ABSTRACT
The Neotropical genus Scleronema is revised based on the re-examination of the type specimens and 1,713 newly collected specimens. Scleronema is diagnosed from other trichomycterids by the following unambiguous derived characters: fleshy flap at the base of the maxillary barbell; skin flap in the posterior margin of the opercle; articulation between the autopalatine and the vomer ventrally located, with the medial margins of the autopalatines very close to each other; and autopalatine with an interrupted or not interrupted ossified arch-shaped process on its dorsal surface forming a canal. Scleronema minutum and S. operculatum are redescribed, S. angustirostre is considered a junior synonym of S. minutum, and six new species are described. A lectotype is designated for Trichomycterus minutus. The type localities of S. angustirostre, S. minutum, and S. operculatum are reviewed in order to correct erroneous information cited in articles and catalogs subsequent to the original descriptions. Species of Scleronema are geographically distributed in the La Plata basin and Atlantic coastal drainages from Southern Brazil, Southern Paraguay, Northeastern Argentina and Uruguay. They inhabit rivers or streams with sand- or gravel-bottoms across the Pampa grasslands. We provide evidences to recognize two putative monophyletic units within the genus, namely the S. minutum species group and the S. operculatum species group, and discuss the distribution patterns of their species.
Keywords:
Biodiversity; Identification key; Taxonomy; Trichomycterinae; Uruguayan savanna ecoregion
RESUMO
O gênero Neotropical Scleronema é revisado com base na análise do material-tipo e outros 1.713 espécimes recentemente coletados. Scleronema é diagnosticado de outros tricomicterídeos pelos seguintes caracteres derivados não ambíguos: aba de pele na base do barbilhão maxilar; aba de pele na margem posterior do opérculo; articulação entre o autopalatino e o vômer posicionada ventralmente, deixando as margens mediais dos autopalatinos muito próximas entre si; e o autopalatino com um processo com formato de arco na sua superfície dorsal. Scleronema minutum e S. operculatum são redescritas, S. angustirostre é considerada um sinônimo júnior de S. minutum e seis novas espécies são descritas. Um lectótipo é designado para S. minutum. As localidades-tipo de S. angustirostre, S. minutum e S. operculatum são revisadas com o intuito de corrigir informações errôneas citadas em artigos e catálogos após suas descrições. As espécies de Scleronema distribuem-se na bacia do rio da Plata e drenagens costeiras atlânticas no sul do Brasil, sul do Paraguai, nordeste da Argentina e Uruguai, habitando rios e riachos ao longo do Pampa com fundos de areia ou cascalho. Dois grupos de espécies supostamente monofiléticos, o grupo S. minutum e o grupo S. operculatum, são reconhecidos no gênero e os padrões de distribuição de suas espécies são discutidos.
Palavras-chave:
Biodiversidade; Chave de identificação; Savana Uruguaia; Taxonomia; Trichomycterinae
INTRODUCTION
Trichomycteridae is a monophyletic group of freshwater catfishes endemic to the Neotropical region which is classified into eight subfamilies: Copionodontinae, Glanapteryginae, Sarcoglanidinae, Stegophilinae, Trichomycterinae, Trichogeninae, Tridentinae, and Vandelliinae (de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.). Trichomycterids are easily recognized by their highly modified opercular system, involving the interopercular and opercular bones equipped with odontodes (de Pinna, 1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.; Adriens et al., 2010Adriaens D, Baskin JN, Coppens H. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in. In: Nelson JS, Schultze HP, Wilson MVH, editors. Origin and Phylogenetic Interrelationships of Teleosts. München: Verlag Dr. Friedrich Pfeil; 2010. p.337-62.), and comprise the second richest family of Siluriformes with 328 species (Fricke et al., 2020Fricke R, Eschmeyer WN, Fong JD. Species by family/subfamily [Internet]. San Francisco: California Academy of Science; 2020 [updated 2020 Mar 02; cited 2020 Mar 09]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
http://researcharchive.calacademy.org/re...
). The subfamily Trichomycterinae contains the majority of these species, being composed of nine genera: Bullockia Arratia, Chang, Menu-Marque, Rojas, 1978, CambevaKatz, Barbosa, Mattos, Costa, 2018Katz AM, Barbosa MA, Mattos JLO, Costa WJEM. Multigene analysis of the catfish genus Trichomycterus and description of a new South American trichomycterine genus (Siluriformes, Trichomycteridae). Zoosyst Evol. 2018; 94(2):557-66. https://doi.10.3897/zse.94.29872
https://doi.10.3897/zse.94.29872...
, Eremophilus Humboldt, 1805, Hatcheria Eigenmann, 1909, Ituglanis Costa, Bockmann, 1993, Rhizosomichthys Miles, 1943, Scleronema Eigenmann, 1917, Silvinichthys Arratia, 1998, and Trichomycterus Valenciennes, 1832.
Though trichomycterines are usually known to preferentially occur in mountain rivers and rapids, species of Scleronema (Tab. 1) occupy areas of low altitudes in the subtropical region of the La Plata basin (Paraná, Paraguay, and Uruguay rivers) and coastal drainages from Southern Brazil and Uruguay. Their distributions encompass almost the entire area of the South American “Campos” - called “Pampa biome” in Brazil according to IBGE (2004Instituto Brasileiro de Geografia e Estatística (IBGE). Mapa de biomas do Brasil [Internet]. Rio de Janeiro; 2004. Available from: http://www.ibge.gov.br/home/presidencia/noticias/21052004biomashtml.shtm
http://www.ibge.gov.br/home/presidencia/...
, 2019Instituto Brasileiro de Geografia e Estatística (IBGE). Biomas do Brasil 1:250 000 [Internet]. Rio de Janeiro; 2019. Available from: https://www.ibge.gov.br/geociencias/informacoes-ambientais/15842-biomas.html?=&t=acesso-ao-produto
https://www.ibge.gov.br/geociencias/info...
) - an ecological region composed mainly of natural grasslands and herbs with sparse shrubs and tree formations generally in the banks of rivers and streams (Pallarés et al., 2005Pallarés OR, Berretta EJ, Maraschin GE. The south american campos ecosystem. In: Suttie J, Reynolds SG, Batello C, editors. Grasslands of the world. Rome: Food and agriculture organization of the United Nations; 2005. p.171-219.). Among the Brazilian biomes, the Pampa has the lowest percentage of legally protected areas and, according to ICMBio (2016Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). SISBIO-Estatísticas [Internet]. Brasília; 2016. Available from: http://www.icmbio.gov.br/sisbio/estatisticas.html
http://www.icmbio.gov.br/sisbio/estatist...
), having had the fewest number of issued research permits.
Scleronema has long been defined by the large base of the maxillary barbel and by the presence of a skin flap in the posterior margin of the opercle, two unusual characters proposed by Eigenmann (1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.) and corroborated by subsequent authors (e.g., Eigenmann, 1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.; Tchernavin, 1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
; de Pinna, 1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25., 1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.; Arratia, 1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.). Hitherto, some phylogenetic analyses using morphological (Wosiacki, 2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.) and molecular data (Ochoa et al., 2017aOchoa LE, Roxo FF, DoNascimiento C, Sabaj MH, Datovo A, Alfaro M, Oliveira C. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae. Mol phylogenet evol. 2017a; 115:71-81. http://dx.doi.org/10.1016/j.ympev.2017.07.007
http://dx.doi.org/10.1016/j.ympev.2017.0...
; Katz et al., 2018Katz AM, Barbosa MA, Mattos JLO, Costa WJEM. Multigene analysis of the catfish genus Trichomycterus and description of a new South American trichomycterine genus (Siluriformes, Trichomycteridae). Zoosyst Evol. 2018; 94(2):557-66. https://doi.10.3897/zse.94.29872
https://doi.10.3897/zse.94.29872...
) recovered the monophyly of Scleronema.
Currently, the genus Scleronema encompasses three sand-dwelling species: S. angustirostre (Devincenzi, 1942Devincenzi GJ, Teague GW. Ictiofauna del Río Uruguay Medio. Anales del Museo de Historia Natural de Montevideo. 1942; 5(4)1-100.), S. minutum (Boulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35.), and S. operculatumEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.. However, the diversity of the genus seems to be larger in view of the number of undescribed taxa listed for the genus in fish catalogs (de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.; Becker et al., 2013Becker FG, De Fries LCC, Ferrer J, Bertaco VA, Luz-Agostinho KDG, Silva JFPWW et al
. Fishes of the Taquari-Antas river basin (Patos Lagoon basin), southern Brazil. Braz J Biol. 2013; 73(1):79-90. https://doi.org/10.1590/S1519-69842013000100010
https://doi.org/10.1590/S1519-6984201300...
; Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
). Even identifications of Scleronema species given in papers focused on higher-level relationships within Trichomycteridae (Wosiacki, 2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.; Ochoa et al., 2017aOchoa LE, Roxo FF, DoNascimiento C, Sabaj MH, Datovo A, Alfaro M, Oliveira C. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae. Mol phylogenet evol. 2017a; 115:71-81. http://dx.doi.org/10.1016/j.ympev.2017.07.007
http://dx.doi.org/10.1016/j.ympev.2017.0...
; Katz et al., 2018Katz AM, Barbosa MA, Mattos JLO, Costa WJEM. Multigene analysis of the catfish genus Trichomycterus and description of a new South American trichomycterine genus (Siluriformes, Trichomycteridae). Zoosyst Evol. 2018; 94(2):557-66. https://doi.10.3897/zse.94.29872
https://doi.10.3897/zse.94.29872...
), as well as some species recorded in local fish inventories (e.g., Burns et al., 2015Burns MDM, Corrêa F, Cheffe MM, Foster J, Lopes JB, Santos JDM. The fish fauna of Turuçu river, Patos-Mirim lagoon system, Rio Grande do Sul state, southern Brazil. Panam J Aquat Sci. 2015; 10(4):315-22.; Corrêa et al., 2015Corrêa F, Oliveira EFD, Tuchtenhagen T, Pouey J, Piedras S. Ichthyofauna of the hydrographic basin of the Chasqueiro Stream (Mirim Lagoon system, southern Brazil): generating subsidies for conservation and management. Biota Neotrop . 2015; 15(4):e0006. https://doi.org/10.1590/1676-0611-BN-2015-0006
https://doi.org/10.1590/1676-0611-BN-201...
) are uncertain due to the lack of a taxonomic review of the genus. To add some uncertainty to species definition and boundaries, information on type localities and vouchers for the three species of Scleronema given in checklists of trichomycterids or siluriforms (i.e., de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.; Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
; Wosiacki, de Pinna, 2007Wosiacki WB, de Pinna MCC. Família Trichomycteridae. In: Buckup PA, Menezes NA, Ghazzi MAS, editors. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional; 2007. p.67-75.) are inaccurate, therefore requiring revisions.
