20 species of Hypostomus are recorded in the Lower La Plata Basin (KoerberKOERBER S and WEBER C. 2014. The Hypostominae (Siluriformes: Loricaridae) of Argentina. Ichthyol Contrib PecesCriollos 29: 1-10. and Weber 2014, LitzLITZ TO and KOERBER S. 2014. Check List of the Freshwater Fishes of Uruguay (CLOFF-UY). Ichthyol Contrib PecesCriollos 28: 1-40. and Koerber 2014, MirandeMIRANDE JM and KOERBER S. 2015. Check List of the Freshwater Fishes of Argentina (CLOFF-AR). Ichthyol Contrib PecesCriollos 36: 1-68. and Koerber 2015), but this is likely to be underestimated. This gap in basic biological data makes obtaining thorough knowledge of the evolutionary and biogeographical events shaping this group of catfish a challenge.
The present study contributes to a more complete assessment of species diversity and expands available biological data on Hypostomus species inhabiting the Lower La Plata Basin. This work focuses on H. commersoniValenciennes 1836VALENCIENNES A. 1834-39. Poissons [plates]. In: A. d’Orbigny. Voyage dans l’Amérique méridionale. Pls. 1-16., H. cordovae (Günther 1880), and H. laplatae (Eigenmann 1907EIGENMANN CH. 1907. On a collection of fishes from Buenos Aires. Proc Washigt Acad Sci 8: 449-458.), species that have been little studied since their original description. The descriptions are outdated and imprecise, and subsequent taxonomic work has often been undertaken without examination of the type material or was based on a small number of specimens or a limited distribution range. Here, an integrated approach, based on taxonomic history, geographic distribution, morphology, morphometrics, live color pattern, and ecological and molecular phylogenetic data of the type-series, together with newly collected topotypes of these species, were used to re-evaluate their taxonomy.
MATERIALS AND METHODS
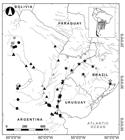
Fishes were collected with gillnets, trammel nets, hand nets, and cast nets. Locations sampled are presented in Fig. 1. A tissue sample was taken from each specimen for molecular analysis, preserved in 96% ethanol, and stored at -20°C. Voucher specimens were fixed in 4% formalin and deposited in the ichthyology collection of the Fundación de Historia Natural Félix de Azara, Buenos Aires, Argentina (CFA-IC). Specimens and photographs of type series were examined from the AMNH, American Museum of Natural History, New York; BMNH, British Natural History Museum, London; CAS, California Academy of Sciences, San Francisco; MACN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Buenos Aires; MCP, Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre; MFA-ZV, Museo Provincial “Florentino Ameghino,” Santa Fe; MHNG, Muséum d’Histoire Naturelle, Genève; MNHN, Museum National d’Histoire Naturelle, Paris; MLP, Museo de La Plata, Buenos Aires, and ZMB, Zoological Museum of Berlin, Berlin (see Appendix).
Map of collection localities of Hypostomus commersoni (triangle), H. spiniger (star), H. cordovae (circles) and H. laplatae (squares). White symbols are type localities and grey symbols are specimens used only in molecular analyses.
Total DNA was obtained using the salt-extraction protocol (AljanabiALJANABI SM and MARTINEZ I. 1997. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res 25: 4692-4693. and Martinez 1997). The PCR amplification of the control region (D-loop) of the mitochondrial DNA was performed as in CardosoCARDOSO YP, BRANCOLINI F, PROTOGINO L and LIZARRALDE M. 2011. Actinopterygii, Siluriformes, Loricariidae, Hypostomus aspilogaster (Cope, 1894). Distribution extension and first record for Argentina. Check List 7: 596-598. et al. (2011). The PCR products were purified and sequenced by Macrogen Inc. (Korea). New sequences were deposited in GenBank for: H. commersoni (JF290450 to JF290458, MG457220 to MG457223, MG457234 to MG457242), H. cordovae (KX852401 to KX852410), H. laplatae (KX852411 to KX852418), and H. spiniger (MG457224 to MG457233). Also, published data for Hypostomus (Montoya-BurgosMONTOYA-BURGOS JI. 2003. Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Mol Ecol 12: 1855-1867. 2003, Cardoso et al. 2012, 2016) were used, so some specimens were incorporated only in the molecular analyses (see Fig. 1). In total, 109 sequences were edited and manually aligned using BioEdit 7.0.1 (HallHALL T. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symposium Ser 41: 95-98. 1999). Two phylogenetic analyses were conducted, regardless of species assignment. Appropriate substitution models were selected with the Akaike information criterion (AIC) and the phylogeny was inferred using maximum likelihood (ML) implemented in MEGA.7 (KumarKUMAR S, STECHER G and TAMURA K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 33: 1870-1874. et al. 2016). Confidence values for the limits of the ML tree were computed with 1000 bootstrap replications (FelsensteinFELSENSTEIN J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783-791. 1985). Subsequently, Bayesian inference (BI) analysis was conducted in MrBayes 3.2.2 (HuelsenbeckHUELSENBECK JP and RONQUIST F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754-755. and RonquistRONQUIST F, TESLENKO M, VAN DER MARK P, L AYRES D, DARLING A, HÖHNA S and HUELSENBECK JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61: 539-542. 2001, Ronquist et al. 2012) on CIPRES Science Gateway (MillerMILLER MA, PFEIFFER W and SCHWARTZ T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE): 1-8. et al. 2010). Four chains were run simultaneously (three heated, one cold) for 30 million generations, with sampling every 500 generations. Following graphic analysis of the evolution of the likelihood scores, the first 25% of generations were discarded as burn-in. The remaining trees were used to calculate the consensus tree.
Measurements were made using an Essex digital electronic calipers with 0.1 mm accuracy following the methods of BoesemanBOESEMAN M. 1968. The genus Hypostomus Lacépède, 1803, and its Surinam representatives (Siluriformes, Loricariidae). Zool Verh 99: 1-89. (1968) and WeberWEBER C. 1985. Hypostomus dlouhyi, nouvelle espéce de poisson-chat cuirassé du Paraguay (Pisces Siluriformes, Loricariidae). Rev Suisse Zool 92: 955-968. (1985). Morphometric characteristics are expressed as percentage of standard length (SL) or head length (HL). Analysis included 21 continuous morphometric variables and 12 discrete meristic variables of 91 specimens (Tables I and II). Missing data were estimated using the least-squares method with SL as explanatory variable. All measurements were standardized according to SL and log-transformed to control for size. This transformation, equivalent to the additive log ratio of AitchinsonAITCHINSON J. 1986. The Statistical Analysis of Compositional Data. London: Chapman & Hall, 416 p. (1986), controls for size effect, preserves and linearizes allometric growth, and prevents spurious correlations of simple ratios (AtchleyATCHLEY R, GASKINS CT and ANDERSON D. 1976. Statistical properties of Ratios. I. Empirical results. Syst Zool 25: 137-148. et al. 1976, Atchley and Anderson 1978ATCHLEY WR and ANDERSON D. 1978. Ratios and the statistical analysis of biological data. Syst Zool 27: 71-78., HillsHILLS M. 1978. On ratios - a response to Atchley, Gaskins, and Anderson. Syst Zool 27: 61-62. 1978). The data were submitted to a between-class analysis (BCA), a particular case of principal component analysis (PCA) with respect to instrumental variables in which there is only a single factor as explanatory variable. In this case, species was used as the explanatory factor. To explore the more closely related species, a second analysis, based on 30 specimens of H. commersoni, 31 of H. spiniger, five H. affinis (SteindachnerSTEINDACHNER F. 1877. Die Süsswasserfische des südöstlichen Brasilien (III). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe 74. 1877), two H. interruptus (Miranda Ribeiro 1918) and three H. ancistroides (Ihering 1911) was submitted to further BCA. Both analyses were performed with the ade4 1.4-14 (DrayDRAY S and DUFOUR AB. 2007. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22: 1-20. and Dufour 2007) package in R 3.4.1 (DR Core team 2014R CORE TEAM. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
http://www.R-project.org/...
).
Assessment of the conservation threat level of the analyzed species was primarily based on the application of the IUCN Red List Criterion B (IUCN 2001IUCN. 2001. IUCN Red List categories and criteria. Version 3.1. IUCN Species Survival Commission, Gland, Switzerland and Cambridge, U.K.). This criterion focuses on two spatial measures related to the distribution of the species: area of occupancy (AOO) and extent of occurrence (EOO) (IUCN 2017). Both measures were performed with the ConR (DaubyDAUBY G. 2017. Computation of Parameters Used in Preliminary Assessment of Conservation Status. Available from: https://cran.r-project.org/web/packages/ConR/ConR.pdf. (Date of access - September 2017).
https://cran.r-project.org/web/packages/...
2017) package in R 3.4.1 (R Core team 2014) according to IUCN (2017IUCN STANDARDS and PETITIONS SUBCOMMITTEE. 2017. Guidelines for using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee.Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (Date of access - March 2017).
http://www.iucnredlist.org/documents/Red...
).
RESULTS
PHYLOGENETIC ANALYSES
Mitochondrial D-loop sequences were obtained from several specimens recently collected in Argentina and Brazil, covering a wide distribution range in the Lower La Plata Basin and Dos Patos Lagoon. The alignment of the mitochondrial D-loop region comprised 584 positions (from which 167 were variable) and 100 sequences of Hypostomus, plus nine outgroups. The best-fit substitution model found for the dataset was GTR+I+G. Evolutionary relationships within the genus (Fig. 2) were similar to those reported by Montoya-Burgos (2003) and Cardoso et al. (2012, 2016), with four main lineages: D1, D2, D3, and D4 (Fig. 2). However, in the analyses, the statistical support was generally low among species, generating polytomies in the BI tree due to the collapse of some branches supported by posterior probabilities < 0.5. Nevertheless, in both the ML and BI analyses, all specimens collected in the Lower Río de la Plata Basin appeared in four strongly supported and reciprocally monophyletic clades. These phylogenetic results indicated that the specimens identified as H. commersoni are organized into two non-sister subclades. One subclade comprises the specimens collected in the Uruguay River and Dos Patos Lagoon, corresponding to the species previously recognized as H. spiniger, which is revalidated here and is the name that will be used henceforth (see Remarks section of Results and Discussion).
