Abstracts
PURPOSE: To study the effect of the modulation of inositol hexaphosphate (IP6) in the biological immunohistochemistry expression of cellular signaling marker apoptosis, in model of carcinogenesis of colon induced by azoxymethane (AOM). METHODS: Wistar rats (N=112) distributed in 4 groups (n=28): Control; B, AOM (5 mg kg-1, 2x, to break week 3); C, IP6 (in water 1%, six weeks); D, IP6+AOM. Weekly euthanasia (n=7), from week three. Immunohistochemistry of ascendant colon with biological marker inositol 1,4,5 triphosphate receptor type III (Itpr3). Quantification of the immune-expression with use of computer-assisted image processing. Analysis statistics of the means between groups, weeks in groups, groups in weeks, and established significance when p<0.05. RESULTS: One proved significant difference between groups in the expression of Itpr3, p<0.0001; with Itpr3 reduction of BxD group, p<0.001. CONCLUSION: Inositol hexaphosphate promotes modulation of biological markers with reduction of Itpr3 in carcinogenesis of colon.
Apoptosis; Azoxymethane; Cancer; Colon; Inositol 1,4,5 trisphosphate receptors; Computer-assisted image processing; Phytic acid; Rats
OBJETIVO: Estudar os efeitos da modulação do inositol hexafosfato (IP6) na expressão imunoistoquímica de marcador biológico de sinalização celular de apoptose, em modelo de carcinogênese induzida pelo azoximetano (AOM). MÉTODOS: Ratos Wistar (N=112) distribuídos em 4 grupos (n=28): A, controle; B, AOM (5 mg Kg-1, 2x, a partir semana 3); C, IP6 (em água a 1%, seis semanas); D, IP6+AOM. Eutanásia semanal (n=7), a partir de semana três. Imunoistoquímica de colo ascendente com marcador biológico inositol 1,4,5 trisphosphate receptor type III (Itpr3). Quantificação da imunoexpressão com uso de processamento imagem assistida computador. Análise estatística da expressão média entre grupos, semanas em grupos e grupos em semanas, e estabelecido significância quando p<0.05. RESULTADOS: Evidenciou-se diferença significante entre grupos na expressão de Itpr3, p<0.0001; com diminuição Itpr3 de grupo BxD, p<0.001. CONCLUSÃO: O inositol hexafostato promove a modulação de marcador biológico com diminuição Itpr3 em carcinogênese de colo.
Apoptose; Ácido fítico; Azoximetano; Câncer; Colo; Receptores de inositol 1,4,5-trifosfato; Processamento imagem assistida computador; Ratos
ARTIGO ORIGINAL
EXPERIMENTAL ONCOLOGY
Apoptotic effects of inositol hexaphosphate on biomarker Itpr3 in induced colon rat carcinogenesis1 1 Research performed at Post-graduation Program in Surgery and Experimentation, Department of Surgery, Federal University of São Paulo (UNIFESP-SP), São Paulo, Brazil.
Efeito de apoptose do inositol hexafosfato no marcador biológico Itpr3 em carcinogênese induzida de colo em ratos
Guido MarksI; Djalma José FagundesII; Celso Massaschi YnouyeIII; Elenir Rose Jardim Cury PontesIV; Luiz Carlos TakitaV; Eva Glória Siufi do AmaralV; Roberto TeruyaV; Manoel Catarino PaesV; José Lacerda BrasileiroV; Ricardo Dutra AydosIII
IMD, Master, Pos-Graduate, PhD, Post-graduation Program in Surgery and Experimentation, Department of Surgery, UNIFESP-SP, São Paulo, Brazil
IIMD, Master, PhD, Associate Professor of Surgery, Division of Operative Technique and Experimental Surgery, Department of Surgery, UNIFESP-SP, Brazil
IIIPhD, Associate Professor, Department of Surgery, UFMS-MS, Brazil
IVPhD, Associate Professor, Department of Food Technology and Public Health, UFMS-MS, Brazil
VMD, Master, Assistant Professor, Department of Surgery, UFMS-MS, Brazil
Correspondence Correspondence: Guido Marks Rua Pedro Celestino 2541 79002372 Campo Grande, MS, Brasil. Fone/fax: (67).30274030 gmarks@brturbo.com.br
ABSTRACT
PURPOSE: To study the effect of the modulation of inositol hexaphosphate (IP6) in the biological immunohistochemistry expression of cellular signaling marker apoptosis, in model of carcinogenesis of colon induced by azoxymethane (AOM).