In this paper, we provide a systematic revision of the genus Scleronema, advanced by the first author during his Doctoral Thesis (Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.), based on the examination of all available type specimens and on a dense sampling of new specimens from the entire geographical distribution of the genus, as well as other trichomycterids.
MATERIAL AND METHODS
Specimens examined are housed at the following fish collections: AMNH, American Museum of Natural History, New York, U.S.A.; ANSP, Academy of Natural Sciences of Drexel University, Philadelphia, U.S.A.; BMNH, Natural History Museum, London, U.K.; FML, Fundación Miguel Lillo, Tucumán, Argentina; FMNH, Field Museum of Natural History, Chicago, U.S.A.; LIRP, Laboratório de Ictiologia de Ribeirão Preto, Faculdade de Filosofia, Letras e Ciências Humanas, Universidade de São Paulo, São Paulo, Brazil; MACN, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina; MCP, Museu de Ciências e Tecnologia, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre, Brazil; MHNM, Museo Nacional de Historia Natural y Antropología, Montevideo, Uruguay; MLP, Museu de La Plata, Instituto de Limnologia, La Plata, Argentina; MPEG, Museu Paraense Emílio Goeldi, Belém, Brazil; MNRJ, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; MZUSP, Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil; UFRGS, Departamento de Zoologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; UMMZ, University of Michigan Museum of Zoology, Ann Arbor, U.S.A.; UNICTIO, Laboratório de Ictiologia, Universidade do Vale do Rio dos Sinos, São Leopoldo, Brazil; USNM, National Museum of Natural History, Smithsonian Institution, Washington D.C., U.S.A.; ZVC-P, Departamento de Zoologia Vertebrados, Universidad de Montevideo, Montevideo, Uruguay.
Specimens identified by “c&s” were cleared and counter-stained for bone and cartilage according to protocol of Taylor, Van Dyke (1985Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9:107-19.); some of these were dissected on the right side of the head. Radiographs (“xr”) were taken of some specimens and additional ones were obtained in the Database of the All Catfish Species Inventory (Morris et al., 2006Morris PJ, Yager HM, Sabaj Pérez MH. ACSImagebase: A digital archive of catfish images compiled by participants in the All Catfish Species Inventory [Internet]. 2006 Available from: http://acsi.acnatsci.org/base.
http://acsi.acnatsci.org/base...
). Osteological illustrations were prepared based on photographs of c&s specimens in a stereomicroscope with a Nikon AZ100M camera attached.
Morphometrics. Measurements were taken point-to-point using a digital caliper to the nearest 0.1 mm and presented as percentages of standard length (SL) or head length (HL) for its subunits. Measurements follow Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) for barbel length; Bockmann, Sazima (2004Bockmann FA, Sazima I. Trichomycterus maracaya, a new catfish from the upper rio Paraná, southeastern Brazil (Siluriformes: Trichomycteridae), with notes on the T. brasiliensis species-complex. Neotrop Ichthyol. 2004; 2(2):61-74. https://doi.org/10.1590/S1679-62252004000200003
https://doi.org/10.1590/S1679-6225200400...
) for total length, body width, distance between snout tip and posterior nare, intranarial length, anterior internarial width, and posterior internarial; Wosiacki, de Pinna (2008Wosiacki WB, de Pinna MCC. Trichomycterus igobi, a new catfish species from the Rio Iguaçu drainage: the largest head in Trichomycteridae (Siluriformes: Trichomycteridae). Neotrop Ichthyol . 2008; 6(1):17-23. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) for length and depth of the caudal peduncle and for supraorbital pore s6 distance; and Ferrer, Malabarba (2011Ferrer J, Malabarba LR. A new Trichomycterus lacking pelvic fins and pelvic girdle with a very restricted range in southern Brazil (Siluriformes: Trichomycteridae). Zootaxa, 2011: 2912:59-67. http://dx.doi.org/10.11646/zootaxa.2912.1.5
http://dx.doi.org/10.11646/zootaxa.2912....
) for scapular girdle width. Remaining measurements follow Costa (1992Costa WJEM. Description de huit nouvelles espèces du genre Trichomycterus (Siluriformes: Trichomycteridae), du Brésil oriental. Rev Fr Aquariol . 1992; 18(4):101-10.).
Counts. Vertebral count excludes those in the Weberian complex; the compound caudal centrum was counted as a single element. Abdominal vertebrae are those without haemal arch and haemal spine, sensude Pinna (1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.) explained by Datovo, Bockmann (2010Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol . 2010; 8(2):193-246. https://doi.org/10.1590/S1679-62252010000200001: 237). Counts of fin rays include the unbranched ones plus branched ones. Number of branchiostegal rays, odontodes, pterygiophores, ribs, teeth, vertebrae and procurrent rays (those anterior to unbranched rays of dorsal, anal, and caudal fins) were counted only in c&s and xr specimens. Rays of paired fins and branchiostegal rays were counted in both sides of specimens and, if variable, the counts of each side separated by a slash.
Nomenclature. Nomenclature and homologies of laterosensory canal system and associated pores follow Rizzato, Bichuette (2016Rizzato PP, Bichuette ME. The laterosensory canal system in epigean and subterranean Ituglanis (Siluriformes: Trichomycteridae), with comments about troglomorphism and the phylogeny of the genus. J Morphol. 2016; 278(1):4-28. https://doi.org/10.1002/jmor.20616
https://doi.org/10.1002/jmor.20616...
). Nomenclature for caudal skeleton morphology follows Lundberg, Baskin (1969Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1-49.) and for remaining bones follows, whenever possible, de Pinna (1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.) with few variations discussed by Arratia (1998Arratia G. Silvinichthys, a new genus of trichomycterid catfishes from the Argentinian Andes, with redescription of Trichomycterus nigricans. Ichthyol Explor Freshw. 1998; 9(4):347-70.), Bockmann, Sazima (2004Bockmann FA, Sazima I. Trichomycterus maracaya, a new catfish from the upper rio Paraná, southeastern Brazil (Siluriformes: Trichomycteridae), with notes on the T. brasiliensis species-complex. Neotrop Ichthyol. 2004; 2(2):61-74. https://doi.org/10.1590/S1679-62252004000200003
https://doi.org/10.1590/S1679-6225200400...
) and Bockmann et al. (2004Bockmann FA, Casatti L, de Pinna MCC. A new species of trichomycterid catfish from the rio Paranapanema basin, southeastern Brazil (Teleostei: Siluriformes), with comments on the phylogeny of the family. Ichthyol Explor Freshw . 2004; 15(3):225-42.) who named the “lacrimal” as “antorbital” and the “supraoccipital” as “parieto-supraoccipital”. Nomenclature for bones not cited in de Pinna (1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.) follows Bockmann, Sazima (2004Bockmann FA, Sazima I. Trichomycterus maracaya, a new catfish from the upper rio Paraná, southeastern Brazil (Siluriformes: Trichomycteridae), with notes on the T. brasiliensis species-complex. Neotrop Ichthyol. 2004; 2(2):61-74. https://doi.org/10.1590/S1679-62252004000200003
https://doi.org/10.1590/S1679-6225200400...
).
Species accounts. All obtainable type specimens were measured and radiographed. Asterisk indicates lots with specimens measured for species descriptions. Number of specimens with each count given in parentheses, also noting the count observed in the holotypes, paratypes, lectotypes, or paralectotypes. The synonym lists were based on the analysis of the vouchers cited in the literature whenever possible. Alternatively, species identity was confirmed by photographs or illustrations provided in publications or through exclusive geographical distribution of some taxa. External characters have preference in the diagnosis rather than internal osteological traits observed only in c&s or xr specimens. If necessary, doubtful and incomplete geographical coordinates were corrected or estimated according to the available information and use of the Google Earth software. Type localities of S. angustirostre, S. minutum and S. operculatum are clarified following the recommendation 76A of the International Code of Zoological Nomenclature (ICZN, 1999International Commission on Zoological Nomenclature (ICZN). International code of zoological nomenclature. 4th ed. London: International trust for zoological nomenclature Natural History Museum [Internet]. London; 1999. Available from: https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/
https://www.iczn.org/the-code/the-intern...
), as well as a lectotype is designated for Trichomycterus minutus according to its Article 74.
RESULTS
Scleronema Eigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.
ScleronemaEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.: 691. -Eigenmann, 1918: 260, 269 (distribution notes); 277 (relationships); 278 (diagnosis in key); plate 36 (distribution map). -Tchernavin, 1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
: 234, 235, 272 (review). -Myers, 1944Myers GS. Two extraordinary new blind nematognath fishes from the Rio Negro, representing a new subfamily of Pygidiidae, with a rearrangement of the genera of the family, and illustrations of some previously described genera and species from Venezuela and Brazil. Proc Calif Acad Sci. 1944; 23(40):591-602.: 597 (diagnosis in key). -Gosline, 1945Gosline WA. Catálogo dos nematognatos de água-doce da América do sul e central. Bol Mus Nac Rio de Janeiro Zool. 1945; 33:1-138.: 55 (listed). -Fowler, 1954Fowler HW. Os peixes de água doce do Brasil. São Paulo: Arq Zool Estado São Paulo. 1954; (vol 2).: 37 (listed). -Myers, Weitzman, 1966Myers GS, Weitzman SH. Two remarkable new trichomycterid catfishes from the Amazon basin in Brazil and Colombia. J Zool Lond. 1966; 149(3):277-87.: 278, 284, 285 (phylogenetic relationships). -Ringuelet et al., 1967Ringuelet RA, Arámburu RH, Arámburu AA. Los peces argentinos de agua dulce. La Plata: Comisión de Investigación Científica; 1967.: 351 (diagnosis in key). -Baskin, 1973Baskin JN. Structure and relationships of the Trichomycteridae. [PhD Thesis]. New York: City University of New York; 1973.: 79, 98 (listed, notes on mouth width). -Castello et al., 1978Castello HP, Ehrlich MD, Wais IR, Puig A. Adiciones a la fauna de los peces de los rios Paraná medio y Bermejo. Rev Mus Argent Cienc Nat “Bernardino Rivadavia”. 1978; 12(9):119-35.: 124-125 (listed, diagnosis). -Burgess, 1989Burgess WE. An atlas of freshwater and marine catfishes: a preliminary survey of the Siluriformes. Neptune City: T.F.H. Publications; 1989.: 307, 313, 321 (listed, diagnosis in key). -Arratia, 1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.: 400 (diagnosis). -de Pinna, 1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.: 9, 24, 25, 28, 29; fig. 21, 30, 31, 35 (putative apomorphic characters, phylogenetic relationships, phylogenetic tree). -de Pinna, 1992: 213 (notes on dorsal-fin position). -Costa, Bockmann, 1993Costa WJEM, Bockmann FA. Un nouveau genre néotropical de la famille des Trichomycteridae (Siluriformes: Loricarioidei). Rev Fr Aquariol. 1993; 20(2): 43-46.: 45 (phylogenetic relationships). -Arratia, Huaquin, 1995Arratia G, Huaquín L. Morphology of the lateral line system and of the skin of diplomystids and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonn Zool Monogr. 1995; 36:1-110.: 21; fig. 9b, 25, 27, 28, 29, 30 (notes on laterosensory canal system; drawing of the neurocranium and laterosensory canal system from dorsal view). -Eschmeyer, 1998Eschmeyer WN. Catalog of fishes. California Academy of Sciences, San Francisco; 1998.: 2123 (listed). -Arratia, 1998Arratia G. Silvinichthys, a new genus of trichomycterid catfishes from the Argentinian Andes, with redescription of Trichomycterus nigricans. Ichthyol Explor Freshw. 1998; 9(4):347-70.: 366, 367; fig. 14e, 368 (phylogenetic relationships, drawing of the neurocranium and laterosensory canal system from dorsal view). -de Pinna, 1998: 299; fig. 10 (phylogenetic relationships, synapomorphies). -Nion et al., 2002Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres communes. Montevideo: Dinara; 2002.: 15 (listed). -Wosiacki, 2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.: 227, 324; fig. 87 (synapomorphies, phylogenetic relationships). -de Pinna, Wosiacki, 2003: 278 (listed). -Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
: 413 (listed). -Datovo, Bockmann, 2010Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol . 2010; 8(2):193-246. https://doi.org/10.1590/S1679-62252010000200001: 233; fig. 37, 235-238 (phylogenetic tree, phylogenetic relationships). -Adriaens et al., 2010Adriaens D, Baskin JN, Coppens H. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in. In: Nelson JS, Schultze HP, Wilson MVH, editors. Origin and Phylogenetic Interrelationships of Teleosts. München: Verlag Dr. Friedrich Pfeil; 2010. p.337-62.: 349, 357; fig. 10 (notes on length of the interopercular odontodes and body elongation). -DoNascimiento, 2015DoNascimiento C. Morphological evidence for the monophyly of the subfamily of parasitic catfishes Stegophilinae (Siluriformes, Trichomycteridae) and phylogenetic diagnoses of its genera. Copeia. 2015; 103(4):933-60. https://doi.org/10.1643/CI-14-132
https://doi.org/10.1643/CI-14-132...