Maximum likelihood tree based on the mitochondrial D-loop region. Numbers next to branches are bootstrap values (1000 pseudoreplicates) followed by Bayesian posterior probabilities when these are above 50% or 0.5. Specimens of H. laplatae, H. cordovae, H. commersoni and H. spiniger sequenced in this work are in bold and their field number are given.
The phylogenetic tree shows H. cordovae, H. commersoni, and H. spiniger grouped in lineage D2 (Fig. 2). This lineage also contains other species from the La Plata Basin (e.g. H. boulengeri, H. formosae, H. ancistroides, and H. derby); H. watwata and H. plecostomus from the Guyana Shield; and H. affinis, H. punctatus, and H. interruptus from Eastern South America coastal rivers. According to the present analysis H. spiniger forms a clade with H. affinis, H. ancistroides, and H. interruptus instead of being the sister group of H. commersoni.
Specimens of H. laplatae were grouped within the D4 lineage, principally composed of species from La Plata Basin: H. aspilogaster, H. luteus, H. isbrueckeri, H. uruguayensis, H. latifrons, H. arecuta, H. ternetzi, H. albopunctatus, and H. latirostris.
MORPHOMETRIC ANALYSES
The first morphological dataset, comprising the four analyzed species, showed a clear structure on the first two axes of the BCA (Fig. 3c). The species were chiefly aligned along the first axis (Fig. 3a). On the negative side of axis 1, H. cordovae corresponded to high number of dentary and premaxillary teeth, inter-dorsal distance, and caudal-peduncle length (Fig. 3b). On the positive side of axis 1, H. commersoni and H. spiniger are characterized by high values of snout length, inter-orbital width, pelvic-fin spine length, orbit diameter. On the negative side of axis 2, H. laplatae corresponded to high values for dorsal plates below the dorsal-fin base, lateral scute series, plates bordering the supraoccipital, and postanal plates. The second morphological dataset was based on the five most closely related species of the D2 lineage defined in the phylogenetic tree. This BCA was mainly structured on the first two axes (Fig. 4c). The first axis separated the specimens of H. commersoni and H. spiniger, while the second separated H. affinis, H. ancistroides, and H. interruptus (Fig. 4a). On the negative side of axis 1, H. commersoni corresponded to high number of dentary and premaxilla teeth, number of plates between adipose and caudal fins, number of plates between dorsal and adipose fins, thoracic length, mandibular ramus length, caudal-peduncle depth, and orbit diameter (Fig. 4b).
Between-class Analysis (BCA) of morphological data of H. laplatae, H. cordovae, H. commersoni and H. spiniger. a. Projection of individuals’s scores onto first factorial plane of BCA, axis 1 horizontal and axis 2 vertical; b, Correlation of the variables labelled as in Tables I and II; c, Eigenvalues.
Between-class Analysis (BCA) of morphological data of H. commersoni, H. spiniger, H. affinis, H. punctatus and H. ancistroides. a. Projection of individuals’s scores onto first factorial plane of BCA, axis 1 horizontal and axis 2 vertical; b, Correlation of the variables labelled as in Tables I and II; c, Eigenvalues.
Hypostomus commersoni Valenciennes 1836
Hypostomus commersoni Valenciennes 1836: Pl. 7. Type locality: Río de La Plata, Montevideo, Uruguay.
Hypostomus commersoni Valenciennes 1836, in Valenciennes 1835-47: pl. 7 (Fig. 5). Type locality: not stated (considered to have come from La Plata River, Uruguay). Holotype or lectotype: MNHN a-9444 (Fig. 5). Name made available from caption to plate, with as illustrated specimen the holotype (if recognizable). Described in Cuvier and Valenciennes (1840b: 495, 366 of Strasbourg deluxe edition).
Hypostomus commersoni: (a) holotype, MNHN A-9444, Dorsal view. (Photograph: Zawadzki, Claudio); (b) Live specimen from Arroyo El Morejón, Campana, Buenos Aires.
DIAGNOSIS
Hypostomus commersoni can be differentiated from all remaining species of the genus, except H. affinis, H. punctatus, H. ancistroides, and H. spiniger, by having the following features: bifid teeth (vs. spoon-shaped teeth), dark spots on a light background (vs. light spots on a dark background or not spotted), possession of four rough lateral ridges on flanks (vs. lacking strong ridges on flanks), 26-29 lateral series plates (vs. fewer than 28 in several species or 31-32 in H. laplatae), 1-2 plates bordering the posterior margin of the supraoccipital bone (vs. 3-4 in H. laplatae). Hypostomus commersoni can be distinguished from H. affinis, H. punctatus, and H. ancistroides by inter-dorsal distance (14.4-19.5% vs. 18.9-21.0% in SL in H. affinis, H. punctatus, and H. ancistroides) and head depth (49.0-71.6% vs. 48.2-49.9 in HL in H. affinis, H. punctatus, and H. ancistroides). It can be differentiated from H. spiniger by having fine lateral ridges with odontodes posteriorly inclined (vs. large lateral ridges with odontodes in all directions), weak lateral ridges in caudal peduncle (vs. strong lateral ridges in caudal peduncle), mid lateral ridge from first plate (vs. mid lateral ridge from 2nd or 3rd plates). Adult of H. commersoni (more than 120mm SL) can be also distinguished from adult of H. spiniger by having a strong ridge on temporal plate (vs. very weak ridge on temporal plate) (Fig. 6).
Details of diagnostic characters between (a, c, e) H. spiniger and (b, d, f) H. commersoni. (a, b) lateral ridges; (c, d) lateral ridges in caudal peduncle; (e, f) mid lateral-rigde origin and rigde in temporal plate.
According to the BPA (Fig. 4), other morphological characters might differentiate H. commersoni from H. spiniger but the range of these features partially overlaps: dentary and premaxillary teeth, plates between adipose and caudal fins, plates between dorsal and adipose fins, thoracic length, mandibular ramus length, caudal-peduncle depth, and orbit diameter, and by lower values of predorsal length and dorsal-fin spine length.
DESCRIPTION
Morphometric and meristic data in Table I. Overall view of body shape in Fig. 5. Dorsal profile rather straight from snout tip to interorbital area. Dorsal plates between end of dorsal-fin and adipose-fin spine flattened. Body width at cleithral region greater than head depth. Head covered dorsally with plates. Mouth rounded, lower lip not reaching transversal through gill openings, ventral surface covered with numerous small papillae. Dorsal-fin rays II,7; slightly convex. Adipose-fin spine curved inward. Pectoral-fin rays I,6; posterior border straight. Pectoral-fin spine slightly curved inward surpassing pelvic-fin origin when depressed. Pelvic-fin rays I,5; posterior border slightly curved. Pelvic-fin spine surpassing anal-fin origin when depressed. Anal-fin rays I,4; tip reaching seventh plate after its origin. Anal-fin rays progressively increasing in size, third branched ray usually longest. Caudal-fin rays I,14,I. Lower caudal-ray longer than upper.
Morphometric and meristic data of Hypostomus commersoni and H. spiniger. Range, mean (or mode for the meristic data), standard deviation (SD) and number of specimens (n). Morphometric and meristic variables used in the BCA analysis (Figs. 3 and 4) are showed with abbreviations in squared brackets.
COLORATION
Background color of head and trunk dark brown or gray with small, irregular-shaped black spots on head and larger and more widely-spaced spots on dorsum, some specimens with more prominent dots. All fins dark brown or gray with inter-radial membranes hyaline and irregularly-shaped small black spots. Abdominal region light brown or gray, usually with vermiculated spots but some specimens without. Coloration is more pronounced in live specimens.
GEOGRAPHIC DISTRIBUTION
Hypostomus commersoni inhabits the Lower Paraná, including the Iguazú (GaravelloGARAVELLO JC, BRITSKI HA and ZAWADZKI CH. 2012. The cascudos of the genus Hypostomus Lacépède (Ostariophysi: Loricariidae) from the rio Iguaçu basin. Neotrop Ichthyol 10: 263-283. et al. 2012), Paraguay, Río de la Plata, and Dulce Rivers. In a thorough survey of Hypostomus species from the Paraíba do Sul River, MazzoniMAZZONI R, CARAMASCHI U and WEBER C. 1994. Taxonomical revision of the species of Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae) from the Lower rio Paraiba do Sul, State of Rio de Janeiro, Brazil. Rev Suisse Zool 5: 3-18. et al. (1994) concluded that H. commersoni does not occur in this river basin, and that previous citations of this species in this river were misidentifications. Similarly, OyakawaOYAKAWA OT, AKAMA A and ZANATAAM. 2005. Review of the genus Hypostomus Lacépède, 1803 from rio Ribeira de Iguape basin, with description of a new species (Pisces, Siluriformes, Loricariidae). Zootaxa 921: 1-27. et al. (2005) confirmed its absence in the Ribeira de Iguape River system. The present analysis of Hypostomus specimens from the Uruguay River and the Dos Patos Lagoon system previously identified as H. commersoni revealed that these specimens exhibit several discriminating features when compared to those from the Río de la Plata, Paraná, and Paraguay rivers. The examined specimens from the Uruguay River and the Dos Patos Lagoon system previously attributed to H. commersoni are herein identified as H. spiniger (HenselHENSEL R. 1870. Beiträge zur Kenntniss der Wirbelthiere Südbrasiliens. (Fortsetzung). Archiv Naturgesch 36: 50-91. 1870).