METHODS: Wistar rats (N=112) distributed in 4 groups (n=28): Control; B, AOM (5 mg kg-1, 2x, to break week 3); C, IP6 (in water 1%, six weeks); D, IP6+AOM. Weekly euthanasia (n=7), from week three. Immunohistochemistry of ascendant colon with biological marker inositol 1,4,5 triphosphate receptor type III (Itpr3). Quantification of the immune-expression with use of computer-assisted image processing. Analysis statistics of the means between groups, weeks in groups, groups in weeks, and established significance when p<0.05.
RESULTS: One proved significant difference between groups in the expression of Itpr3, p<0.0001; with Itpr3 reduction of BxD group, p<0.001.
CONCLUSION: Inositol hexaphosphate promotes modulation of biological markers with reduction of Itpr3 in carcinogenesis of colon.
Key words: Apoptosis. Azoxymethane. Cancer. Colon. Inositol 1,4,5 trisphosphate receptors. Computer-assisted image processing. Phytic acid. Rats.
RESUMO
OBJETIVO: Estudar os efeitos da modulação do inositol hexafosfato (IP6) na expressão imunoistoquímica de marcador biológico de sinalização celular de apoptose, em modelo de carcinogênese induzida pelo azoximetano (AOM).
MÉTODOS: Ratos Wistar (N=112) distribuídos em 4 grupos (n=28): A, controle; B, AOM (5 mg Kg-1, 2x, a partir semana 3); C, IP6 (em água a 1%, seis semanas); D, IP6+AOM. Eutanásia semanal (n=7), a partir de semana três. Imunoistoquímica de colo ascendente com marcador biológico inositol 1,4,5 trisphosphate receptor type III (Itpr3). Quantificação da imunoexpressão com uso de processamento imagem assistida computador. Análise estatística da expressão média entre grupos, semanas em grupos e grupos em semanas, e estabelecido significância quando p<0.05.
RESULTADOS: Evidenciou-se diferença significante entre grupos na expressão de Itpr3, p<0.0001; com diminuição Itpr3 de grupo BxD, p<0.001.
CONCLUSÃO: O inositol hexafostato promove a modulação de marcador biológico com diminuição Itpr3 em carcinogênese de colo.
Descritores: Apoptose. Ácido fítico. Azoximetano. Câncer. Colo. Receptores de inositol 1,4,5-trifosfato. Processamento imagem assistida computador. Ratos.
Introduction
Inositol 1,4,5-trisphosphate receptor (Itpr) is the primary cytosolic target responsible for the initiation of intracellular calcium (Ca2+) signaling. To fulfill this function, the Itpr depends on interaction with accessory subunits and regulatory proteins. Specific interactions between these modulatory proteins and the Itpr have been described, making it clear that the controlled modulation of the Itpr by its binding partners is necessary for physiological cell regulation. The functional coupling of these modulators with the Itpr can control apoptosis, intracellular pH, the initiation and regulation of neuronal Ca2+ signaling, exocytosis, and gene expression. The pathophysiological relevance of Itpr modulation is apparent when the functional interaction of these proteins is enhanced or abolished by mutation or overexpression. The subsequent deregulation of the Itpr leads to pathological changes in Ca2+ signaling, signal initiation, the amplitude and frequency of Ca2+ signals, and the duration of the Ca2+ elevation. Consequences of this deregulation include abnormal growth and apoptosis1.