: 938, 941, 943, 944, 951 (notes on osteological characters). -Fernández et al., 2015: 6 (distribution in Argentina). -Nion et al., 2016Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres comunes; segunda edición corregida y ampliada. Montevideo: Dinara ; 2016.: 15 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 61-62; figs. 47-50 (synapomorphies, phylogenetic relationships). -Ochoa et al., 2017aOchoa LE, Roxo FF, DoNascimiento C, Sabaj MH, Datovo A, Alfaro M, Oliveira C. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae. Mol phylogenet evol. 2017a; 115:71-81. http://dx.doi.org/10.1016/j.ympev.2017.07.007
http://dx.doi.org/10.1016/j.ympev.2017.0...
: 74-79 (phylogenetic relationships). -Katz et al., 2018Katz AM, Barbosa MA, Mattos JLO, Costa WJEM. Multigene analysis of the catfish genus Trichomycterus and description of a new South American trichomycterine genus (Siluriformes, Trichomycteridae). Zoosyst Evol. 2018; 94(2):557-66. https://doi.10.3897/zse.94.29872
https://doi.10.3897/zse.94.29872...
: 560-564 (phylogenetic relationships). -Ochoa et al., 2020Ochoa LE, Datovo A, DoNascimiento C, Roxo FF, Sabaj MH, Chang J et al
. Phylogenomic analysis of trichomycterid catfishes (Teleostei: Siluriformes) inferred from ultraconserved elements. Sci Rep. 2020; 10:2697. http://doi.org/ 10.1038/s41598-020-59519-w
http://doi.org/ 10.1038/s41598-020-59519...
: fig. 3 (phylogenetic relationships).
Type species. Scleronema operculatumEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703. (by original designation).
Diagnosis. Scleronema is phylogenetically diagnosed by four synapomorphies: 1) fleshy flap at the base of the maxillary barbel (Fig. 1) vs. fleshy flap absent or extending beyond the base of the maxillary barbel; 2) skin flap in the posterior margin of the opercle (Fig. 1) vs. skin flap absent or covering practically the entire opercle; 3) articulation between the autopalatine and the vomer ventrally located, with the medial margins of the autopalatines very close to each other (Fig. 2) vs. articulation between the autopalatine and the vomer situated laterally, with the medial margins of the autopalatines not close to each other; and 4) autopalatine with an interrupted or not interrupted ossified arch-shaped process on its dorsal surface forming a canal (Fig. 3) vs. arch-shaped process absent.
Identification key. The geographical distribution of the species in the key are given according to the names and limits of the freshwater ecoregions of Abell et al. (2008Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N et al
. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience. 2008; 58(5):403-14. https://doi.org/10.1641/B580507
https://doi.org/10.1641/B580507...
) and illustrated in the Fig. 4. The two species groups mentioned in key (Scleronema minutum species group and S. operculatum species group) are further commented in the Discussion section.
| Lateral view of A. Scleronema macanuda, new species, paratype (ZVC-P 9374; 74.5 mm SL) and B. S. milonga, new species, holotype (MCP 54165; 37.8 mm SL) showing the thick fleshy flap in the base of the maxillary barbel (red arrows), the skin flap in the posterior margin of the opercle (black arrows), the dorsal membrane in the caudal peduncle (white arrows), and the vertical black bar in the caudal fin (blue arrow).
| Ventral view of the autopalatine (au) and vomer (vo) of Scleronema operculatum (UFRGS 19654) showing the articulation ventrally located between these two bones (black arrow). Scale bar = 1 mm.
| Dorsal view of the autopalatine (au) and maxilla (ma) of Scleronema minutum (ZVC-P 12491) showing the arch-shaped process on its dorsal surface (black arrow). Scale bar = 2 mm.
| Geographical distribution of the genus Scleronema. Circles and squares indicate species of the S. minutum group and S. operculatum group, respectively. Some symbols represent more than one collection locality. AR = Argentina; BR = Brazil; PY = Paraguay; UY = Uruguay.
Identification key to the species of Scleronema
1a. Body compressed; maxillary barbel with thinner portion shorter than wider one; fleshy flap at the base of the maxillary barbel located anteriorly, thick, prolonged up to the snout and with distal margin straight; skin flap posterior to opercle pointed and long; caudal fin with a vertical black bar distally (Fig. 1A) .................... 2 (Scleronema operculatum species group)
1b. Body roughly cylindrical; maxillary barbel with thinner portion longer than wider one; fleshy flap at the base of the maxillary barbel located posteriorly, thin, restricted to the maxilla and with distal margin rounded; skin flap posterior to opercle rounded and short; caudal fin lacking a vertical black bar (Fig. 1B) .................... 3 (Scleronema minutum species group)
2a. Lateral surface of body with a midlateral series of 6-9 rounded black blotches larger than opercle (Figs. 1A, 9, 11); tip of maxillary barbel extending between anterior and posterior margins of interopercle .................... Scleronema macanuda (Laguna dos Patos and lower rio Uruguay)
2b. Lateral surface of body with a midlateral series of 10-14 rounded black blotches smaller than or equivalent in size to opercle (Figs. 21, 22, 25A); tip of maxillary barbel never surpassing anterior margin of interopercle .................... Scleronema operculatum (lower rio Uruguay)
3a. Lateral surface of body with diffuse brown spots or rounded black blotches as large as or smaller than opercle .................... 4
3b. Lateral surface of body with rounded black blotches larger than opercle .................... 5
4a. Lateral surface of body with diffuse, scattered brown spots (Figs. 6, 7); infraorbital line of the laterosensory system with pores i10 and i11 and no additional pores associated .................... Scleronema guapa (lower rio Uruguay)
4b. Lateral surface of body with rounded black blotches (Figs. 12, 13A); infraorbital line of the laterosensory system with pores i10 and i11 and additional pores associated (Fig. 5A) .................... Scleronema mate (Laguna dos Patos)
5a. Pore i10 of the sphenotic canal of the laterosensory system absent; 8 pterygiophores in the dorsal fin .................... Scleronema teiniagua (lower rio Uruguay)
5b. Pore i10 of the sphenotic canal of the laterosensory system present (Fig. 5B-D); 9-10 pterygiophores in the dorsal fin .................... 6
6a. Pore s6 of the frontal canal of the laterosystem canal absent (Fig. 5B) .................... Scleronema ibirapuita (lower rio Uruguay)
6b. Pore s6 of the frontal canal of the laterosystem canal present (Fig. 5C-D) .................... 7
7a. Pore s3 of the frontal canal of the laterosensory system absent (Fig. 5C); dorsal-fin origin located at vertical through origin of pelvic fin or slightly posterior .................... Scleronema milonga (lower rio Uruguay)
7b. Pore s3 of the frontal canal of the laterosensory system present (Fig. 5D); dorsal-fin origin located at vertical through half-length of pelvic fin .................... Scleronema minutum (Laguna dos Patos, lower rio Uruguay and lower rio Paraná)
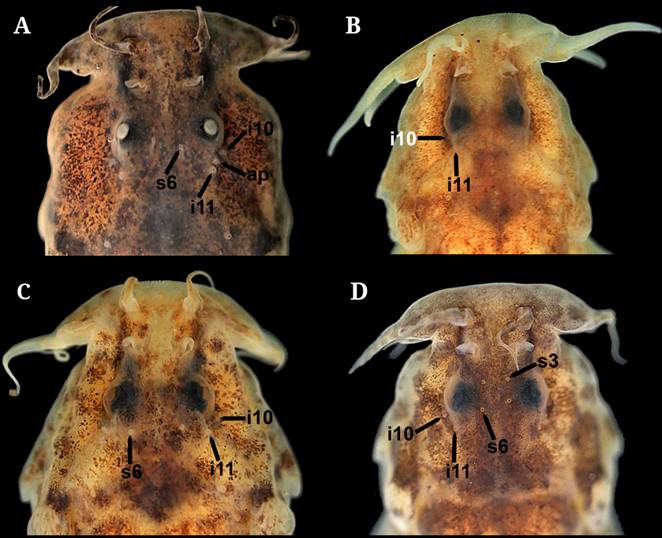
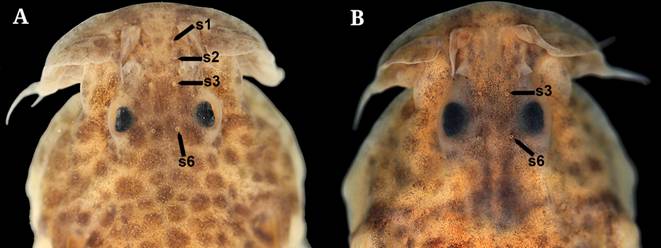
| Dorsal view of the head of A. Scleronema mate, new species, paratype (UFRGS 17418; 34.9 mm SL), B. S. ibirapuita, new species, paratype (UFRGS 27402; 49.4 mm SL), C. S. milonga, new species, paratype (LIRP 16775; 35.3 mm SL), and D. S. minutum (UFRGS 19651; 46.4 mm SL) showing the pores of the laterosensory system. “Ap” = additional pore.