ECOLOGICAL NOTES
The substrate of the rivers from which specimens were obtained was composed chiefly of muddy sand. Hypostomus commersoni was found in moderately oxygenated waters (6.1-9.1 mg/l) with moderate current. Water turbidity was 23.7-442 NTU, conductivity 1.087-2.654 μS/cm, pH 7.2-9.2, and temperature 16.8-27.8°C.
CONSERVATION STATUS
Populations of H. commersoni studied here were from a broad region. The estimated values for IUCN criterion B were: EOO = 551.286 km² and AOO = 100 km², categorizing H. commersoni as a Species of Least Concern.
REMARKS
Valenciennes (1836) based the description of H. commersoni on two specimens from São Francisco Basin (Brazil) and two specimens from Río de la Plata (Uruguay). Weber (1986)WEBER C. 1986. Revision de Hypostomus boulengeri (Eigenmann and Kennedy), et deux espèces nouvelles de poissons-chats du Paraguay (Pisces, Siluriformes, Loricariidae). Rev Suisse Zool 93: 979-1007. after revision of type-series in the Museum National d’Histoire de Paris, designated MNHN a-9444 as lectotype and restricted the type-locality to Río de la Plata, Montevideo, Uruguay. Hypostomus spiniger from Cadeira River was described by Hensel (1870), but Steindachner (1877) considered this species a junior synonym of H. commersoni. Hypostomus limosus from Rio Grande do Sul was described by EigenmannEIGENMANN CH and EIGENMANN RS. 1888. Preliminary notes on South American Nematognathi. I. Proc Calif Acad Sci (Series 2) 1: 119-172. and Eigenmann (1888). MalabarbaMALABARBA LR. 1989. Histórico sistemático e lista comentada das espécies de peixes de água doce do sistema da Laguna dos Patos, Rio Grande do Sul, Brasil. Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia 2: 107-179. (1989) added H. limosus as another junior synonym of H. commersoni.ReisREIS RE, WEBER C and MALABARBA LR. 1990. Review of the genus Hypostomus Lacèpéde, 1803 from Southern Brazil, with descriptions of the three new species (Pisces, Siluriformes, Loricariidae). Rev Suisse Zool 97: 729-766. et al. (1990) re-described H. commersoni, but they reviewed only specimens from the Uruguay River and Dos Patos Lagoon system in Southern Brazil; and outside this region examined only the lectotype. Garavello et al. (2012) also re-described H. commersoni, but examined only specimens from the Iguazú Basin. Based on the morphological and molecular results, specimens previously identified as H. commersoni can be clearly separated into two species: Hypostomus commersoni inhabiting the Paraná, Paraguay, and Río de La Plata and a second species inhabiting the Uruguay River and Dos Patos Lagoon system which is considered here as H. spiniger described by Hensel (1870).
MATERIAL EXAMINED
Argentina: CFA-IC-5543, 3, 40.8-60.2 mm SL, Pozo Añatuya, Santiago del Estero.CFA-IC-5539, 7, 61.3-214.0 mm SL, Tacañitas, Santiago del Estero. CFA-IC-5730, 1, 286.8 mm SL, Tacañitas, Santiago del Estero. CFA-IC-5517, 18, 45.5-85.2 mm SL, Saladillo River and Ruta 92 between Colonia Dora and Los Telares, Santiago del Estero. CFA-IC-3150, 4, 42.3-75.9 mm SL, Corrientes. CFA-IC-3161, 4, 38.4-2.5 mm SL, Entre Ríos.CFA-IC-3381, 3, 338.4-349.1 mm SL, San Nicolás de los Arroyos, Delta of the Paraná River, Buenos Aires. CFA-IC-3380, 9, 290.3-327.1 mm SL, Vuelta of Obligado, Delta of the Paraná, Buenos Aires. CFA-IC-3379, 15, 256.3-329.8 mm SL, San Pedro, Delta of the Paraná River, Buenos Aires. CFA-IC-3005, 1, 285.6 mm SL, Villa Paranacito, Entre Ríos. MLP 9400, 5, 55.6-197.7 mm SL, Reconquista River, Buenos Aires. MLP 7687, 9, 43.5–71.6 mm SL, Golf Club Lakes of Palermo, Buenos Aires. MLP 10003, 1, 70.9 mm SL, Stream Balta, approximately 100 of the bridge RN5, Mercedes, Buenos Aires. MLP 10025, 1, 71.2 mm SL, Stream La Choza, General Rodríguez, Buenos Aires. MLP 10026, 1, 71.5 mm SL, idem previous location.CFA-IC-3372,16, 332.3-366.7 mm SL, Club de Regatas, Ensenada, Rio Santiago, Buenos Aires. CFA-IC-3373, 1, 408.5 mm SL, Los Talas, Berisso, Buenos Aires.
Hypostomus spiniger (Hensel 1870)
Plecostomus spiniger Hensel, 1870: 73. Type locality: Rio Cadea (=Cadeia River), Rio Grande do Sul, Brazil. Holotype: ZMB 7444 (Fig. 7).
Hypostomus spiniger: (a) holotype, ZMB 7444 (Photographs: Allen, Mark); (b) Live specimen from Uruguay River Chajarí, Entre Ríos.
Plecostomus limosus Eigenmann and Eigenmann, 1888: 167. Type locality: Rio Grande do Sul, Brazil. Lectotype: MCZ 7869, designated by Reis et al. (1990: 737). Name made available by diagnostic features in key: 168.
DIAGNOSIS
Hypostomus spiniger can be differentiated from all remaining species of the genus, except H. affinis, H. punctatus, H. ancistroides, and H. commersoni, by having the following features: bifid teeth (vs. spoon-shaped teeth), dark spots on a light background (vs. light spots on a dark background or not spotted), the presence of four rough lateral ridges on flanks (vs. lacking strong ridges on flanks), 26-29 lateral series plates (vs. fewer than 28 in several species or 31-32 in H. laplatae), 1-2 plates bordering the posterior margin of the supraoccipital bone (vs. 3-4 in H. laplatae). Hypostomus spiniger can be distinguished from H. affinis, H. punctatus, and H. ancistroides by inter-dorsal distance (14.7-20.4% vs. in SL 18.9-21.0% in H. affinis, H. punctatus, and H. ancistroides) and head depth (48.8-76.4% vs. 48.2-49.9% in HL in H. affinis, H. punctatus, and H. ancistroides). It can be differentiated from H. commersoni by having large lateral ridges with odontodes posteriorly inclined (vs. fine lateral ridges with odontodes in all directions), strong lateral ridges in caudal peduncle (vs. weak lateral ridges in caudal peduncle), mid lateral ridge from 2nd or 3rd plate (vs. mid lateral ridge from first plate). Adult of H. spiniger (more than 120mm SL) can be also distinguished from adult of H. commersoni by having a very weak ridge on temporal plate (vs. strong ridge on temporal plate) (Figs. 6-8).
According to the BPA (Fig. 4), other morphological characters might differentiate H. spiniger from H. commersoni but the range of these features partially overlaps: dentary and premaxillary teeth, plates between adipose and caudal fins, plates between dorsal and adipose fins, thoracic length, mandibular ramus length, caudal-peduncle depth, and orbit diameter, and by lower predorsal length and dorsal-fin spine length.
DESCRIPTION
Morphometric and meristic data in Table I. Overall view of body shape in Figs. 7 and 8. Dorsal profile nearly straight from snout tip to inter orbital area. Dorsal plates between the insertion of the dorsal fin and adipose fin. Body width at cleithral region greater than head depth. Head covered dorsally with plates. Mouth rounded, lower lip not reaching transversal line through gill openings, ventral surface covered with numerous small papillae. Dorsal-fin rays II,7; margin straight. Adipose-fin spine curved toward the body. Pectoral-fin rays I,6; posterior border straight. Pectoral-fin spine curved slightly inward. Pelvic-fin rays I,5; posterior border slightly curved. Pelvic-fin spine extends past anal-fin origin when depressed. Anal-fin rays I,4; tip reaching seventh plate posterior to the origin. Anal-fin rays progressively increasing in length, third branched ray usually longest. Caudal-fin rays I,14,I.
COLORATION
Background color of head and trunk dark brown or gray, small, irregular black spots on head and larger and more widely spaced spots on dorsum, some specimens with more prominent spots. All fins dark brown or gray, inter radial membranes hyaline with irregular small black spots. Abdominal region pale brown or gray, usually with circular or vermiculated spots. Coloration was more pronounced in live specimens.
GEOGRAPHIC DISTRIBUTION
Hypostomus spiniger inhabits the Uruguay River and Dos Patos system. The southernmost sampled locality was Gualeguaychú, Entre Ríos, Argentina.
ECOLOGICAL NOTES
Based on the locality of sample CFA-IC-3370 and CFA-IC-3371. The substrate is mainly composed of sand. Hypostomus spiniger was found in oxygenated waters (8.4-9.0 mg/l) with moderate current. Water turbidity was 22.4-74.2 NTU., conductivity 567-1909 μS/cm, pH 7.6-8.3, and temperature 19.8-25.3°C.
CONSERVATION STATUS
Hypostomus spiniger is reported from a wide region in Argentina and Brazil, and also occurs in Uruguay. The estimated values for criterion B were: EOO = 251.934 km2 and AOO = 72 km2. According to IUCN criteria H. spiniger can be categorized as a Species of Least Concern.