Itpr was identified in chromosome 20, localization 20p12, geneID 25679, and participates of the pathway phosphatidylinositol signaling system. The amplitude, frequency, and sub cellular localization of Ca2+ signals play an important role in determining cellular responses. Itpr´s require the second messenger inositol 1,4,5-trisphosphate (IP3) for activation but they are also regulated physiologically via cross-talk with other signaling pathways. Among the signaling pathways that modulate Itpr function are those involving kinases and phosphatases. The Itpr3 acts as a messenger for inositol triphosphate, mediating the pathway signalling of calcium, this why isoform Itpr3 loses feedback of inhibition with cytosol calcium2. However, has evidence that the calcium increases the sensitivity of the Itpr3 in unbroken cells and supports the concept that this isoform can act as a target for signaling calcium (hormone-induced)3.
Failure of apoptosis may be of particular importance in the development of colorectal cancer. Evidence indicates that the regulatory pathways of apoptosis are frequently disabled in colorectal carcinoma (CRC), with an increased threshold for its activation and a progressive disorder of apoptotic homeostasis during carcinogenesis as genomic instability progressively increases4. As a consequence, genetically defective cells escape apoptotic deletion with the possible survival of clones possessing biologically significant mutations. Apoptosis is one Ca2+-mediated event that may be influenced differently by each Itpr isoform. A number of studies have shown that reducing Itpr expression inhibits apoptosis5 however, type Itpr3 was selectively increased during apoptosis6.
Elucidation of the critical events associated with carcinogenesis provides the opportunity to prevent cancer development through induction of apoptosis, particularly by bioactive agents or functional foods7.
Thus, understanding the modulation of death receptors by chemopreventive agents and their implications in chemoprevention may provide a rational approach for using such agents alone or in combination with other agents to enhance death receptor-mediated apoptosis as a strategy for effective chemoprevention of cancer8. This study was conducted to test the ability of inositol hexaphosphate9 (IP6; or phytic acid) to inhibit alteration in biomarkers Itpr3 in azoxymethane (AOM) induced colon tumorigenesis when IP6 was fed to rats during the short carcinogenesis.
Methods
The study was evaluated by the Animal Ethics Committee of UFMS, approved, and certified by Protocol 064/2004 and to authenticate by Research Ethics Committee of UNIFESP-Hospital São Paulo by protocol 0183/06.
Sample
112(N) male Wistar rats (Rattus norvegicus albinus), eight-week-old, weighted 117 ± 29g, were selected from the Central Animal Facilities of UFMS.
Environment
The experiment was carried out in the Laboratory of Experimental Carcinogenesis of the Central Animal Facilities of UFMS. Polypropylene cages (standard size for 5 rats) with galvanized wire lids were used, each housing four animals randomly selected by draw. The animals were then acclimated to the housing conditions for 7 days under artificial light (130 to 325 lux) with light/dark cycles of 12h each, mean temperature of 22 ± 3°C, and mean humidity of 58 ± 13%. They were fed ad libitum with rat chow (Nuvilab CR1, Nuvital Nutrientes e Produtos Veterinários Ltda, Curitiba, Brazil) and filtered water.
Experimental design
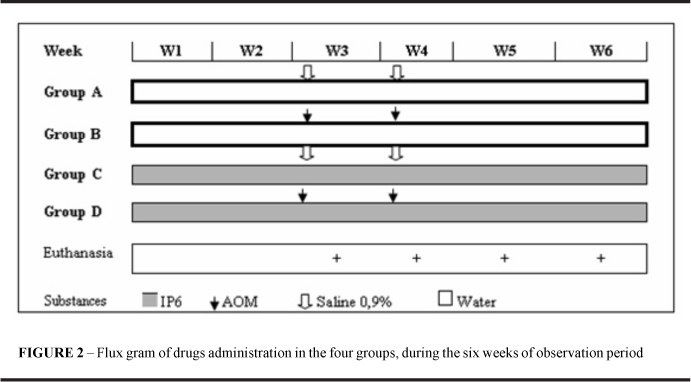
The 112 rats were distributed into four groups by draw. Tail tattoos, made with an indelible black ink pen, were used to identify groups (A through D) and individual animals (A through Q) within each group. Groups distribution was as follows: group A (control): 28(n) untreated animals; group B (AOM): 28(n), receiving AOM alone; group C(IP6): 28(n), receiving IP6 alone; group D (IP6+AOM): 28(n) animals receiving IP6 and AOM.