Scleronema guapa, new species
urn:lsid:zoobank.org:act:78F28718-7E96-401E-A8C5-AC73A4555E3E
Scleronema sp. n. 4 -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 85-89; figs. 47-50 (phylogenetic relationships, taxonomy).
Holotype. UFRGS 23500, 36.4 mm SL, Brazil, Rio Grande do Sul State, Rosário do Sul, sanga Santo Antônio, tributary of rio Ibicuí da Armada, rio Ibicuí basin, lower rio Uruguay, 30°17’41”S 54°59’17”W, 8 Sep 2013, A. Duarte, J. Ferrer, L. R. Malabarba, M. Loureiro & M. Volcan.
Paratypes. 63 especimens from Brazil, Rio Grande do Sul State, rio Ibicuí basin, lower rio Uruguay: LIRP 16770, 5, 28.4-32.6 mm SL, collected with holotype. LIRP 16769, 5, 33.0-35.3 mm SL, Alegrete, arroio Jacaquá, 29°51’39”S 55°20’56”W, 1 May 2007, B. Klotzel, L. E. Lanés & M. Volcan. MCN 20231, 5, 29.7-36.9 mm SL, Alegrete, arroio Jacaquá, 29°51’39”S 55°20’56”W, 1 May 2007, B. Klotzel, L. E. Lanés & M. Volcan. MCP 25224*, 3, 26.7-31.7 mm SL, São Francisco de Assis, arroio Taquari, 29°23’46”S 55°08’52”W, 27 Set 1999, J. F. P. Silva, R. E. Reis & V. A. Bertaco. UFRGS 14034*, 4, 29.5-37.6 mm SL, São Gabriel, sanga do Areal, tributary of rio Santa Maria, 8 Oct 2007, 30°09’50”S 54°43’58”W, J. F. P. Silva. UFRGS 18087*, 27 (1 c&s), 16.2-33.6 mm SL, collected with holotype. UFRGS 19307*, 1, 29.2 mm SL, Alegrete, unnamed stream tributary of arroio São João, 29°46’07”S 55°23’53”W, 28 Oct 2013, C. Hartmann, L. Poldigaiski, M. Dalmolin, R. B. Dala-Corte & T. Guimarães. UFRGS 19649, 2, 31.6-40.0 mm SL, Santana do Livramento, arroio Capivara, tributary of sanga da Divisa, 30°59’42”S 55°24’12”W, 14 May 2014, C. Hartmann, M. Dalmolin, R. B. Dala-Corte & T. Guimarães. UFRGS 19655*, 10 (1 c&s), 29.8-42.3 mm SL, Alegrete, arroio Jacaquá, 29°51’39”S 55°20’56”W, 1 May 2007, B. Klotzel, L. E. Lanés & M. Volcan.
Diagnosis. Scleronema guapa is distinguished from all congeners by the lateral surface of body with diffuse, scattered brown spots, sometimes grouped forming irregular and small marks at midlateral line (vs. lateral surface of body with a midlateral line of black or brown rounded blotches). Scleronema guapa is further distinguished from S. macanuda and S. operculatum by the maxillary barbel longer than half-length of the head (vs. smaller than half-length of the head); the tips of pectoral-fin rays not extending beyond the interadial membrane (vs. extending beyond the interadial membrane), the skin flap in the posterior margin of the opercle rounded and short (vs. skin flap pointed and long); the fleshy flap at the base of the maxillary barbel located posteriorly, thin, restricted to the maxilla and distal margin rounded (vs. fleshy flap located anteriorly, thick, prolonged up to the snout and with distal margin straight); and by the caudal fin uniformly brown (vs. caudal fin with a transversal black bar distally). Scleronema guapa is further distinguished from S. ibirapuita, S. milonga, and S. teiniagua by having the pore s3 of the supraorbital line of the laterosensory system (vs. pore s3 absent).
Description. Based on specimens ranging from 16.2 to 42.3 mm SL; 2 c&s (one dissected). Morphometric data for 20 type specimens in Tab. 2.
| Morphometric data of Scleronema guapa, new species (data of holotype included in the range). N = number of specimens; SD = standard deviation.
External morphology. Greatest height and width of body in half-length of trunk. Body elongate, trunk roughly cylindrical gradually compressed towards to caudal fin. Dorsal profile of trunk convex and ventral profile straight to slightly convex. Dorsal and ventral profiles of caudal peduncle straight. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin. Head depressed and wide, usually trapezoid-shaped from dorsal view, wider posteriorly; square-shaped in specimens with muscles of cheeks well developed. Dorsal and ventral profiles of head straight to slightly convex. Anterior snout profile usually rounded from dorsal view (holotype with anterior snout profile straight; Fig. 6). Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
| Scleronema guapa just after the fixation in formalin, holotype (UFRGS 23500; 36.4 mm SL), Brazil, Rio Grande do Sul State, Rosário do Sul, sanga Santo Antônio, tributary to rio Ibicuí da Armada, lower rio Uruguay.
| Scleronema guapa just after the fixation in formalin, paratype (UFRGS 18087, 32.0 mm SL), Brazil, Rio Grande do Sul, Rosário do Sul, sanga Santo Antônio, tributary to rio Ibicuí da Armada, lower rio Uruguay.
Barbels with large bases and tapering gradually towards tips. Nasal barbel long; emerging from posterolateral edge of anterior nostril extending between anterior and posterior margins of eye. Maxillary barbel long; emerging from edge of upper lip and extending between anterior and posterior margins of interopercle. Basal portion of maxillary barbel wide with thin fleshy flap dorsally and distal margin rounded. Maxillary barbel with thinner portion longer in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Ventral surface of lower lip with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap short and rounded. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 6(n = 5), 6/7(n = 9) or 7(n = 48; including holotype) rays; first ray unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture usually covered by branchial membrane anteriorly. Some specimens with intumescence above anterior portion of pectoral fin and axillary pore not visible. Pelvic fin with distal margin convex when expanded, 4(n = 1), 4/5(n = 2) or 5(n = 59; including holotype) rays; first ray unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight to slightly convex when expanded, 7(n = 2), 8(n = 5), 9(n = 46; including holotype), or 10(n = 9) rays; usually first two rays unbranched. Dorsal-fin origin located at vertical through half-length of pelvic fin. Anal fin with distal margin slightly convex when fin expanded, 5(n = 2) or 6(n = 60; including holotype); usually first two rays unbranched. Anal-fin origin located at vertical through last third of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 11(n = 5), 12(n = 57; including holotype) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 9(n = 1) procurrent rays dorsally and 8(n = 1) procurrent rays ventrally. Procurrent rays of dorsal, anal, and caudal fins rarely visible.
Osteology. Premaxilla with 14-19(n = 2) teeth arranged in two rows. Dentary with 28-32(n = 1) teeth arranged in one to three rows. Opercle with 9-14(n = 2) odontodes and interopercle with 10-14(n = 2) odontodes. Hyoid arch with 6(n = 1) or 6/7(n = 1) branchiostegal rays. Free vertebrae 35(n = 1) or 36(n = 1); abdominal vertebrae 3(n = 1). Ribs 11(n = 2). First complete haemal arch in 4th(n = 1) free vertebra, first haemal spine in 12th(n = 1) free vertebra. Dorsal fin with 9(n = 2) pterygiophores; first one inserted anteriorly to neural spine of 16th(n = 2) vertebra. Anal fin with 6(n = 2) pterygiophores; first one inserted anteriorly to haemal spine of 20th(n = 2) vertebra.
Laterosensory system. Data for 61 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with nasal canal usually absent and frontal canal usually with pores s3 and s6. Infraorbital line with antorbital segment invariably absent and sphenotic canal usually with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused to each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short and usually with one pore each (po1 and po2, respectively). Trunk canal short usually with two pores.
Coloration in alcohol. Lateral surface of body with brown spots over light yellow background (Fig. 6). Occasionally, smaller specimens with spots grouped forming small blotches in midlateral line of trunk (Fig. 7). Dorsal surface of body usually with brown spots irregularly distributed or with vermicular brown marks extending ventrally to dorsolateral surface of trunk over light yellow background. Ventral surface of body light yellow with few brown blotches in caudal peduncle. Dorsal and laterodorsal surfaces of head with small brown spots over light yellow background. Anterior portion of opercle black. Ventral surface of body light yellow. Barbels uniformly yellow or intercalated with brown areas. Pectoral and dorsal fins hyaline or with rays of anterior portion faintly brown. Pelvic and anal fins hyaline. Caudal fin with rays faintly brown and distal margin hyaline. Caudal fin with vertical light brown stripe basally (Figs. 6-7).
| Distribution of pores of the laterosensory system for the species of Scleronema. Asterisks indicate the pattern present in the type specimens of Scleronema minutum and S. operculatum and the holotype for other species. Additional pores are present in the infraorbital line or posteriorly to the pore s6 of the supraorbital line.
Coloration in life. Coloration in life similar to that of specimens preserved in ethyl alcohol, but more intense (Figs. 6-7).
Geographical distribution. Scleronema guapa is endemic to the rio Ibicuí basin, a tributary to the left bank of rio Uruguay, State of Rio Grande do Sul, southern Brazil (Fig. 4).
Ecological notes. Scleronema guapa inhabits rivers and streams, with fine sand-bottoms. The species is usually collected syntopically with S. operculatum.
Etymology. The species epithet “guapa” is a regional adjective used to describe a beautiful person, an allusion to the beauty of the new species.
Conservation status. Scleronema guapa has an Extent of Occurrence (EOO) less than 5,000 km2, but no specific threats were detected and the species can be classified as LC according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Additional material examined. 55 specimens from Brazil, rio Ibicuí basin, lower rio Uruguay: MCP 17436, 6, 24.6-29.5 mm SL, São Francisco de Assis, rio Jaguari. MCP 48738, 1 (c&s), not measured (specimen with axial skeleton and fins damaged), São Francisco de Assis, rio Jaguari. MCP 26866, 1, 38.8 mm SL, Rosário do Sul, arroio do Salso, tributary of rio Ibicuí da Armada. MCP 25192, 14, 20.0-21.7 mm SL, São Francisco de Assis, rio Inhacunda. MCP 27505, 1, 23.2 mm SL, São Francisco de Assis, arroio Taquari, tributary of rio Miracatu. MCP 54170, 19, 17.6-27.0 mm SL, São Francisco de Assis, unnamed stream tributary of rio Inhacundá. MCP 54169, 13, 17,3-26,3 mm SL, Jaguari, arroio do Tigre, tributary of rio Jaguari.