REMARKS
Hensel (1870) described H. spiniger as a new species, based on dry specimens from the Cadea (Cadeira, Cadeia) River, in Rio Grande do Sul State. Steindachner (1877) proposed H. spiniger as a junior synonym of H. commersoni. Later, Eigenmann and Eigenmann (1888:168) described H. limosus based on four specimens from the Rio Grande do Sul, name made available by diagnostic features in a key. Malabarba (1989) restricted the type locality of H. limosus to the Dos Patos Lagoon and considered this species a junior synonym of H. commersoni. Reis et al. (1990) described the lectotype of H. limosus and reviewed several specimens of H. commersoni from the Uruguay River and Dos Patos Lagoon system from Southern Brazil, as well as the type-series of H. spiniger. However, their examination was limited to the dry lectotype of H. commersoni from Río de La Plata; no other specimens from this basin or from the Paraná or Paraguay basins were analyzed. The morphological and molecular results show clear differences between the specimens from Uruguay/Dos Patos Lagoon and Paraná/Paraguay/Río de La Plata. Of the species described from the Dos Patos system, H. spiniger has priority by date, thus it is considered a valid species, with H. limosus being its junior synonym.
MATERIAL EXAMINED
Brazil: ZMB 7444, holotype of Plecostomus spiniger, examined by photograph, Cadeia River, Rio Grande do Sul. MCZ 7869 lectotype of Plecostomus limosus, examined by photograph, Rio Grande do Sul. BMNH 1904.1.28.1 paralectotype of Plecostomus limosus examined by photograph. MHNG 2517.62, 1, 127.5 mm SL, Ibicuí da Foxina. MCP10530, 1, 118.4 mm SL, Guaiba Lagoon Guiaba in Ponta do Jacare, Rio Grande do Sul. MCP10496, 1, 118.9 mm SL, Uruguay Basin, Conceição River, Rio Grande do Sul. MCP27618, 1, 84.6 mm SL, Uruguay Basin, Stream Carai-Passo, in São Francisco de Assis/ Manoel Viana, Rio Grande do Sul.
Argentina: MLP5132, 1, 52.5 mm SL, San Javier, Misiones. CFA-IC-, 1, 56.7 mm SL, Ayui River, Corrientes. CFA-IC-3745, 1, 92.1 mm SL, Tres Cerros in route 114, Corrientes. CFA-IC-3369, 1, 273 mm SL, route 14 to 15 km of Mocoretá, Corrientes. CFA-IC-3370, 1, 283 mm SL, Santo Tomé in route 94, Corrientes. CFA-IC-3371, 4, 295-325 mm SL, route 14 to 11 km of Chajari, Entre Ríos. CFA-IC-5829, 3, 65-157 mm SL, Stream El Doctor, Entre Ríos. CFA-IC-5305, 1, 198 mm SL, Stream El Pelado, Entre Ríos. CFA-IC-5854, 3, 103-242 mm SL, Stream Urquiza, Entre Ríos. CFA-IC-5960, 1, 86.7 mm SL, Concepción del Uruguay, Entre Ríos. MLP10067, 1, 126 mm SL, Stream without name to 10 km of Concepcion del Uruguay, Entre Ríos. MLP10068, 1, 76.4 mm SL, Stream without name to 10 km of Concepcion del Uruguay, Entre Ríos. MLP10069, 1, 63 mm SL, Stream without name to 10 km of Concepcion del Uruguay, Entre Ríos. CFA-IC-3324, 1, 82.7 mm SL, Stream Urquiza, Entre Ríos. CFA-IC-6904, 3, 66.7-79.2 mm SL, Stream La Capilla, Gualeguaychú, Entre Ríos.
Hypostomus cordovae (Günther 1880)
Plecostomus cordovae Günther 1880: 11-12.Type locality: Córdoba, Argentina. Holotype: BMNH 1878.4.4.1 (Fig. 9)
Hypostomus cordovae: (a) holotype, BMNH 1878.4.4.1. Dorsal view. (Photograph: Natural History Museum, London); (b) Live specimen from Quinto River, Córdoba.
DIAGNOSIS
Hypostomus cordovae can be differentiated from all remaining species of the genus by having the following features: bifid teeth (vs. spoon-shaped teeth), dark spots on a light background (vs. light spots on a dark background or not spotted), absence of four rough lateral ridges on flanks (vs. presence strong ridges on flanks in H. commersoni, H. spiniger and H. laplatae), 28-30 lateral series plates (vs. fewer than 28 in several species or 31-32 in H. laplatae), height body (13.3-18.1% vs. 17.7-24.7% in SL in H. commersoni, H. spiniger and H. laplatae), and head length (25.9-30.7 vs. 27.5-38.7 in SL in H. commersoni, H. spiniger and H. laplatae).
DESCRIPTION
Morphometric and meristic data in Table II. Overall view of body shape in Fig. 9. Dorsal profile of body rises smoothly from snout to supraoccipital process, continuing to dorsal fin and descending to distal end of caudal peduncle. Height of body less than cleithral width. Body in cleithral region wider than depth of head. Body width constant to base of pelvic fins and narrows toward base of caudal fin. Head roughly triangular in dorsal view, with flattened area between orbits. Head covered with plates dorsally, except for area on snout tip. Slightly rounded snout with crest. Labial disc rounded, upper lip with small papillae and lower lip covered by more than two rows of papillae, of which size decreases toward lip margin. Ventral surface of head and body completely covered by small plates. Lateral line on median series of plates complete. Caudal peduncle compressed, especially posterior to adipose-fin spine. Dorsal and ventral surfaces of caudal peduncle slightly flattened. Dorsal fin I,7; dorsal-fin spine usually shorter than head length, posterior edge slightly convex or nearly straight. Dorsal-fin base without dermal ossifications, extended dorsal fin not reaching origin of adipose fin. Pectoral fins I,6; distal edge nearly straight, when extended reaching past origin of pelvic fins. In adult males, pectoral fins with well-developed odontodes. Pelvic fin I,5; extend past anal-fin origin when extended, distal edge rounded. Anal fin, I,4; external edge almost straight. Caudal fin, I,14,I; posterior margin concave. Ventral-most caudal-fin ray usually longer than dorsal-most.
Morphometric and meristic data of Hypostomus cordovae and H. laplatae. Range, mean (or mode for the meristic data), standard deviation (SD) and number of specimen (n). Morphometric and meristic variables used in the BCA analysis (Figs. 3) are showed with abbreviations in brackets.
COLORATION
Background color of dorsal and lateral regions of body dark gray with roughly circular black spots. Spots small and closely spaced on head, larger and more widely spaced posterior. Ventral region with light gray background color and circular, irregular, and kidney-shaped spots. In small specimens, spots forming series of crosswise bands on body: first on head, second at origin of dorsal-fin spine, third at sixth dorsal-fin branched ray, fourth anterior to adipose-fin spine, and final on 3rd or 4th plate of caudal peduncle. Fins with black dots on inter radial membrane. In some specimens, dorsal fin with variable number of zigzagging black bands (Fig. 9). Dots on pectoral fin smaller than those on other fins. Black dots more prominent in live specimens. Background color of body yellowish brown in specimens from Segundo River Basin (Argentina).
GEOGRAPHIC DISTRIBUTION
Hypostomus cordovae occurs in the Primero, Segundo, Cuarto, and Quinto Rivers in the Córdoba Province; in the Horcones River in Santiago del Estero; the Juramento River in Salta; and in Del Cajón, Calera, and Salí Rivers in Tucumán Province. This species is also reported in Catamarca, Jujuy, Santa Fe (in the Carcarañá River), and San Luis (LiottaLIOTTA J. 2017. Base de datos de peces de aguas continentales de Argentina. Publicación electrónica. Available from: http://www.pecesargentina.com.ar (Date of access - June 2017).
http://www.pecesargentina.com.ar...
2017). RingueletRINGUELET RA, ARÁMBURU RH and ALONSO DE ARÁMBURU A. 1967. Los peces argentinos de agua dulce. Comisión de Investigaciones Científicas de la Provincia de Buenos Aires. et al. (1967) cited H. cordovae from Ituzaingó, Corrientes (Paraná River), but the examination of this material allowed us to confirm that the specimens were misidentified.
ECOLOGICAL NOTES
Specimens were recently obtained from seven rivers. The substrate of those rivers was mainly composed of sandstone boulders with patches of coarse sand and pebbles. Hypostomus cordovae was found in well-oxygenated waters (6.9-11.2 mg/l) with strong current. Water turbidity was 1.29-75.1 NTU, conductivity 77-4.530 μS/cm, pH 7.8-8.8, and temperature 20.7-31.4°C.
CONSERVATION STATUS
Populations of H. cordovae are known from a wide region in Argentina. They are endemic to this country, and not considered under imminent threat. The estimated values for criterion B were: EOO = 58.445 km2 and AOO = 48 km2, thus, according to IUCN criteria, H. cordovae can be categorized as a species of Least Concern (LC).
REMARKS
The name H. paranensis was used by Weyenbergh (1877)WEYENBERGH H. 1877. Algunos nuevos pescados del Museo Nacional y algunas noticias ictiológicas. Actas Acad Nac Ciencias Exactas Córdoba 3: 1-37. for a single specimen of Hypostomus from the marshes around Santa Fe. This author did not designate type material and only mentioned that “La especie del Paraná me parece distinta por la forma y el menor número y tamaño de las manchitas negras, los que produce en general otro color” (The Paraná species seems different because of the shape and the smaller number and size of the black spots, which usually produces a different pattern). According to Eschmeyer et al. (2017) there are (several) syntypes in the MNAC (supposedly referring to the MACN), however, no specimens of Hypostomus paranensis are included in the collection or in the database of this museum.