Administration of substances
IP6 (antitumoral substance, C6H6O24P6 Na12. Sigma, cat. P3168) was administered as a 1% solution in drinking water ad libitum to groups C and D, for six weeks. AOM (carcinogenic substance, C2H6N2O. Sigma, cat. A9517, lot 014K0719) was administered subcutaneously at 5 mg/kg of body weight to groups B and D in the beginning of the third and fourth weeks. At the same times to the groups A and C were given 0.9% saline solution subcutaneously at 10 ml/kg of body weight. The volumes were equivalent to those of diluted AOM administered to groups B and D (Figure 2).
Euthanasia
At the beginning of the third, fourth, fifth or sixth week, the animals were identified, weighed, and subjected to euthanasia by intraperitoneal thiopental (150 mg/kg of body weight), about 3 hours post-administration of AOM in groups B and D.
Collecting colon samples
Each rat was positioned in dorsal decubitus and submitted to midline laparotomy. The ileocecal region was then identified and the terminal ileum and ascendant colon were excised en bloc.
Histological procedure
Each colon and ileum sample was opened along the antimesenteric border and the mucosa was rinsed with Ringer's solution. A 1-cm long segment was resected from the ascendant colon, inicially in the ileocolic transition. The segment of colon wall was sandwiched between the two plates of a hinged perforated double holder in order to be maintained straightened, and the resulting set was immersed in 10% buffered formaldehyde solution for 24 hours. Each sample was embedded in paraffin (with sample identification being preserved) and sectioned with a microtome (2-micrometer-thick sagital sections of colon wall). The resulting sections were mounted on silanized glass slides.
Immunohistochemistry procedure
The colon was prepared with biological marker inositol 1,4,5 trisphosphate receptor type III (Itpr3, primary antibodies, Santa Cruz Biotechnology Inc., cat. SC7277, lot G1604; 1:100 dilution) and followed by secondary antibody anti-rat (Dako LSABTM, K609 product, lot 15068); revealer of color DAB (diaminobenzidine, DakoTM, K3468 product, lot 01317, in 1:10 dilution); Itpr3 expression was obtained as brown color areas.
Microscopy, image capture and photographic optimization
Optical microscopy was performed with a microscope (NikonTM, mod. Elipse E200) equipped with 10X binocular lenses and a 40X/0.65 objective. The microscope was coupled to a video camera (SamsungTM, mod. SCC131) connected to a microcomputer (DuronTM 750-MHz processor, 128-MB RAM, 20-GB hard disk, Microsoft WindowsTM 98 SE) through a video board (Pinnacle StudioTM PCTV, USB). The captured image was saved for in separated archives with previous codification. All arquives were submitted for optimization of the photographic quality with use of Adobe PhotoshopTM Software 7.0 / image / adjusted / canal RGB / curves / automatic.
Itpr3 measure
Itpr3 was quantified by computer-assisted image processing with Image LabTM software. Ten single images from each sample were captured, always centered on the crypt. The optimized image crypts were randomly captured, saved and codified scheme. The measure were done by double-blind method. The quantified brown coloration density corresponds to Itpr3 expression in percentage area (using a RGB filter, blue background, 0-to-147 color interval) in the selected crypt (Figure 3).
Statistical analysis
For data analysis was used the statistical program Epi-InfoTM and BioEstatTM. A 95% confidence interval and a 5% significance level (p<0.05) were adopted. ANOVA was used for comparing: (a) the mean weights of animals in each group versus all groups at the beginning of the experiment; (b); the mean values of Itpr3 expression, as revealed by immunohistochemistry processing, is paired comparison .Variance Test the mean differences between the initial and final weights in all groups. Kruskal-Wallis test to comparing: in group, week versus week; in week, group versus group. Dunn test or Student-Newman-Keuls test to comparing: in week, differences between weeks.