Scleronema ibirapuita, new species
urn:lsid:zoobank.org:act:CBB4C41B-EEC8-4CDE-81DC-C99D4D4E2E1B
Scleronema sp. -Bertaco, Azevedo, 2013Bertaco VA, Azevedo MA. Fishes from Rio Ibirapuita basin, environmental protection area of Ibirapuita, Pampa biome. Check List. 2013; 9(5):966-72. http://dx.doi.org/10.15560/9.5.966: 969 (listed).
Scleronema sp. n. 2 -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 90-94; figs. 47-50 (phylogenetic relationships, taxonomy).
Holotype. MCN 19470, 39.5 mm SL, Brazil, Rio Grande do Sul State, Santana do Livramento, “Área de Proteção Ambiental Ibirapuitã” Conservation Unit, rio Ibirapuitã Chico, tributary of rio Ibirapuitã, rio Ibicuí basin, lower rio Uruguay, 30º33’29”S 55º31’03”W, 28 Aug 2012, C. L. Castilho, M. A. Azevedo & V. A. Bertaco.
Paratypes. 45 specimens from Brazil, Rio Grande do Sul State, rio Ibirapuitã basin, rio Ibicuí basin, lower rio Uruguay: MCP 11171*, 6 (1 c&s), 28.3-39.4 mm SL, Rosário do Sul, unnamed stream tributary of rio Ibirapuitã, 30º11’S 55º39’W, 13 Nov 1986, C. Lucena, L. Bergmann & P. Azevedo. MCN 2632*, 33 (2 c&s), 19.4-37.7 mm SL, Santana do Livramento, rio Ibirapuitã Chico, 24 Jul 1975, M. E. F. Beurmann, M. I. Vieira & P. C. Braun. MCN 19545*, 2, 35.2-42.5 mm SL, Santana do Livramento, rio Ibirapuitã at Passo do Cerrito, “Área de Proteção Ambiental Ibirapuitã” Conservation Unit, 30º37’37”S 55º40’57”W, 30 Aug 2012, C. L. Castilho, M. A. Azevedo & V. A. Bertaco. UFRGS 17386, 3, 26.7-35.8 mm SL, Santana do Livramento, rio Ibirapuitã at Passo do Cerrito, “Área de Proteção Ambiental Ibirapuitã” Conservation Unit, 30º37’37”S 55º40’57”W, 30 Aug 2012, C. L. Castilho, M. A. Azevedo & V. A. Bertaco. UFRGS 27402*, 1, 39.4 mm SL, Santana do Livramento, arroio Passo das Pedras, “Área de Proteção Ambiental Ibirapuitã” Convervation Unit, 30º32’38”S 55º25’58”W, 29 Aug 2012, C. L. Castilho, M. A. Azevedo & V. A. Bertaco.
Diagnosis. Scleronema ibirapuita is distinguished from all congeners with the exception of S. teiniagua by the absence of the pores s1, s2, s3, and s6 of the supraorbital line of the laterosensory system (vs. presence of, at least, the pore s6). Scleronema ibirapuita differs from S. teiniagua by having the pore i10 of the infraorbital line of the laterosensory system (vs. pore i10 absent) and nine pterygiophores in the dorsal fin (vs. eight).
Description. Based on specimens ranging from 19.4 to 42.5 mm SL; 3 c&s (one dissected). Morphometric data for 18 type specimens in Tab. 4.
| Morphometric data of Scleronema ibirapuita, new species (data of holotype included in the range). N = number of specimens; SD = standard deviation.
External morphology. Greatest height and width of body in half-length of trunk or in dorsal-fin origin. Body elongate, trunk roughly cylindrical gradually compressed towards to caudal fin. Dorsal and ventral profiles of trunk straight to slightly convex. Dorsal and ventral profiles of caudal peduncle straight. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin. Head depressed and wide, trapezoid-shaped from dorsal view, wider posteriorly. Dorsal and ventral profiles of head straight. Anterior snout profile usually rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel long; emerging from posterolateral edge of anterior nostril briefly surpassing posterior margin of eye. Maxillary barbel long; emerging from edge of upper lip and extending up to posterior margin of interopercle or briefly surpassing. Basal portion of maxillary barbel wide with thin skin fold dorsally and distal margin rounded. Maxillary barbel with thinner portion longer in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Ventral surface of lips with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap short and rounded. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 6(n = 1), 6/7(n = 3), or 7(n = 39; including holotype) rays; first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture usually covered by branchial membrane anteriorly. Some specimens with intumescence above anterior portion of pectoral fin and axillary pore not visible. Pelvic fin with distal margin convex when expanded, 4/5(n = 2) or 5(n = 41; including holotype) rays; first one always unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight to slightly convex when expanded, 8(n = 2), 9(n = 39; including holotype), or 10(n = 2) rays; first two or three rays unbranched. Dorsal fin with 2(n = 2) procurrent rays. Dorsal-fin origin located at vertical through half-length of pelvic fin. Anal fin with distal margin slightly convex when expanded, 6(n = 43; including holotype) rays; usually first two rays unbranched. Anal fin with 2(n = 2) procurrent rays. Anal-fin origin located at vertical through last third to posterior edge of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 11(n = 2) or 12(n = 41; including holotype) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Procurrent rays of dorsal, anal and caudal fins rarely visible. Caudal fin with 12(n = 1) or 13(n = 1) procurrent rays dorsally and 9(n = 1) or 10(n = 1) procurrent rays ventrally.
Osteology. Premaxilla with 26-28(n = 1) teeth arranged in three rows. Dentary with 30-32(n = 1) teeth. Opercle with 12-13(n = 2) odontodes and interopercle with 16-18(n = 2) odontodes. Hyoid arch with 6(n = 2) branchiostegal rays. Free vertebrae 33(n = 1) or 35(n = 2); abdominal vertebrae 3(n = 1). Ribs 12(n = 3). First complete haemal arch in 4th(n = 1) free vertebra, first haemal spine in 12th(n = 1) free vertebra. Dorsal fin with 9(n = 3) pterygiophores; first one inserted anteriorly to neural spine of 14th(n = 2) or 15th(n = 1) vertebra. Anal fin with 6(n = 3) pterygiophores; first one inserted anteriorly to haemal spine of 18th(n = 1) or 20th(n = 2) vertebra.
Laterosensory system. Data for 43 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with nasal canal invariably absent and frontal canal usually absent (Fig. 5B) (one of 43 specimens with pore s6). Infraorbital line with antorbital segment invariably absent and sphenotic canal with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused to each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short usually with one pore associated each (po1 and po2, respectively). Trunk canal short with two pores.
Coloration in alcohol. Lateral surface of body with midlateral line of 5-8 round brown blotches larger than opercle over light yellow background (Fig. 8); blotches of some individuals becoming fade or absent towards caudal peduncle. Dorsal surface of body with 5-6 rectangular brown blotches extending ventrally to laterodorsal surface of body. Ventral surface of body light yellow with few brown blotches in caudal peduncle. Dorsal surface of head almost entirely black. Laterodorsal surface of head with numerous brown round blotches over light yellow background. Anterior portion of opercle black. Ventral surface of head light yellow with few small brown blotches in lower lip, sometimes forming thin stripe. Barbels uniformly yellow or intercalated with brown areas. Pectoral and anal fins with rays faintly brown and distal margins hyaline. Pelvic fin hyaline. Dorsal and caudal fins with vertical light brown stripe basally, rays faintly brown, and distal margins hyaline (Fig. 8).
| Scleronema ibirapuita, new species, holotype (MCN 19470; 39.5 mm SL), Brazil, Rio Grande do Sul, Santana do Livramento, “Área de Proteção Ambiental (APA) Ibirapuitã”, rio Ibirapuitã Chico, rio Ibicuí drainage, lower rio Uruguay.
Geographical distribution. Scleronema ibirapuita occurs in the rio Ibirapuitã basin, a tributary to the left bank of rio Ibicuí (Brazil), and in the río Arapey (Uruguay; see remarks); lower rio Uruguay basin (Fig. 4).
Ecological notes.Scleronema ibirapuita inhabits rivers and streams with sand- or gravel-bottoms. The species has not been collected with congeners. The stomach of one specimen had immature aquatic insects and unidentified plant remains.
Etymology. The species epithet “ibirapuita” is given in reference to the Conservation Unit “Área de Proteção Ambiental Ibirapuitã”, where the new species can be found and that includes its type locality. A noun in apposition.
Conservation status. Scleronema ibirapuita has an Extent of Occurrence (EOO) of less than 5,000 km2, but no specific threats were detected to the species. In addition, S. ibirapuita is widespread in streams and rivers draining a Federal Conservation Unit (Área de Proteção Ambiental Ibirapuitã). Thus, the species can be classified as Least Concern (LC) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Remarks. Scleronema ibirapuita has only one record for the río Arapey basin collected in 1972, Uruguay (ZVC-P 5123; five specimens). Although these specimens have the diagnostic pattern of the laterosensory system for the species, they are listed herein as non-type specimens.
Additional material examined. ZVC-P 5123, 5, 26.1-32.2 mm SL, Uruguay, Salto, río Arapey Grande near Termas, lower río Uruguay.
Scleronema macanuda, new species
urn:lsid:zoobank.org:act:6931E764-6A53-4014-848A-C794FAB57E00
(Figs. 1a, 9, 10a, 11; Tabs. 3, 5)
Scleronema operculatum [nonEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.] -Vaz-Ferreira, Soriano, 1960Vaz-Ferreira R, Soriano BS. Dos Trichomycteridae (Pisces, Siluroidei) poco conocidos. Rev Fac Humanid Cienc (Univ Repub, Montevideo). 1960; 18:315-38.: 6-11 (brief description), figs. 1, 3, 4, 5 (drawings of a specimen from lateral view and detail of head).
Scleronema minutum [nonBoulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35.] -Carvalho et al., 2012Carvalho FR, Malabarba LR, Lenz AJ, Fukakusa CK, Guimarães TFR, Sanabria JA, Moraes AC. Ictiofauna da Estação Experimental Agronômica da Universidade Federal do Rio Grande do Sul, sul do Brasil: composição e diversidade. Rev Bras Biocien. 2012; 10(1):26-47.: 32 (listed), 45; fig. 17M (photo in lateral view), 46 (diagnosis in key).