Later, Gunther (1880)GÜNTHER A. 1880. A contribution to the knowledge of the fish fauna of the Río de la Plata. J Nat Hist 6: 7-13. described Plecostomus cordovae on the basis of a specimen originating from Córdoba. BergBERG C. 1895. Sobre peces de agua dulce nuevos o poco conocidos de la República Argentina. An del Mus Nac Buenos Aires IV: 121-165. (1895) proposed that P. cordovae is synonymous with Plecostomus commersonii affinis (Eigenmann and Eigenmann 1888) and mentioned that this species “se halla en los mismos ríos y arroyos que la especie típica Plecostomus commersoni (C. V.) Gunth” (…is found in the same rivers and streams as the type species Plecostomus commersoni (C. V.) Gunth). Several decades later, Ringuelet et al. (1967) and LópezLÓPEZ HL and MIQUELARENA A. 1991. Los Hypostominae (Pisces: Loricariidae) de Argentina. Programa de Fauna de Agua Dulce. PROFADU (CONICET), Museo de La Plata 40: 1-64. and Miquelarena (1991) recognized H. cordovae as valid, considering H. paranensis as a junior synonym. WeberWEBER C. 2003. Subfamily Hypostominae. In: Reis RE, Kullander SO and Ferraris CJ Jr (Eds), Check List of the freshwater fishes of South and Central America, Edipucrs, Porto Alegre, p. 351-372. (2003) considered the name H. paranensis as “nomen oblitum” and H. cordovae as a “nomen protectum”, however this was suggested without providing justification. More recently, FerrarisFERRARIS CJ JR. 2007. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa 1418: 1-628. (2007), based on the priority principle, established that H. paranensis is a valid species, and that H. cordovae is a junior synonym of H. paranensis. Recently, H. paranensis was included as a valid species on the list of species of Hypostomus from Argentina (Koerber and Weber 2014). Based on the analysis of the literature and according to the International Code of Zoological Nomenclature (1999)ICZN - INTERNATIONAL COMMISSION ON ZOOLOGICAL NOMENCLATURE. 1999. International Code of Zoological Nomenclature [the Code]. 4th ed., London.The International Trust for Zoological Nomenclature, c/o Natural History Museum., the name H. paranensis is considered available. However, the exhaustive examination of specimens from the region surrounding the cities of Santa Fe and Córdoba strongly suggests that H. paranensis must be considered as species inquirenda. The morphological and molecular results show that all specimens from the Paraná River near Santa Fe City can be identified as H. commersoni. The examination of the holotype of H. cordovae and the new material from Córdoba indicates that this species can be clearly differentiated and is valid.
MATERIAL EXAMINED
BMNH 1878.4.4.1, holotype of Plecostomus cordovae, examined by photograph (Fig. 9), Córdoba. CFA-IC-3140, 16, 40-171.7 mm SL (10, 42.1-171.7 mm SL), Horcones River, Santiago del Estero. CFA-IC-3131, 17, 37.5-70.9 mm SL (9, 37.5-70.9 mm SL), del Cajón River, Tucumán. CFA-IC-3041-3053, 13, 40-216.8 mm SL (8, 48.1-216.8 mm SL), Salí River, Calera River, Tucumán. CFA-IC-11914, 2, 56-68 mm SL, Primero River, in front of Isla Los Patos, Córdoba. CFA-IC-3368, 10, 114.2-231.4 mm SL, Mar Chiquita Basin, Anisacate River, Segundo River, Córdoba. CFA-IC-3734, 2, 65-87 mm SL, Cuarto River, Córdoba. CFA-IC-3367, 10, 73.6-247.5 mm SL, Quinto River, near to Villa Sarmiento, Córdoba.
Hypostomus laplatae (Eigenmann 1907)
Plecostomus laplatae Eigenmann, 1907: 450-451, pl. 21 (Figs. 1-3). Type locality: Buenos Aires, Argentina. Paratype: CAS 77342 (Fig. 10).
Hypostomus laplatae: (a) paratype, CAS 77342. Dorsal view. (Photograph: California Academy of Sciences, San Francisco); (b) Live specimen from Lower Paraná River, San Pedro, Buenos Aires.
Plecostomus taeniatus Regan, 1908: 358. Type locality: Río La Plata, Argentina.
Plecostomus commersonoides Marini, Nichols and La Monte, 1933: 3-4. Type locality: Dársena Norte, Buenos Aires, Argentina.
DIAGNOSIS
Hypostomus laplatae can be differentiated from all remaining species of the genus by having the following features: bifid teeth (vs. spoon-shaped teeth), dark spots on a light background (vs. light spots on a dark background or not spotted), possession of four rough lateral ridges on flanks (vs. lacking strong ridges on flanks), 31-32 lateral series plates (vs. fewer than 30 in several species), 3-4 plates bordering the posterior margin of the supraoccipital bone (vs. 1-2 in H. commersoni and H. spiniger) and inter-orbital width (30.5-38.6% vs. 36.2-56.3% in HL in H. commersoni and H. spiniger).
DESCRIPTION
Morphometric and meristic data in Table II. Overall view of body shape in Fig. 10. Dorsal profile of body convex from snout to supraoccipital bone, then continuing straight to origin of dorsal fin. Body height less than cleithral width. Cleithral region wider than head height. Body width constant to base of pelvic fin, then narrowing toward base of caudal fin. Head with flattened area between orbits. Slightly rounded snout, more than half length of head. Snout completely covered by small plates. Rounded labial disc, upper lip with small papillae and lower lip covered by more than two rows of papillae that became smaller toward labial margin. Bifid teeth. Relatively small eye; weak crest anterior to eye. Supraoccipital bone bordered by medial plate and 2-4 lateral plates. Smaller irregularly-shaped plates on head. Ventral surface covered by small plates including between pectoral fins and anterior to anus. Compressed caudal peduncle, mainly anterior to adipose-fin spine, length greater than depth. Dorsal and ventral surfaces of caudal peduncle slightly flattened. Dorsal fin I,7; posterior border slightly convex or almost straight. Pectoral fins I,6; border almost straight, extending past origin of pelvic fins. In adult males, pectoral-fin spines has well developed odontodes. Pelvic fins I,5, extending past origin of anal fin, rounded distal border. Anal fin, I,4; external border nearly straight. Caudal fin I,14,I; with concave margin. Lower caudal-ray longer than upper.
COLORATION
Body and head dark grayish or brown background, with roughly circular spots of various sizes. Smaller and more numerous spots on head, more irregular spots in dorsal body. Ventral surface grayish-white, with irregular-shaped black spots. Fins with black spots on inter radial membrane, rays, and base, with exception of anal and caudal fins, uniformly colored; some specimens with a darker distal portion. Body and head more grayish background in live specimens.
DISTRIBUTION
Hypostomus laplatae occurs in the Río de la Plata (type locality) and the Lower Paraná River. The species has been reported in the provinces of Salta and Corrientes (Liotta 2017), but no collected specimens are available to corroborate the identification in these localities.
ECOLOGICAL NOTES
Specimens were recently obtained from two rivers, the substrates of which are primarily sand. Hypostomus laplatae was found in oxygenated waters (5.4-7.4 mg/l) with moderate current. Water turbidity was 260-392 NTU, conductivity 543-2,435 μS/cm, pH 7.8-8.1, and temperature 15.3-20.4°C.
CONSERVATION STATUS
Hypostomus laplatae is recorded in a limited region of the Lower Paraná River and Río de la Plata. The estimated values for criterion B were EOO = 15.827 km² and AOO = 20 km². Following the IUCN criteria (IUCN 2017), a given species can be categorized as “Endangered” if, in addition to small EOO (B1) and AOO (B2), two out of three sub criteria were found: (i) to be severely fragmented or occupy a limited number of locations (ii) present continuing generation decline; and (iii) present extreme fluctuations. Thus, H. laplatae can be classified as Near Threatened.
REMARKS
Plecostomus laplatae was described based on specimens from Buenos Aires (Eigenmann 1907). Forty years later, GoslineGOSLINE WA. 1947. Contributions to the classification of the loricariid catfishes. Arq do Mus Nac Rio de Janeiro 41: 79-134. (1947) considered H. taeniatus and H. commersonoides as junior synonyms of H. laplatae, both also described from Buenos Aires. The holotype and paratypes of Plecostomus commersonoides (AMNH 12243 and AMNH 12244) were not found in the collection (checked recently). Gosline (1947) also suggested that H. rachovii (ReganREGAN CT. 1913. Description of a new loricariid fish of the genus Plecostomus from Rio Janeiro. Ann Mag Nat Hist (Series 8) 12: 555. 1913) could be related to the mentioned species, without having seen material of those species. López and Miquelarena (1991) presented a revised description of H. laplatae based on four specimens (not found in the collection of Museo de La Plata) from the Río de la Plata. Weber (2003) analyzed the syntypes of H. taeniatus and the holotype of H. rachovii and considered them synonyms, adding H. rachovii to the list of junior synonyms of H. laplatae. Ferraris (2007) stated that the synonymy of H. taeniatus and H. rachovii is probably an error.
MATERIAL EXAMINED
Argentina:Plecostomus laplatae: CAS 77342, paratype, examined by photograph (Fig. 10), Buenos Aires. Plecostomus taeniatus: BMNH 1908.8.29.17, syntype, examined by photograph, Río de la Plata. Hypostomus laplatae: CFA-IC-3011, 1, 343 mm SL, Villa Paranacito, Entre Ríos. CFA-IC-3020, 1, 345 mm SL, Vuelta de Obligado, Buenos Aires. CFA-IC-3007, 1, 371 mm SL, San Pedro, Buenos Aires. CFA-IC-3008, 1, 257 mm SL, San Pedro, Buenos Aires. CFA-IC-3010, 3, 385-466 mm SL, Río de la Plata, Punta Lara, Buenos Aires. CFA-IC-3018, 1, 370 mm SL, Río de la Plata, Punta Lara, Buenos Aires. CFA-IC-3013, 3, 385-461 mm SL, Río de la Plata, Punta Lara, Buenos Aires. CFA-IC-3012, 1, 385 mm SL and CFA-IC-3015, 2, 423-441 mm SL, Río de la Plata, Punta Lara, Buenos Aires.