Results
General observations
No significant differences were found when the mean weight gains were compared between groups (p=0.2274, ANOVA) and with the weight profit (p=0.9131, Variance test). No deaths occurred in the experimental period. Three samples failed to be collected because of losses during microtomy or because sectioning made them unusable.
Effect on Itpr3 with administration of AOM
In Group B, significant difference occurred between the weeks (Table 1, W3xW4xW5xW6, p=0.0005). There was an increase of Itpr3 expression of week 3 and 4 for the week 5 (Table 1,W3xW5, p<0.001; W4xW5, p<0.001) and subsequent significant reduction of week 5 for week 6 (Table 1,W5xW6, p<0.001). Significant increase in Itpr3 expression was found on week 5 with administration of AOM (Table 1, Group AxB, p<0.001).
Effect on Itpr3 with administration of IP6
Significant difference between Groups was showed (Table 1, AxBxCxD, p<0.0001); a significant reduction of Itpr3 expression ocurred after AOM+IP6 administration (Table 1, BxD, p<0.001). The Itpr3 expression increased significantly in group D (Table 1, AxD, p=0.0164) in comparison with group A (without any drug). The comparison of weeks in each group evidenced: a) in week 5, significant difference between Groups (Table 1, AxBxCxD, p<0.0001), with reduction in the Itpr3 expression when is administred IP6+AOM (Table 1, BxD, p<0.001); b) in week 6, significant difference between Groups (Table 1, AxBxCxD, p=0.0435), with significant reduction in the Itpr3 expression when the IP6 is administred (Table 1, AxC, p=0.0334).
Discussion
The use of experimental carcinogenesis model of shortness and average duration does not demonstrate difference in the installation of aberrant crypt foci (ACF) in colon10. AOM is recognized as carcinogen inductor of ACF in colorectal tumor, being this used as structure type endpoint in carcinogenesis of colon. The multiple and repeated injection of carcinogen in rats results in increase in the tumor colorectal incidence.
The biomarker use as endpoint is gaining ample acceptance in the precocious diagnosis in experimentations of short term of chemoprevention of the cancer in the place of traditional endpoints intermediate (ex., ACF) of the cancer. Biomarkers genetic molecular can be used as tools in the risk identification, screening and evaluation of cancer cure. Biomarkers presents high potential to identify involved changes in the development of new boarding's in the chemoprevention, and is promising seek area. Biomarkers in cancer prevention are increasingly important tools in primary prevention and in intervention by chemo preventive agents. Biomarkers can be utilized as indicators of exposures, effects and individual susceptibility to cancer. Sampling of biomarkers in relation to exposure may have a great impact on the reliability of mechanism of action11.
This image analysis algorithm provides a robust and flexible method for objective immunohistochemical analysis of samples stained with up to three different stains using a laboratory microscope, standard RGB camera setup. Quantification of the different stains was not significantly influenced by the combination of multiple stains in a single sample. The color deconvolution algorithm resulted in comparable quantification independent of the stain combinations as long as the histochemical procedures did not influence the amount of stain in the sample due to bleaching because of stain solubility and saturation of staining was prevented12 and were used in the quantification of immunohistochemistry13.
Genotoxic carcinogens, such as azoxymethane, alkylate DNA, forming DNA adducts, induction of mutation of gene K-ras and gene b-catenin (G-to-A transitions). K-ras mutations at codon 12 may contribute to induce hyperplastic changes, while b-catenin mutations seem to be involved in generation of dysplastic lesions. These carcinogens cause DNA adducts, resulting in initiating mutations and subsequent development of tumours14. DNA damage due to carcinogens causes 'nuclear anomalies' in the proliferative compartment of the crypt colon. Some damaged cells are repaired but a few are not. This failure to delete such mutated cells may give rise to an abnormal clone with the potential to progress to cancer. It has been proposed that this reactive apoptotic response to DNA damage is the biological mechanism for protection against tumorigenesis15.