Scleronema sp. -Ferrer, Malabarba, 2011Ferrer J, Malabarba LR. A new Trichomycterus lacking pelvic fins and pelvic girdle with a very restricted range in southern Brazil (Siluriformes: Trichomycteridae). Zootaxa, 2011: 2912:59-67. http://dx.doi.org/10.11646/zootaxa.2912.1.5
http://dx.doi.org/10.11646/zootaxa.2912....
: 66 (material examined). -DoNascimiento, 2012DoNascimiento C. Sistemática y relaciones filogenéticas de la subfamília de bagres parasíticos Stegophilinae (Siluriformes: Trichomycteridae). [PhD Thesis]. Caracas: Universidad Central de Venezuela; 2012.: 329 (material examined; phylogenetic relationships).
Scleronema sp. n. 5 -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 77-84; figs. 47-50 (phylogenetic relationships, taxonomy).
Scleronema aff. operculatum -Carvalho, 2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.: 21 (diet, ecomorphology and reproduction).
Holotype. MCN 20230, 71.8 mm SL, Brazil, Rio Grande do Sul State, Sentinela do Sul, irrigation water channel draining to arroio Velhaco, laguna dos Patos system, 30º43’13”S 51º39’28”W, 15 Jul 2010, M. A. Azevedo & T. V. Aguzzoli.
| Scleronema macanuda, new species, holotype (MCN 20230; 71.8 mm SL), Brazil, Rio Grande do Sul Sentinela do Sul, irrigation water channel draining to arroio Velhaco, laguna dos Patos system.
Paratypes. 229 specimens. Brazil, Rio Grande do Sul State, laguna dos Patos system: LIRP 16771, 2, 68.8-79.9 mm SL, Jaguarão, arroio Telha Chico, 32°13’52”W 53°26’13”W, L. Poldigaiski, M. Camana, R. B. Dala-Corte, T. Guimarães & V. Bastazini, 11 Jan 2014. MCN 12660*, 7 (1 c&s), 15.8-52.3 mm SL, São Lourenço do Sul, arroio Evaristo, 31°11’22”S 52°11’39”W, 14 Jun 1996, K. M. Grosser, M. R. da Costa & S. C. Freitas. MCN 12670, 13, 31.0-41.8 mm SL, São Lourenço do Sul, arroio Evaristo, 31°09’42”S 52°10’05”W, 14 Jun 1996, K. M. Grosser, M. R. da Costa & S. C. Freitas. MCN 14811*, 1, 47.1 mm SL, Mariana Pimentel, arroio Ribeiro Pequeno, 31 Jul 1997, L. F. Guterrez, P. Guterrez & W. R. Koch. MCP 17257*, 3 (1 c&s), 31.0-41.8 mm SL, São Sepé, rio São Sepé, rio Vacacaí basin, 30º11’06”S 53º33’35”W, A. R. Cardoso, A. Ramires & J. F. Pezzi, 24 Jun 1994. MCP 23034, 1, 46.2 mm SL, São Sepé, rio São Sepé, rio Vacacaí basin, 30º11’06”S 53º33’35”W, A. R. Cardoso, A. Ramires & J. F. Pezzi, 24 Jun 1994. MCP 54168*, 8 (1 c&s), 25.1-67.2 mm SL, Viamão, stream at Praia da Pedreira, Parque Estadual de Itapuã Conservation Unit, 30°21’30”S 51°02’48”W, 23 Jun 1999, C. A. Lucena, C. Porto, J. P. Silva & V. A. Bertaco. MCP 54167*, 29 (2 c&s), 24.6-84.0 mm SL, Pedro Osório, arroio Mata Olho, rio Piratini basin, 31º54’56”S 53º00’17”W, 20 Nov 1999, C. A. Lucena, E. Pereira, V. Bertaco & Z. M. Lucena. UFRGS 3955*, 5, 39.7-49.9 mm SL, Barra do Ribeiro, arroio Ribeirinho, 30°21’15”S 51°25’43”W, 22 Set 1986, Malabarba et al. UFRGS 7615*, 7, 23.3-43.5 mm SL, Encruzilhada do Sul, unnamed stream tributarty of rio Camaquã, 30°53’60”S 52°32’19”W, 15 Jul 2005, A. Schaan, G. Neves, J. Anza, J. Ferrer & J. Giora. UFRGS 7616*, 1, 41.2 mm SL, Canguçu, rio Camaquã, 16 Jul 2005, 30°56’24”S 52°38’45”W, A. Schaan, G. Neves, J. Anza, J. Ferrer & J. Giora. UFRGS 7618*, 6 (1 c&s), 25.4-44.5 mm SL, Canguçu, unnamed stream tributary of rio Camaquã, 30°57’49”S 52°39’26”W, 16 Jul 2005, A. Schaan, G. Neves, J. Anza, J. Ferrer & J. Giora. UFRGS 8770*, 10 (1 c&s), 29.2-49.6 mm SL, Rio Pardo, unnamed stream, rio Pardo basin, 12 Jul 2006, J. Anza. UFRGS 8972*, 4, 25.6-48.7 mm SL, Encruzilhada do Sul, arroio Abranjo, rio Camaquã basin, 30°53’60”S 52°32’19”W, 1 Dez 2006, J. Anza & R. Hirano. UFRGS 12484, 5, 31.0-41.8 mm SL, Camaquã, arroio Velhaco, 30°45’02”S 51°38’09”W, 26 Mar 2010, J. Ferrer & J. Wingert. UFRGS 12580*, 4 (2 c&s), 30.4-41.7 mm SL, Camaquã, arroio Velhaco, 30°45’02”S 51°38’09”W, 26 Mar 2010, J. Ferrer & J. Wingert. UFRGS 13099*, 5 (1 c&s), 41.7-53.3 mm SL, Eldorado do Sul, arroio Calombos, rio Jacuí basin, 30°06’02”S 51°41’41”W, 30 Abr 2010, J. Giora & J. Ferrer. UFRGS 14966, 4, 39.3-64.0 mm SL, Eldorado do Sul, arroio Calombos, rio Jacuí basin, 30°06’02”S 51°41’41”W, 6 May 2011, C. K. Fukakusa. UFRGS 17417, 8, 25.6-45.5 mm SL, Eldorado do Sul, arroio Calombos, rio Jacuí basin, 30°06’02”S 51°41’41”W, 17 Mar 2013, J. Ferrer. UFRGS 19304, 8, 37.3-81.9 mm SL, Santana da Boa Vista, arroio das Neves, 30°51’17”S 53°13’38”W, 13 Dez 2013, C. Hartmann, L. Poldigaiski, M. Dalmolin, R. B. Dala-Corte & T. Guimarães. UFRGS 19322 14, 39.8-77.9 mm SL, Jaguarão, unnamed stream tributary of arroio Telha Chico, 32º14’28”S 53º27’17”W, L. Poldigaiski, M. Camana, R. B. Dala-Corte, T. Guimarães & V. Bastazini, 12 Jan 2014. UFRGS 19387, 4, 41.5-75.6 mm SL, Jaguarão, unnamed stream tributary of arroio Quilombo, 32°15’09”S 53°23’44”W, L. Poldigaiski, M. Camana, R. B. Dala-Corte, T. Guimarães & V. Bastazini, 13 Jan 2014. Rio Negro basin, lower rio Uruguay: LIRP 16772, 10, 36.6-59.0 mm SL, Bagé, rio Piraí, 31º28’31”S 54º24’34”W, 17 Mar 2016, J. Chuctaya, J. Ferrer, L. R. Malabarba & M. C. Malabarba. MPEG 34068, 5, 32.0-71.5 mm SL, Bagé, rio Piraí, 31º28’31”S 54º24’34”W, 17 Mar 2016, J. Chuctaya, J. Ferrer, L. R. Malabarba & M. C. Malabarba. UFRGS 20739, 2, 38.5-38.7 mm SL, Bagé, arroio do Acampamento, tributary of rio Piraí, 31°15’03”S 54°21’08”W, 13 Mar 2015, B. Collares, B. Meneses, L. de Fries & T. Guimarães. UFRGS 21635, 40 (10 c&s), 22.2-75.6 mm SL, Bagé, rio Piraí, 31º28’31”S 54º24’34”W, 17 Mar 2016, J. Chuctaya, J. Ferrer, L. R. Malabarba & M. C. Malabarba. Uruguay, laguna Merín basin, laguna de los Patos system: ZVC-P 14526*, 2, 47.1-76.8 mm SL, Treinta y Tres, río Tacuarí at Paso del Dragón, 32°45’51”S 53°43’10”W, 24 Fev 2001, F. Scasso, F. Teixeira, M. Loureiro & N. Marchand. ZVC-P 14525*, 6, 23.4-70.7 mm SL, Treinta y Tres, río Olimar, 33°15’27”S 54°23’06”W, 21 Fev 2001, F. Scasso, F. Teixeira, M. Loureiro & N. Marchand. ZVC-P 14524*, 1, 73.2 mm SL, Lavalleja, río Cebollatí at Paso del Rey, 33°44’19”S 54°53’03”W, 2 Fev 2001, F. Scasso, F. Teixeira, M. Loureiro & N. Marchand. ZVC-P 8951*, 2, 51.4-93.6 mm SL, Treinta y Tres, río Tacuarí at Paso del Dragón, 32°45’51”S 53°43’10”W, 21 Fev 2001, F. Scasso, F. Teixeira, M. Loureiro & N. Marchand. ZVC-P 13639, 1, 50.9 mm SL, Treinta y Tres, río Tacuarí at Paso del Dragón, 32°45’51”S 53°43’10”W, 6 Dez 2013, A. Duarte, D. Hernández, E. Burress, M. Loureiro & S. Serra. Río Negro basin, lower río Uruguay: UFRGS 21923*, 1, 49.5 mm SL, Rivera, arroyo Batovi, 31°06’58”S 55°24’56”W, 27 May 2005, F. Cantera, L. R. Malabarba, P. Lehmann & V. Bertaco. ZVC-P 7531*, 5, 37.5-55.1 mm SL, Flores, arroyo Grande, 33°14’56”S 57°15’44”W, 20 Nov 2006, A. D’Anatro, F. Teixeira de Mello, I. González, M. Loureiro & S. Oviedo. ZVC-P 9374*, 1, 74.5 mm SL, Durazno, río Yi at Paso San Borja, 33°23’50”S 56°24’12”W, 23 Ago 2005, I. González-Bergonzoni. ZVC-P 14523*, 5, 50.8-70.3 mm SL, Durazno, río Yi at Paso San Borja, 33°23’50”S 56°24’12”W, 23 Nov 2005, A. D’Anatro, F. Teixeira de Mello, I. González, M. Loureiro & S. Oviedo.