DISCUSSION
The identification of Hypostomus species is a challenge, given the complex taxonomy of the group and the sparse morphological information available for many species. Thus application of multisource approaches (ecology, distribution, morphology, molecular phylogeny) takes advantage of complementarity among disciplines and is crucial to detecting cryptic diversity within the genus (DayratDAYRAT B. 2005. Towards integrative taxonomy. Biol J Linnean Soc 85: 407-415. 2005). Valid taxonomic identification is critical to estimate the diversity of freshwater environments, including the Neotropical Region. Discoveries of new fish species contribute not only to evaluate regional faunal diversity but to reconstruct the geohistory of South American river basins.
SPECIES DIVERSITY AND TAXONOMIC IMPLICATIONS
Several of the many Hypostomus species identified and described in recent years were previously grouped under a single species name. In the present study, an exhaustive examination of H. commersoni specimens revealed the existence of a cryptic form which was resurrected and re-described as H. spiniger. Significant differences were documented between H. commersoni and H. spiniger: In the molecular analyses they formed separate monophyletic groups instead of being sister species. They show high levels of genetic and morphological differentiation and occur in different river basins without overlap of their distributional ranges. Comparison with other species inhabiting the Lower La Plata Basin and other watersheds, confirm that H. spiniger, although considered for several years a junior synonym of H. commersoni, is a valid species. Revalidation of formerly described species is being made possible by the examination of large numbers of specimens and robust analyzes. Similar results were found by BertacoBERTACO VA and LUCENA CAS. 2010. Redescription of the Astyanax obscurus (Hensel, 1870) and A. laticeps (Cope, 1894) (Teleostei: Characidae): two valid freshwater species originally described from rivers of Southern Brazil. Neotrop Ichthyol 8: 7-20. and Lucena (2010) when they analyzed two species of Astyanax from the Dos Patos system: Astyanax obscurus and A. laticeps were once considered junior synonyms of other species and recently re-validated. Interestingly, Astyanax obscurus was described by Hensel (1870) from the same type locality as H. spiniger (Cadeia River).
Hypostomus cordovae and H. paranensis are little-known species described more than a century ago and only cited in fish catalogues. The species were considered synonyms by several authors (López and Miquelarena 1991, Weber 2003, Ferraris 2007) without clear explanations. Based on morphological and molecular data of specimens from near Santa Fe (type locality), and on the original description of H. paranensis (without assigned type material), this name should be considered as species inquirenda. Thus, the name H. paranensis remains available in the event that further taxonomic studies reveal diagnostic characters to differentiate it from other species.
The examination of the holotype of H. cordovae and the new material from its type locality allows us to consider this species as valid. The molecular data showed that H. cordovae is closely related to H. commersoni; nevertheless, they are readily distinguished morphologically. The differentiation of these species is crucial, since they have the widest distribution of any fish species in the La Plata Basin. HaroHARO JG and BISTONI MA. 2007. Peces de Córdoba. Primera edición. Córdoba. Universidad Nacional de Córdoba, 246 p. and Bistoni (2007) stated that both species would be present in the Dulce and Cuarto Rivers. Both species were sampled in the Dulce and Salado Rivers but no evidence of sympatric distribution were found. Hypostomus cordovae was found exclusively in the headwaters of the rivers, on stony substrates and well-oxygenated, fast-flowing water, while H. commersoni is found primarily in lentic environments with muddy substrates. The ecological characteristics of H. cordovae support the premise that H. paranensis described from the marshes around Santa Fe cannot be considered the same species.
In this study freshly collected specimens of H. laplatae were compared with the species considered synonyms. The material concurs with the original description by Eigenmann (1907), and allows to conclude that H. taeniatus and H. commersonoides are the same species. However, the holotype of H. rachovii from Rio de Janeiro shows significant morphological differences: a narrower snout, wider interorbital distance, larger dorsal-fin base and a caudal fin with dark bars. These morphological features and the thousand kilometers separating the type locality of H. rachovii from the type locality of H. laplatae, suggest that H. rachovii is not a conspecific of H. laplatae. Similarly, Mazzoni et al. (1994) suggested H. rachovii as a junior synonym of H. punctatus Valenciennes 1840, also described from Rio de Janeiro. Hypostomus laplatae appears confined to the Lower Paraná River and the Río de la Plata. These data show that it is sympatric with H. aspilogaster, H. uruguayensis, and H. commersoni, and is one of the species with a southern distribution.
SPECIES CONSERVATION STATUS
The IUCN Red List provides information on which species are most threatened and provides a basis for conservation priority. It is also a powerful tool for persuading governments to protect threatened species and their habitats (IUCN 2017). The Red List is a valuable compendium of information on threats to species, but coverage of little-studied freshwater taxa in the list is limited (BarlettaBARLETTA M, JAUREGUIZAR A, BAIGÚN CRM, FONTOURA N, AGOSTINHO A, ALMEIDA-VAL V, VAL A, TORRES R, JIMENES L and GIARRIZZO T. 2010. Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J Fish Biol 76: 2118-2176. et al. 2010). This is probably due to lack of knowledge of their taxonomic identity, distribution, evolution, and population dynamics (BaigúnBAIGÚN CRM, COLAUTTI DC, LÓPEZ HL, VAN DAMME PA and REIS RE. 2012. Application of extinction risk and conservation criteria for assessing fish species in the lower La Plata River basin, South America. Aquat Conserv Mar Freshw Ecosyst 22: 181-197. et al. 2012). These gaps in the knowledge of freshwater taxa need to be recognized, as biased and under-represented information compromises our capacity to describe existing biodiversity and to make accurate predictions of potential change (HortalHORTAL F, DE BELLO J, DINIZ-FILHO AF, LEWINSOHN TM, LOBO JM and LADLE RJ. 2015. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol S 46: 523-549. et al. 2015). For these reasons, works about species distribution and check-lists of different regions are still very important (CabreraCABRERA MB, BOGAN S, POSADAS P, SOMOZA G, MONTOYA-BURGOS JI and CARDOSO YP. 2017. Risks associated with introduction of poeciliids for control of mosquito larvae: first record of the non-native Gambusia holbrooki in Argentina. J Fish Biol 91: 704-710. et al. 2017, Cardoso and BoganCARDOSO YP and BOGAN S. 2015. Expanding the distribution of Trichomycterus barbouri (Siluriformes, Trichomycteridae) for Argentina. Rev del Mus Argentino Ciencias Nat Nueva Ser 17: 147-151. 2015, Bogan and Cardoso 2017BOGAN S and CARDOSO YP. 2017. Review of the distribution of Glanidium ribeiroi Haseman, 1911 in Argentina (Siluriformes: Auchenipteridae). Rev del Mus Argentino Ciencias Nat Nueva Ser 19: 1-7.).
Based on criterion B, H. laplatae has small values of EOO and AOO and can consider into the Near Threatened category (IUCN 2017). The distribution range of this species is the greater urban area of La Plata Basin, impacting the quality of its water (PelusoPELUSO L, ABELANDO M, APARTÍN CD, ALMADA P and RONCO AE. 2013. Integrated ecotoxicological assessment of bottom sediments from the Paraná basin, Argentina. Ecotox Environ Safe 98: 179-186. et al. 2013). The Lower Paraná and Río de la Plata basins present high concentrations of solid waste, heavy metals, hydrocarbons, and persistent organic compounds, especially concentrated in sediments (FREPLATAFREPLATA. 2005. Análisis Diagnóstico Transfronterizo del Río de la Plata y su Frente Marítimo. Protección Ambiental del Río de la Plata y su Frente Marítimo: Prevención y Control de la Contaminación y Restauración de Hábitats. Documento Técnico. Proyecto PNUD/GEF RLA/99/G31. Montevideo, Uruguay. 2005, GalindoGALINDO G, SAINATO C, DAPEÑA C, FERNÁNDEZ-TURIEL JL, GIMENO D, POMPOSIELLO MC and PANARELLO HO. 2007. Surface and groundwater quality in the northeastern region of Buenos Aires Province, Argentina. J South Am Earth Sci 23: 336-345. et al. 2007, Peluso et al. 2013). The conservation status of H. laplatae, with benthic habits, may be dramatically affected by the environmental conditions of its limited distribution range. Indeed, Hypostomus laplatae suffers continuing decline in habitat quality, this partial meeting of the criteria will make this species Near Threatened. Currently there are not data to evaluate the criteria i and iii for this species which is essential and should be analyzed in subsequent studies.
BIOGEOGRAPHY OF HYPOSTOMUS IN THE LOWER LA PLATA BASIN
To the known species from the Lower La Plata Basin may potentially be added H. itacua Valenciennes 1836, which can be considered an “incertae sedis species” (Koerber and Weber 2014), along with several reported unidentified Hypostomus sp (unpublished data). The phylogenetic tree (Fig. 2) concurred with those presented previously (Montoya-Burgos 2003, Cardoso et al. 2012, 2016) and shows that H. laplatae belongs to a different lineage from H. commersoni, H. spiniger, and H. cordovae. This indicates that the diversity of Hypostomus in the Lower La Plata Basin is significant, not only in number of species, but also in number of phylo-groups. Elucidating phylogenetic relationships among Hypostomus of La Plata Basin is important for a better understanding of the biogeography of this basin.