The redox status of the protein is another parameter that seems to be very important for safeguarding the normal functioning of the channel Itpr. It can be concluded that oxidative stress may result in aberrant activation of the Itpr3 channel and depletion of the Ca2+ stores, thereby disturbing normal Ca2+ signaling16. Although IP3 is necessary to open native Itpr, activation of these channels is complex and their open probability actually depends on the ambient Ca2+ concentration. Up to ~500 nM, Ca2+ works synergistically with IP3 to activate Itpr. At higher concentrations, cytosolic Ca2+ inhibits Itpr-receptor opening. The inhibition of Itpr by Ca2+ is thought to be a crucial mechanism for terminating channel activity and thus preventing pathological Ca2+ rises.
Although some cells and tissues express a single or predominant isoform of the receptor, most cells instead express two or all three Itpr isoforms. Itpr is an intracellular Ca2+ channel that is for the largest part expressed in the endoplasmic reticulum. Its precise sub cellular localization is an important factor for the correct initiation and propagation of Ca2+ signals. The relative position of the Itpr´s, and thus of the IP3-sensitive Ca2+ stores, to mitochondria, nucleus or plasma membrane determines in many cases the physiological consequences of IP3-induced Ca2+ release. Most cell types express more than one Itpr isoform and their sub cellular distribution is cell-type dependent. Moreover, it was demonstrated that depending on the physiological status of the cell redistribution of Itpr´s and/or of IP3-sensitive Ca2+ stores could occur. This indicates that the cell must be able to regulate not only Itpr expression but also its distribution. The various proteins potentially determining Itpr localization and redistribution will therefore be discussed17.
Significant increase of Itpr3 was showed after AOM administration and is concordant with: (a) acute apoptotic reply to the AOM in colon and initialization of tumorigenesis18; (b) Itpr3 selectively is increased in apoptosis6, has active participation of apoptosis in a sample diversity, it stress oxidative results in aberrant activation of the Itpr canal166 and the selective increase during apoptosis of linphocites6.
The comparison of weeks in each group evidenced: Group B, rise in the Itpr3 expression of week 3 for 5 week and posterior significant reduction of week 5 to 6. The initial increase is evidences original and justifies it reduction in the Itpr3 expression: a) when apoptosis is induced for the extrinsically or intrinsic pathway, the induction will be more efficient through the inhibition of the Itpr3 and the transmission of calcium signals for mitochondria19; b) chronic activation of hydrolysis of phosphoinosídes can reduce Itpr320; c) evidence of that Itpr3 reduction inhibits apoptosis5.
The comparison between groups to each week evidenced: a) in week 5, with increase in the expression of Itpr3 of the control in comparison to the AOM and reduction in the Itpr3 expression when is administred associated IP6+AOM, demonstrating to be the effective IP6 in neutralizing the AOM; b) in week 6, significant reduction in the Itpr3 expression when administred IP6 alone in comparison to the control however, was showed that the Itpr3 expression is bigger in group AOM in comparison to the IP6. The related results are associated: a) the Itpr3 demands a higher concentration of IP3 (resultant of IP6 hydrolysis to reach the maximum reaction of 3.2 microM against 0.5 microM for the Itpr1, for comparison effect). Increase in the activity in the canal of membrane in the presence of high concentration of IP3 can be important during periods of drawn out stimulation, considering that the alosteric modulation for the ATP can help in the intracellular modulation of the signaling of Ca2+ 21; b) selective expression of a type of particular receiver will influence the sensitivity of Ca2+ in accordance with the cellular concentration of IP3; c) evidence that Itpr3 plays the special rolls in induction of apoptosis19.