Diagnosis. Scleronema macanuda is distinguished from all congeners with the exception of S. operculatum by the following external characters: maxillary barbel smaller than half-length of the head (vs. larger than half-length of the head); tips of the pectoral-fin rays extending beyond the interadial membrane (vs. not extending beyond the interadial membrane), skin flap in the posterior margin of the opercle pointed and long (vs. skin flap round an short); fleshy flap at the base of the maxillary barbel located anteriorly, thick, prolonged up to the snout and with distal margin straight (vs. fleshy flap located posteriorly, thin, restricted to the maxilla and with distal margin rounded); and the caudal fin with a transversal black bar distally (vs. caudal fin with black bar absent). Scleronema macanuda differs from S. operculatum by having a midlateral line of 6-9 rounded black blotches larger than opercle (vs. midlateral line of 10-14 rounded black blotches as large as or smaller than opercle); tip of nasal barbel usually extending beyond anterior margin of eye (vs. tip of nasal barbel never reaching anterior margin of eye), tip of maxillary barbel extending between anterior and posterior margins of interopercle (vs. tip of maxillary barbel never surpassing anterior margin of interopercle).
Description. Based on types ranging from 15.8 to 93.6 mm SL; 11 c&s (3 dissected). Morphometric data for types and non-types in Tab. 5.
| Morphometric data of Scleronema macanuda, new species (data of holotype included in the range). N = number of specimens; SD = standard deviation.
External morphology. Greatest height of body in trunk and greatest width of body in anterior portion of trunk. Body elongate and compressed. Dorsal and ventral profiles of trunk straight to slightly convex. Dorsal and ventral profiles of caudal peduncle straight to slightly convex up to anteriormost procurrent ray insertion. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin (Fig. 1A). Head depressed and wide, trapezoidal from dorsal view, wider posteriorly. Dorsal and ventral profiles of head straight. Anterior snout profile rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel short; emerging from posterolateral edge of anterior nostril and usually extending between anterior and posterior margins of eye (few specimens with nasal barbel surpassing posterior nostril and not reaching anterior margin of eye). Maxillary barbel short; emerging from edge of upper lip and extending between anterior and posterior margins of interopercle. Basal portion of maxillary barbel wide and with thick flashy flap dorsally with distal margin straight. Maxillary barbel with thinner portion smaller in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Lower lip and corners of upper lip with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head and visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap, thin and pointed; some specimens with groove in skin flap. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 7 rays(n = 41; including holotype); first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture usually covered by branchial membrane anteriorly. Rays of pectoral fin extending slightly beyond interadial membrane. Some specimens with wilted skin above anterior portion of pectoral fin or with intumescence, axillary pore not visible. Pelvic fin with distal margin convex when expanded, 5(n = 40) or rarely 6(n = 1) rays; first one always unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight when expanded, 10(n = 9), 11(n = 9), 12(n = 15; including holotype), 13(n = 5) or 14(n = 3) rays; first two rays unbranched. Dorsal fin with two(n = 4) or three(n = 7) procurrent rays. Dorsal-fin origin located at vertical through first half of pelvic fin. Anal fin with distal margin slightly convex when expanded, 6(n = 36; including holotype) or 7(n = 6) rays; first two rays unbranched. Anal fin with one(n = 1) or two(n = 10) procurrent rays. Anal-fin origin located at vertical through last third of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 12(n = 41; including holotype) rays; most external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 11(n = 2), 12(n = 7), 13(n = 1) or 14(n = 1) procurrent rays dorsally and 9(n = 9), 10(n = 1) or 13(n = 1) procurrent rays ventrally. Procurrent rays of dorsal, anal and caudal fins rarely visible.
Osteology. Premaxilla with 19-29(n = 3) teeth arranged in three rows. Dentary with 34-38(n = 1) teeth inserted in one to three rows. Opercle with 9-13(n = 3) odontodes and interopercle with 14-19(n = 3) odontodes. Hyoid arch with 6(n = 11) branchiostegal rays. Free vertebrae 34(n = 2), 35(n = 2), 36(n = 4), 37(n = 2) or 38(n = 1); abdominal vertebrae 3(n = 5). Ribs 8(n = 5), 9(n = 4) or 10(n = 2). First complete haemal arch in 4th(n = 5) free vertebra, first haemal spine in 7th(n = 1), 8th(n = 3) or 9th(n = 2) free vertebra. Dorsal fin with 10(n = 1), 11(n = 5) or 12(n = 4) or 14(n = 1) pterygiophores; first one inserted anteriorly to neural spine of 13th(n = 2), 14th(n = 2), 15th(n = 3), 16th(n = 3) or 17th(n = 1) vertebra. Anal fin 5(n = 2) or 6(n = 9) pterygiophores; first one inserted anteriorly to haemal spine of 19th(n = 1), 20th(n = 4), 21st(n = 4) or 22nd(2) vertebra.
Laterosensory system. Data for 107 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line usually with two paired nasal canals (right and left; Fig. 10A); however, one or both canals can be absent. Nasal canal, when present, interrupted (not connecting with frontal canal) with pores s1 and s2. Frontal canal usually with pores s3 and s6. Infraorbital line with antorbital segment invariably absent and sphenotic canal usually with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused to each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short with one pore each (po1 and po2, respectively). Trunk canal short usually with two pores.
| Dorsal view of the head of A. Scleronema macanuda, new species, paratype (UFRGS 21635; 75.6 mm SL) and B. S. operculatum (UFRGS 19646; 56.1 mm SL) showing the pores of the laterosensory system.
Coloration in alcohol. Lateral surface of body with two longitudinal lines (midlateral and laterodorsal) of 6-9 black rounded blotches (distinctly larger than opercle) over light yellow background (Figs. 9, 11). Occasionally, smaller individuals with blotches becoming fade or absent towards to caudal peduncle and larger individuals with scattered small blotches among larger ones (Fig. 11). Longitudinal line of laterodorsal blotches visible from dorsal view. Anterior portion of dorsal surface of body with numerous black rounded blotches. Ventral surface of body uniformly light yellow. Dorsal and lateral surfaces of head and nape area with numerous black rounded blotches or uniformly black. Ventral surface of head light yellow. Dorsal surface of maxillary and rictal barbels weakly black and ventral surface light yellow. Nasal barbel weakly black. Opercle with anterior and dorsal margins black; posterior fold light yellow. Pectoral, pelvic, dorsal and anal fins hyaline. Dorsal fin of some larger individuals with black stripe basally. Caudal fin hyaline with vertical stripe basally and vertical black bar in distal margin, which could extend up to mid-length of fin in larger individuals (Figs. 9, 11).
Coloration in life. Coloration in life similar to that of specimens preserved in ethyl alcohol, but more intense (Fig. 11).
| Specimens in life of Scleronema macanuda, new species, paratypes, (UFRGS 21635; A. 75.6 mm SL, B. 65.7 mm SL), Brazil, Rio Grande do Sul, Bagé, rio Piraí, tributary of rio Negro, lower rio Uruguay.
Geographical distribution. Scleronema macanuda is widely distributed in rivers and streams that drain to laguna dos Patos (Brazil) and lagoa Mirim (Brazil and Uruguay); and in small Atlantic coastal drainages of Uruguay (Fig. 4). In the lower rio Uruguay basin, the species occurs in the rio Negro and tributaries (Brazil and Uruguay) and in arroyo Higueritas (Uruguay). The specimen figured by Vaz-Ferreira, Soriano (1960Vaz-Ferreira R, Soriano BS. Dos Trichomycteridae (Pisces, Siluroidei) poco conocidos. Rev Fac Humanid Cienc (Univ Repub, Montevideo). 1960; 18:315-38.: fig. 1; ZVCP 170) from the mouth of río Queguay in the rio Uruguay (Uruguay) was not analyzed, but can be identified as S. macanuda.
Ecological notes.Scleronema macanuda inhabits rivers and streams usually with sand- bottoms. The species is collected syntopically with S. minutum in some localities. The stomachs of 11 specimens were analyzed and six had immature aquatic Diptera (Chironomidae), Oligochaeta, unidentified plant fragments and sand. Invididuals of S. macanuda larger than 35.0 mm SL are capable to spawn and considered adults by Carvalho (2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.). The diet of immatures of S. macanuda is composed of Chironomidae and Ephemeroptera according to Carvalho (2017).
Etymology. The species epithet “macanuda” is a regional adjective to describe a “large and strong” person, an allusion to being the largest species of the genus.
Conservation status. Scleronema macanuda is frequent and abundant in the rio Negro basin and Laguna dos Patos system. In addition, no specific threats were detected and the species can be classified as Least Concern (LC) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Additional material examined. 75 specimens. Brazil: FMNH 125684, 1, 13.1 mm SL, Santa Maria, rio Vacacaí-Mirim. MCN 12681, 3, 22.6-54.3 mm SL, São Lourenço do Sul, arroio Inhuquipá. MCN 12701, 2, 48.9-50.3 mm SL, Arambaré, arroio Velhaco. MCN 20232, 1, 34.1 mm SL, São Jerônimo, arroio dos Cachorros. MCP 54172, 1, 53.2 mm SL, Barra do Ribeiro, arroio do Ribeiro. MCP 19454, 17, 17.2-39.9 mm SL, Barra do Ribeiro, arroio Petim. MCP 34753, 16, 23.3-54.0 mm SL, Herval, arroio Arambaré. MCP 54166, 3, 42.0-68.5 mm SL, Pedro Osório, unnamed stream tributary of rio Piratini. UFRGS 3954, 1, 27.1 mm SL, Tapes, arroio Velhaco. UFRGS 3957, 1, 34.8 mm SL, Tapes, arroio Velhaco. UFRGS 21296, 6, 14.5-21.2 mm SL, Tapes, arroio Velhaco. UFRGS 8466, 2, 20.1-26.4 mm SL, Eldorado do Sul, arroio Calombos. UFRGS 14967, 1, 52.8 mm SL, Eldorado do Sul, arroio Calombos. UFRGS 18022, 4, 25.9-44.3 mm SL, Eldorado do Sul, arroio Calombos. UFRGS 18023, 3, 30.4-32.8 mm SL, Eldorado do Sul, arroio Calombos. UFRGS 19305, 2, 34.9-40.7 mm SL, Santana da Boa Vista, arroio das Neves UNICTIO 1024, 2, 44.0-49.0 mm SL, Morungava, arroio Demétrio. UNICTIO 1045, 1, 26.8 mm SL, Morungava, arroio Demétrio. Uruguay: FMNH 71372, 2, 35.5-39.1 mm SL, Lavalleja, arroyo Polanco, río Cebollatí, basin. ZVC-P 3522, 1, 67.0 mm SL, Colonia, Nueva Palmira, arroyo Higueritas. ZVC-P 5008, 1, 118.7 mm SL, Cerro Largo, arroyo de las Cañas at Paso de las Cañas. ZVC-P 7179, 3, 28,5-55,6 mm SL, Maldonado, arroyo José Ignacio at Paso Correa.