Non-overlapping distribution ranges were found among the closely related species H. cordovae, H. commersoni, and H. spiniger belonging to the D2 lineage, with a distribution organized in a west-east geographic manner (Fig. 1). In the western site of the Lower La Plata Basin, H. cordovae inhabits the headwater streams in the piedmont, with strong flows and highly-oxygenated water rich in dissolved minerals. The substrate comprises rocks and stones (MiquelarenaMIQUELARENA A, MENNI RC, LÓPEZ HL and CASCIOTTA J. 1990. Ichthyological and limnological observations on the Salí river basin (Tucumán, Argentina). Ichthyol Explor Freshwaters 1: 269-276. et al. 1990, IsasmendiISASMENDI S, TRACANNA B, VENDRAMINI F, NAVARRO M, BARRIONUEVO M and MEONI S. 2007. Caracterización física y química de ríos de montaña (Tafí del Valle-Tucumán-Argentina). Limnetica 26: 129-142. et al. 2007). The species displays a morphology adapted to these environmental conditions, including a long, deep body and large mouth. Hypostomus commersoni and H. spiniger inhabit wide river channels: the Paraná River in the central area of the La Plata Basin, and the Uruguay River in the eastern La Plata Basin, respectively, rivers that are dissimilar in physical and chemical characteristics. The mean values of conductivity and suspended nutrients and solids are higher in the Paraná River than in the Uruguay River (Di Persia and Neiff 1986DI PERSIA D and NEIFF JJ. 1986. The Uruguay River system. In Davies BR and Walker KF (Eds), The Ecology of River Systems. Monographiae Biologicae, W. Junk Publishers, Dordrecht, p. 599-623., Quirós and Cuch 1989QUIROS R and CUCH S. 1989. The fisheries and limnology of the lower Plata Basin. In Dodge DP (Ed), Proceedings of the International Large River Symposium. Canadian Special Publications of Fisheries and Aquatic Sciences (106), Ontario, p. 429-443.). The load of suspended solids contributed by the Paraguay and Bermejo rivers is the source of the high turbidity in the Paraná River (BonettoBONETTO A. 1986. The Parana River system. In Davies BR and Walker KF (Eds), The Ecology of River Systems. Monographiae Biologicae, W. Junk Publishers, Dordrecht, p. 541-589. 1986). In contrast, the Uruguay River presents high clarity associated with slower current and low suspended solids (INCYTHINCYTH. 1978. Caudal sólido transportado por el río Uruguay. CTM Saito Grande 5ta.RDA/ 1.9, 8 pp., 1978). The substrate of the Paraná River consists of medium and fine sand and a large silt-clay fraction (Di Persia and Neiff 1986), while the Uruguay River has a rocky bed (Quirós and Cuch 1989). These characteristics persist until the Río de la Plata interior, where the north coast is sandy, while sediments on the south coast are principally silt-clays (Quirós and Cuch 1989).
Since there are no physical barriers to dispersion in the distribution areas of H. commersoni, H. spiniger, and H. cordovae, with the exception of some endorheic rivers for H. cordovae, the distribution of these species may be related to the contrasting environmental conditions of the tributaries of the Lower La Plata Basin. This raises the question of the processes responsible for the diversification in this clade. Given that the basins occupied by these species had a complex connection-disconnection history (LundbergLUNDBERG JG, MARSHALL LG and GUERRERO J. 1998. The stage for Neotropical fish diversification: a history of Tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena M and Lucena CAS (Eds), Phylogeny and classification of Neotropical fishes. Edipucrs, Porto Alegre, p. 13-48. et al. 1998), two possible speciation events for these three species may be suggested: (i) an ancient allopatric speciation when the basins were separated, according to the hydrogeological hypothesis proposed by Montoya-Burgos (2003) or (ii) recent ecological speciation via dispersion after the connection among basins, as suggested by SilvaSILVA G, ROXO FF, LUJAN NK, TAGLIACOLLO VA, ZAWADZKI CH and OLIVEIRA C. 2016. Transcontinental dispersal, ecological opportunity and origins of an adaptive radiation in the Neotropical catfish genus Hypostomus (Siluriformes: Loricariidae). Mol Ecol 25: 1511-1529. et al. (2016). To help resolve this question, a robust time-calibrated phylogenetic tree of the genus should be constructed to compare time of the separation events with the geological events reported. Understanding the role of hydrogeology and ecology in species divergence is central to the study of fish evolutionary diversification and historical biogeography. In this regard, the present work is an important source of geographic, ecological, and diversity data of the genus Hypostomus from the Lower La Plata Basin that will contribute to a better understanding of the evolution and biogeography of Hypostomus.
ACKNOWLEGMENTS
This study was supported by PICT2014-0580 (to YPC), BARCODE Argentina, by the Swiss 2015-Seed Money Grants for Latin America (to YPC, GS, and JIMB), and by the G. and A. Claraz Foundation (to JIMB). We thank the authorities of the provinces of Santiago del Estero, Santa Fe, Salta, Buenos Aires, Tucumán and Entre Ríos for granting the permission for collecting fishes. We thank the Fundación de Historia Natural Félix de Azara, R. Covain and S. Fisch-Muller for their invaluable collaboration and R. Reis for his helpful comments. We also thank S. Koerber for the translation of the description of H. spiniger from old German.
REFERENCES
- AITCHINSON J. 1986. The Statistical Analysis of Compositional Data. London: Chapman & Hall, 416 p.
- ALJANABI SM and MARTINEZ I. 1997. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res 25: 4692-4693.
- ATCHLEY R, GASKINS CT and ANDERSON D. 1976. Statistical properties of Ratios. I. Empirical results. Syst Zool 25: 137-148.
- ATCHLEY WR and ANDERSON D. 1978. Ratios and the statistical analysis of biological data. Syst Zool 27: 71-78.
- BAIGÚN CRM, COLAUTTI DC, LÓPEZ HL, VAN DAMME PA and REIS RE. 2012. Application of extinction risk and conservation criteria for assessing fish species in the lower La Plata River basin, South America. Aquat Conserv Mar Freshw Ecosyst 22: 181-197.
- BARLETTA M, JAUREGUIZAR A, BAIGÚN CRM, FONTOURA N, AGOSTINHO A, ALMEIDA-VAL V, VAL A, TORRES R, JIMENES L and GIARRIZZO T. 2010. Fish and aquatic habitat conservation in South America: a continental overview with emphasis on neotropical systems. J Fish Biol 76: 2118-2176.
- BERG C. 1895. Sobre peces de agua dulce nuevos o poco conocidos de la República Argentina. An del Mus Nac Buenos Aires IV: 121-165.
- BERTACO VA and LUCENA CAS. 2010. Redescription of the Astyanax obscurus (Hensel, 1870) and A. laticeps (Cope, 1894) (Teleostei: Characidae): two valid freshwater species originally described from rivers of Southern Brazil. Neotrop Ichthyol 8: 7-20.
- BOESEMAN M. 1968. The genus Hypostomus Lacépède, 1803, and its Surinam representatives (Siluriformes, Loricariidae). Zool Verh 99: 1-89.
- BOGAN S and CARDOSO YP. 2017. Review of the distribution of Glanidium ribeiroi Haseman, 1911 in Argentina (Siluriformes: Auchenipteridae). Rev del Mus Argentino Ciencias Nat Nueva Ser 19: 1-7.
- BONETTO A. 1986. The Parana River system. In Davies BR and Walker KF (Eds), The Ecology of River Systems. Monographiae Biologicae, W. Junk Publishers, Dordrecht, p. 541-589.
- CABRERA MB, BOGAN S, POSADAS P, SOMOZA G, MONTOYA-BURGOS JI and CARDOSO YP. 2017. Risks associated with introduction of poeciliids for control of mosquito larvae: first record of the non-native Gambusia holbrooki in Argentina. J Fish Biol 91: 704-710.
- CARDOSO YP, ALMIRÓN A, CASCIOTTA J, AICHINO D, LIZARRALDE M and MONTOYA-BURGOS JI. 2012. Origin of species diversity in the catfish genus Hypostomus (Siluriformes: Loricariidae) inhabiting the Paraná River basin, with the description of a new species. Zootaxa 3453: 69-83.
- CARDOSO YP and BOGAN S. 2015. Expanding the distribution of Trichomycterus barbouri (Siluriformes, Trichomycteridae) for Argentina. Rev del Mus Argentino Ciencias Nat Nueva Ser 17: 147-151.
- CARDOSO YP, BRANCOLINI F, PARACAMPO A, LIZARRALDE M, COVAIN R and MONTOYA-BURGOS JI. 2016. Hypostomus formosae, a new catfish species from the Paraguay River Basin with redescription of H. boulengeri (Siluriformes: Loricariidae). Ichthyol Explor Freshwaters 27: 9-23.
- CARDOSO YP, BRANCOLINI F, PROTOGINO L and LIZARRALDE M. 2011. Actinopterygii, Siluriformes, Loricariidae, Hypostomus aspilogaster (Cope, 1894). Distribution extension and first record for Argentina. Check List 7: 596-598.
- DAUBY G. 2017. Computation of Parameters Used in Preliminary Assessment of Conservation Status. Available from: https://cran.r-project.org/web/packages/ConR/ConR.pdf. (Date of access - September 2017).
» https://cran.r-project.org/web/packages/ConR/ConR.pdf. - DAYRAT B. 2005. Towards integrative taxonomy. Biol J Linnean Soc 85: 407-415.
- DI PERSIA D and NEIFF JJ. 1986. The Uruguay River system. In Davies BR and Walker KF (Eds), The Ecology of River Systems. Monographiae Biologicae, W. Junk Publishers, Dordrecht, p. 599-623.