A striking anticancer effect of IP6 was demonstrated in different experimental models and because it is abundantly present in regular diet, efficiently absorbed from the gastrointestinal tract, and safe, IP6 holds great promise in our strategies for the prevention and treatment of cancer. Uncontrolled proliferation is a hallmark of malignant cells, and IP6 can reduce the cell proliferation rate of many different cell lines of different lineage and of both human and rodent origin. Although normal cells divide at a controlled and limited rate, malignant cells escape from the control mechanisms that regulate the frequency of cell multiplication and usually have lost the checkpoint controls that prevent replication of defective cells. IP6 can regulate the cell cycle to block uncontrolled cell division and force malignant cells either to differentiate or go into apoptosis. IP6 induces G1 phase arrest and a significant decrease of the S phase of human breast, colon, and prostate cancer cell lines. However, a cDNA micro array analysis showed a down-modulation of multiple genes involved in transcription and cell-cycle regulation by IP6. Thus, for cancer prevention, prophylactic intake of IP6 may be not only more effective, but also more practical than gorging on large quantities of fiber9.
The study carried through with biomarkers it anticipates and potencialize the identification of alterations for the carcinogenesis stage of promotion of signaling benefit deriving right-handers of its accomplishment. Beyond adding to the IP6 to the list of Itpr modulators1.
Conclusion
Inositol hexaphosphate promotes modulation of biological markers with reduction of Itpr3 in carcinogenesis of colon.
Acknowledgment
Institution: Federal University of Mato Grosso of South, for the financing of biorreagents; Screenlab Laboratory for the execution of the immunohistochemystri processing. People who participle in this study: Mr. Rodrigo Avelar, loboratory technic and Mr. Renan Albuquerque Marks, student of Computation Science.
References
1. Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006; (363):re15.
2. DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002; 277 (42): 39397-400.
3. O'Neill AF, Hagar RE, Zipfel WR, Nathanson MH, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and calcium. Biochem Biophys Res Commun. 2002; 294 (3): 719-25.
4 . Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35 (4): 495-516.
5. Tantral L, Malathi K, Kohyama S, Silane M, Berenstein A, Jayaraman T. Intracellular calcium release is required for caspase-3 and -9 activation. Cell Biochem Funct. 2004; 22 (1): 35-40.
6. Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996; 273 (5274): 503-7.
7. Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans 2003; 31(Pt 6): 1441-4.
8. Sun SY. Chemopreventive agent-induced modulation of death receptors. Apoptosis. 2005; 10 (6):1203-10.
9.Vucenik I, Shamsuddin AM. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr. 2003; 133 (11 Suppl 1): 3778S-3784S.
10. Rodrigues MAM, Silva LAG, Slavador DMF, de Camargo JLU, Montenegro MR. Aberrant crypt foci and colon cancer: comparison between a short- and medium-term biossay for colon carcinogenesis using dimethylhydrazine in Wistar rats. Braz J Med Biol Res. 2002; 35 (3): 351-355.
11. Tompa A. Application of biomarkers in cancer prevention. Orv Hetil. 2006; 147 (14): 627-36.
12. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001; 23 (4): 291-9.
13. Marks G, Aydos RD, Fagundes DJ, Pontes ERJC, Takita LC, Amaral EGAS et al . Modulação do fator transformador de crescimento beta2 (TGFbeta2) pelo inositol hexafosfato na carcinogênese colônica em ratos. Acta Cir. Bras. [periódico na Internet]. [citado 2007 Ago 25]. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102 -86502006001000012&lng=pt&nrm=iso.
14. Hirose Y, Yoshimi N, Makita H, Hara A, Tanaka T and MoriH. Early alterations of apoptosis and cell proliferation in azoxymethane-initiated rat colonic epithelium. Jpn. J. Cancer Res. 1996; 87, 57582.
15. Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. J Zhejiang Univ Sci B. 2007; 8(6):377-97.
16. Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005; 120 (1): 85-98.
17.Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell. 2004; 96 (1): 3-17.
18. Rogers K.J, Pegg AE. Formation of O6-methylguanine by alkylation of rat liver, colon and kidney DNA following administration of 1,2-dimethylhydrazine. Cancer Res. 1977; 37, 40827.
19. Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005; 280 (49): 40892-900.
20. Wojcikiewicz RJ, Nahorski SR. Chronic muscarinic stimulation of SH-SY5Y neuroblastoma cells suppresses inositol 1,4,5-trisphosphate action. Parallel inhibition of inositol 1,4,5-trisphosphate-induced Ca2+ mobilization and inositol 1,4,5-trisphosphate binding. J Biol Chem. 1991; 266 (33): 22234-41.
21. Hagar RE, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and ATP. Biophys J. 2000; 79(1): 271-8.
Received: September 4, 2007
Review: November 12, 2007
Accepted: December 11, 2007
Conflict of interest: none
Financial source: none
- 1. Choe CU, Ehrlich BE. The inositol 1,4,5-trisphosphate receptor (IP3R) and its regulators: sometimes good and sometimes bad teamwork. Sci STKE. 2006; (363):re15.
- 2. DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002; 277 (42): 39397-400.
- 3. O'Neill AF, Hagar RE, Zipfel WR, Nathanson MH, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and calcium. Biochem Biophys Res Commun. 2002; 294 (3): 719-25.
- 4 . Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35 (4): 495-516.
- 5. Tantral L, Malathi K, Kohyama S, Silane M, Berenstein A, Jayaraman T. Intracellular calcium release is required for caspase-3 and -9 activation. Cell Biochem Funct. 2004; 22 (1): 35-40.
- 6. Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996; 273 (5274): 503-7.
- 7. Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans 2003; 31(Pt 6): 1441-4.
- 8. Sun SY. Chemopreventive agent-induced modulation of death receptors. Apoptosis. 2005; 10 (6):1203-10.
- 9.Vucenik I, Shamsuddin AM. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr. 2003; 133 (11 Suppl 1): 3778S-3784S.
- 10. Rodrigues MAM, Silva LAG, Slavador DMF, de Camargo JLU, Montenegro MR. Aberrant crypt foci and colon cancer: comparison between a short- and medium-term biossay for colon carcinogenesis using dimethylhydrazine in Wistar rats. Braz J Med Biol Res. 2002; 35 (3): 351-355.
- 11. Tompa A. Application of biomarkers in cancer prevention. Orv Hetil. 2006; 147 (14): 627-36.
- 12. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001; 23 (4): 291-9.
- 14. Hirose Y, Yoshimi N, Makita H, Hara A, Tanaka T and MoriH. Early alterations of apoptosis and cell proliferation in azoxymethane-initiated rat colonic epithelium. Jpn. J. Cancer Res. 1996; 87, 57582.
- 15. Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. J Zhejiang Univ Sci B. 2007; 8(6):377-97.
- 16. Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005; 120 (1): 85-98.
- 17.Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell. 2004; 96 (1): 3-17.
- 18. Rogers K.J, Pegg AE. Formation of O6-methylguanine by alkylation of rat liver, colon and kidney DNA following administration of 1,2-dimethylhydrazine. Cancer Res. 1977; 37, 40827.
- 19. Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005; 280 (49): 40892-900.
- 20. Wojcikiewicz RJ, Nahorski SR. Chronic muscarinic stimulation of SH-SY5Y neuroblastoma cells suppresses inositol 1,4,5-trisphosphate action. Parallel inhibition of inositol 1,4,5-trisphosphate-induced Ca2+ mobilization and inositol 1,4,5-trisphosphate binding. J Biol Chem. 1991; 266 (33): 22234-41.
- 21. Hagar RE, Ehrlich BE. Regulation of the type III InsP(3) receptor by InsP(3) and ATP. Biophys J. 2000; 79(1): 271-8.
Publication Dates
-
Publication in this collection
26 Mar 2008 -
Date of issue
Apr 2008
History
-
Accepted
11 Dec 2007 -
Reviewed
12 Nov 2007 -
Received
04 Sept 2007