- DRAY S and DUFOUR AB. 2007. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22: 1-20.
- EIGENMANN CH. 1907. On a collection of fishes from Buenos Aires. Proc Washigt Acad Sci 8: 449-458.
- EIGENMANN CH and EIGENMANN RS. 1888. Preliminary notes on South American Nematognathi. I. Proc Calif Acad Sci (Series 2) 1: 119-172.
- ESCHMEYER WN, FRICKE R and VAN DER LAAN R. 2017. Catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Electronic version accessed 17 Set 2017. [This version was edited by Bill Eschmeyer].
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. - FELSENSTEIN J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783-791.
- FERRARIS CJ JR. 2007. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa 1418: 1-628.
- FREPLATA. 2005. Análisis Diagnóstico Transfronterizo del Río de la Plata y su Frente Marítimo. Protección Ambiental del Río de la Plata y su Frente Marítimo: Prevención y Control de la Contaminación y Restauración de Hábitats. Documento Técnico. Proyecto PNUD/GEF RLA/99/G31. Montevideo, Uruguay.
- GALINDO G, SAINATO C, DAPEÑA C, FERNÁNDEZ-TURIEL JL, GIMENO D, POMPOSIELLO MC and PANARELLO HO. 2007. Surface and groundwater quality in the northeastern region of Buenos Aires Province, Argentina. J South Am Earth Sci 23: 336-345.
- GARAVELLO JC, BRITSKI HA and ZAWADZKI CH. 2012. The cascudos of the genus Hypostomus Lacépède (Ostariophysi: Loricariidae) from the rio Iguaçu basin. Neotrop Ichthyol 10: 263-283.
- GOSLINE WA. 1947. Contributions to the classification of the loricariid catfishes. Arq do Mus Nac Rio de Janeiro 41: 79-134.
- GÜNTHER A. 1880. A contribution to the knowledge of the fish fauna of the Río de la Plata. J Nat Hist 6: 7-13.
- HALL T. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symposium Ser 41: 95-98.
- HARO JG and BISTONI MA. 2007. Peces de Córdoba. Primera edición. Córdoba. Universidad Nacional de Córdoba, 246 p.
- HENSEL R. 1870. Beiträge zur Kenntniss der Wirbelthiere Südbrasiliens. (Fortsetzung). Archiv Naturgesch 36: 50-91.
- HILLS M. 1978. On ratios - a response to Atchley, Gaskins, and Anderson. Syst Zool 27: 61-62.
- HORTAL F, DE BELLO J, DINIZ-FILHO AF, LEWINSOHN TM, LOBO JM and LADLE RJ. 2015. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol S 46: 523-549.
- HUELSENBECK JP and RONQUIST F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754-755.
- ICZN - INTERNATIONAL COMMISSION ON ZOOLOGICAL NOMENCLATURE. 1999. International Code of Zoological Nomenclature [the Code]. 4th ed., London.The International Trust for Zoological Nomenclature, c/o Natural History Museum.
- INCYTH. 1978. Caudal sólido transportado por el río Uruguay. CTM Saito Grande 5ta.RDA/ 1.9, 8 pp.
- ISASMENDI S, TRACANNA B, VENDRAMINI F, NAVARRO M, BARRIONUEVO M and MEONI S. 2007. Caracterización física y química de ríos de montaña (Tafí del Valle-Tucumán-Argentina). Limnetica 26: 129-142.
- IUCN. 2001. IUCN Red List categories and criteria. Version 3.1. IUCN Species Survival Commission, Gland, Switzerland and Cambridge, U.K.
- IUCN STANDARDS and PETITIONS SUBCOMMITTEE. 2017. Guidelines for using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee.Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf (Date of access - March 2017).
» http://www.iucnredlist.org/documents/RedListGuidelines.pdf - KOERBER S and WEBER C. 2014. The Hypostominae (Siluriformes: Loricaridae) of Argentina. Ichthyol Contrib PecesCriollos 29: 1-10.
- KUMAR S, STECHER G and TAMURA K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 33: 1870-1874.
- LIOTTA J. 2017. Base de datos de peces de aguas continentales de Argentina. Publicación electrónica. Available from: http://www.pecesargentina.com.ar (Date of access - June 2017).
» http://www.pecesargentina.com.ar - LITZ TO and KOERBER S. 2014. Check List of the Freshwater Fishes of Uruguay (CLOFF-UY). Ichthyol Contrib PecesCriollos 28: 1-40.
- LÓPEZ HL and MIQUELARENA A. 1991. Los Hypostominae (Pisces: Loricariidae) de Argentina. Programa de Fauna de Agua Dulce. PROFADU (CONICET), Museo de La Plata 40: 1-64.
- LUNDBERG JG, MARSHALL LG and GUERRERO J. 1998. The stage for Neotropical fish diversification: a history of Tropical South American rivers. In: Malabarba LR, Reis RE, Vari RP, Lucena M and Lucena CAS (Eds), Phylogeny and classification of Neotropical fishes. Edipucrs, Porto Alegre, p. 13-48.
- MALABARBA LR. 1989. Histórico sistemático e lista comentada das espécies de peixes de água doce do sistema da Laguna dos Patos, Rio Grande do Sul, Brasil. Comunicações do Museu de Ciências e Tecnologia da PUCRS, Série Zoologia 2: 107-179.
- MAZZONI R, CARAMASCHI U and WEBER C. 1994. Taxonomical revision of the species of Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae) from the Lower rio Paraiba do Sul, State of Rio de Janeiro, Brazil. Rev Suisse Zool 5: 3-18.
- MILLER MA, PFEIFFER W and SCHWARTZ T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE): 1-8.
- MIQUELARENA A, MENNI RC, LÓPEZ HL and CASCIOTTA J. 1990. Ichthyological and limnological observations on the Salí river basin (Tucumán, Argentina). Ichthyol Explor Freshwaters 1: 269-276.
- MIRANDE JM and KOERBER S. 2015. Check List of the Freshwater Fishes of Argentina (CLOFF-AR). Ichthyol Contrib PecesCriollos 36: 1-68.
- MONTOYA-BURGOS JI. 2003. Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Mol Ecol 12: 1855-1867.
- OYAKAWA OT, AKAMA A and ZANATAAM. 2005. Review of the genus Hypostomus Lacépède, 1803 from rio Ribeira de Iguape basin, with description of a new species (Pisces, Siluriformes, Loricariidae). Zootaxa 921: 1-27.
- PELUSO L, ABELANDO M, APARTÍN CD, ALMADA P and RONCO AE. 2013. Integrated ecotoxicological assessment of bottom sediments from the Paraná basin, Argentina. Ecotox Environ Safe 98: 179-186.
- QUIROS R and CUCH S. 1989. The fisheries and limnology of the lower Plata Basin. In Dodge DP (Ed), Proceedings of the International Large River Symposium. Canadian Special Publications of Fisheries and Aquatic Sciences (106), Ontario, p. 429-443.
- R CORE TEAM. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
» http://www.R-project.org/ - REGAN CT. 1913. Description of a new loricariid fish of the genus Plecostomus from Rio Janeiro. Ann Mag Nat Hist (Series 8) 12: 555.
- REIS RE, WEBER C and MALABARBA LR. 1990. Review of the genus Hypostomus Lacèpéde, 1803 from Southern Brazil, with descriptions of the three new species (Pisces, Siluriformes, Loricariidae). Rev Suisse Zool 97: 729-766.
- RINGUELET RA. 1975. Zoogeografía y ecología de los peces de aguas continentales de la Argentina y consideraciones sobre las áreas ictiológicas de América del Sur. Ecosur 2: 1-122.
- RINGUELET RA, ARÁMBURU RH and ALONSO DE ARÁMBURU A. 1967. Los peces argentinos de agua dulce. Comisión de Investigaciones Científicas de la Provincia de Buenos Aires.
- RONQUIST F, TESLENKO M, VAN DER MARK P, L AYRES D, DARLING A, HÖHNA S and HUELSENBECK JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61: 539-542.
- SILVA G, ROXO FF, LUJAN NK, TAGLIACOLLO VA, ZAWADZKI CH and OLIVEIRA C. 2016. Transcontinental dispersal, ecological opportunity and origins of an adaptive radiation in the Neotropical catfish genus Hypostomus (Siluriformes: Loricariidae). Mol Ecol 25: 1511-1529.
- STEINDACHNER F. 1877. Die Süsswasserfische des südöstlichen Brasilien (III). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Classe 74.
- VALENCIENNES A. 1834-39. Poissons [plates]. In: A. d’Orbigny. Voyage dans l’Amérique méridionale. Pls. 1-16.
- WEBER C. 1985. Hypostomus dlouhyi, nouvelle espéce de poisson-chat cuirassé du Paraguay (Pisces Siluriformes, Loricariidae). Rev Suisse Zool 92: 955-968.
- WEBER C. 1986. Revision de Hypostomus boulengeri (Eigenmann and Kennedy), et deux espèces nouvelles de poissons-chats du Paraguay (Pisces, Siluriformes, Loricariidae). Rev Suisse Zool 93: 979-1007.
- WEBER C. 2003. Subfamily Hypostominae. In: Reis RE, Kullander SO and Ferraris CJ Jr (Eds), Check List of the freshwater fishes of South and Central America, Edipucrs, Porto Alegre, p. 351-372.
- WEYENBERGH H. 1877. Algunos nuevos pescados del Museo Nacional y algunas noticias ictiológicas. Actas Acad Nac Ciencias Exactas Córdoba 3: 1-37.
Publication Dates
-
Publication in this collection
25 Apr 2019 -
Date of issue
2019
History
-
Received
8 Feb 2018 -
Accepted
11 Aug 2018