Scleronema mate, new species
urn:lsid:zoobank.org:act:7B4355E7-07B0-4B79-A82A-060C122A16C8
(Figs. 5a, 12, 13a, Tabs. 3, 6)
Scleronema angustirostris [nonDevincenzi, 1942Devincenzi GJ, Teague GW. Ictiofauna del Río Uruguay Medio. Anales del Museo de Historia Natural de Montevideo. 1942; 5(4)1-100.] -Wosiacki, de Pinna, 2007Wosiacki WB, de Pinna MCC. Família Trichomycteridae. In: Buckup PA, Menezes NA, Ghazzi MAS, editors. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional; 2007. p.67-75.: 69 (listed).
Scleronema sp. -Becker et al., 2013Becker FG, De Fries LCC, Ferrer J, Bertaco VA, Luz-Agostinho KDG, Silva JFPWW et al
. Fishes of the Taquari-Antas river basin (Patos Lagoon basin), southern Brazil. Braz J Biol. 2013; 73(1):79-90. https://doi.org/10.1590/S1519-69842013000100010
https://doi.org/10.1590/S1519-6984201300...
: 85 (listed).
Scleronema sp. 6 -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 101-105; figs. 47-50 (phylogenetic relationships, taxonomy).
Holotype. MCP 54183, 49.3 mm SL, Brazil, Rio Grande do Sul, Venâncio Aires, arroio Grande, tributary of arroio Castelhano, rio Taquari basin, laguna dos Patos system, 29º33’S 52º17’W, 14 Oct 1994, C. A. S. Lucena, J. F. P. Silva & Z. M. Lucena.
| Scleronema mate, new species, holotype (MCP 54183; 49.3 mm SL), Brazil, Rio Grande do Sul, Venâncio Aires, arroio Grande, tributary of arroio Castelhano, rio Taquari basin, laguna dos Patos system.
Paratypes. 91 specimens from Brazil, Rio Grande do Sul, laguna dos Patos system: LIRP 16773, 5, 31.4-39.4 mm SL, Rio Pardo, unnamed stream tributary of rio Pardo, 12 Jul 2006, J. Anza. LIRP 16774, 5, 22.9-30.5 mm SL, Cruzeiro do Sul, rio Sampaio, tributary of rio Taquari, 29°31’10”S 52°04’43”W, 27 Mar 2013, J. Ferrer & J. Wingert. MCP 9479, 2, 24.3-30.1 mm SL, Santa Cruz do Sul, rio Pardinho, 29º40’S 52º29’W, 15 Sep 1983, C. A. S. Lucena, L. R. Malabarba & R. E. Reis. MCP 10059*, 1, 36.4 mm SL, Três Coroas, arroio Moreira, rio do Sinos basin, 29º31’S 50º46’W, 27 Jul 1984, C. Rangel et al. MCP 17498*, 13 (1 c&s), 36.2-50.1 mm SL, collected with holotype. UFRGS 17616*, 18 (2 c&s), 18.4-34.3 mm SL, Cruzeiro do Sul, rio Sampaio, tributary of rio Taquari, 29°31’10”S 52°04’43”W, 27 Mar 2013, J. Ferrer & J. Wingert. UFRGS 17418, 2, 34.9-38.1 mm SL, Taquara, rio da Ilha, rio do Sinos basin, 20 Dec 2012, R. Dala-Corte et al. UFRGS 21924*, 22, 23.6-43.5 mm SL, Rio Pardo, unnamed stream tributary of rio Pardo, 12 Jul 2006, J. Anza. Following lots from Caraá, rio do Sinos, 29°46’18”S 50°19’58”W: MCN 19024, 1, 21.1 mm SL, 6 Feb 2007, B. Calegari, M. Azevedo & T. V. Aguzzoli. MCN 19025*, 4, 27.3-39.1 mm SL, 27 Sep 2006, B. Galegari, M. A. Azevedo & R. Hirano. MCN 19026, 1, 22.8 mm SL, 28 Aug 2007, M. A. Azevedo, R. Dala-Corte & T. Aguzzoli. MCN 19027*, 1, 28.8 mm SL, 30 Oct 2006, M. A. Azevedo & R. Hirano. MCN 19028, 8, 19.4-28.2 mm SL, 23 Apr 2006, M. A. Azevedo & T. Aguzzoli. MCN 19029, 5 (1 c&s), 28.8-29.7 mm SL, 1 Oct 2007, M. A. Azevedo, R. Dala-Corte & T. Aguzzoli. MCN 19030, 3, 29.4-30.1 mm SL, 3 Nov 2007, M. A. Azevedo & T. Aguzzoli.
Diagnosis. Scleronema mate is distinguished from all congeners, with the exception of S. operculatum, by having the rounded blotches at the midlateral line as large as or smaller than opercle (vs. rounded blotches larger than opercle or absent). Scleronema mate differs from S. operculatum by the maxillary barbel longer than half-length of the head (vs. shorter than half-length of the head); tips of the pectoral-fin rays not extending beyond the interadial membrane (vs. extending beyond the interadial membrane), skin flap in the posterior margin of the opercle rounded and short (vs. skin flap pointed and long); fleshy flap at the base of the maxillary barbel, thin, restricted to the maxilla and with distal margin rounded (vs. fleshy flap located anteriorly, thick, prolonged up to the snout and with distal margin straight); and by the caudal fin uniformly brown (vs. caudal fin with a transversal black bar distally).
Description. Based on specimens ranging from 18.4 to 50.1 mm SL; 4 c&s (2 dissected). Morphometric data for 16 types in Tab. 6.
| Morphometric data of Scleronema mate, new species (data of holotype included in the range). N = number of specimens; SD = standard deviation.
External morphology. Greatest height and width of body in half-length of trunk. Body elongate, trunk roughly cylindrical gradually compressed towards to caudal fin. Dorsal profile of trunk convex and ventral profile straight. Dorsal and ventral profiles of caudal peduncle straight. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin. Head depressed and wide, trapezoid-shaped from dorsal view, wider posteriorly; square-shaped in specimens with muscles of cheeks well developed. Dorsal and ventral profiles of head straight to slightly convex. Anterior snout profile usually rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel long; emerging from posterolateral edge of anterior nostril extending up to posterior margin of eye or briefly surpassing. Maxillary barbel long; emerging from edge of upper lip and extending up to posterior margin of interopercle or briefly surpassing. Basal portion of maxillary barbel wide with thin fleshy flap dorsally and distal margin rounded. Maxillary barbel with thinner portion longer in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges po steriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Ventral surface of lips with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap short and rounded. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 7(n = 14; including holotype) rays; first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture covered by branchial membrane anteriorly. Some specimens with intumescence above anterior portion of pectoral fin and axillary pore not visible. Pelvic fin with distal margin convex when expanded, 5(n = 14; including holotype) rays; first one always unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight to slightly convex when expanded, 8(n = 1), 9(n = 3), 10(n = 5; including holotype) or 11(n = 4) rays; usually first two rays unbranched. Dorsal fin with one(n = 1) or two(n = 1) procurrent rays. Dorsal-fin origin located at vertical through first third to half-length of pelvic fin. Anal fin with distal margin slightly convex when expanded, 6(n = 13; including holotype) or 7(n = 1) rays; usually first two rays unbranched. Anal fin with two(n = 2) procurrent rays. Anal-fin origin located at vertical through last third to posterior edge of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 10(n = 1), 11(n = 1), 12(n = 10) or 13(n = 2; including holotype) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 13(n = 2) procurrent rays dorsally and 9(n = 2) procurrent rays ventrally. Procurrent rays of dorsal, anal and caudal fins rarely visible.
Osteology. Premaxilla with 21-22(n = 1) teeth arranged in two to three rows. Dentary with teeth inserted in one to three rows. Opercle with 9-12(n = 3) odontodes and interopercle with 9-18(n = 3) odontodes. Hyoid arch with 5/6(n = 3) branchiostegal rays. Free vertebrae 34(n = 1) or 35(n = 2); abdominal vertebrae 3(n = 3). Ribs 11(n = 1) or 12(n = 1). First complete haemal arch in 4th(n = 3) free vertebra, first haemal spine in 12th(n = 2) or 13th(n = 1) free vertebra. Pelvic girdle with basipterygium with anterolateral process absent. Dorsal fin with 9(n = 4) pterygiophores; first one inserted anteriorly to neural spine of 14th(n = 1) or 15th(n = 2) free vertebra. Anal fin with 6(n = 4) pterygiophores; first one inserted anteriorly to haemal spine of 19th(n = 3) free vertebra.
Laterosensory system. Data for 34 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with nasal canal invariably absent and presence of pores s3 and s6 of frontal canal variable (Fig. 5A). Infraorbital line with antorbital segment invariably absent and sphenotic canal usually with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short usually with one pore each (po1 and po2, respectively). Fifteen specimens of 34 with additional pores mainly in infraorbital line. Trunk canal short usually with two pores.
Coloration in alcohol. Lateral surface of body with midlateral line of 4-5 rounded brown blotches as large as or smaller than opercle over light yellow background; blotches becoming fade or absent towards caudal peduncle (Figs. 12, 13A). Dorsal surface of body with 5-6 rectangular brown blotches extending ventrally to laterodorsal surface of body. Ventral surface of body light yellow with few brown blotches in caudal peduncle. Dorsal surface of head almost entirely black. Laterodorsal surface of head with numerous brown rounded blotches over light yellow background. Anterior portion of opercle black. Ventral surface of head light yellow with few small brown blotches in lower lip, sometimes forming thin stripe. Barbels uniformly yellow or intercalated with brown areas. Pectoral, pelvic and anal fins with rays weakly brown and distal margins hyaline. Dorsal and caudal fins with vertical light brown stripe basally, rays weakly brown, and distal margins hyaline (Figs. 12, 13A).
| Small specimens of A. Scleronema mate, new species, paratype (UFRGS 17616; 34.3 mm SL) and B. S. teiniagua, new species, paratype (LIRP 16776; 29.3 mm SL).
Coloration in life. Coloration in life similar to that of specimens preserved in ethyl alcohol, but more intense.
Geographical distribution. Scleronema mate occurs in four tributaries of the rio Jacuí (rio Caí, rio Pardo, rio Taquari, and rio dos Sinos), laguna dos Patos system, Brazil (Fig. 4).
Ecological notes. Scleronema mate inhabits rivers and streams with sand- and gravel-bottoms. In the rio Pardo basin, the species is collected syntopically with S. macanuda.
Etymology. The species epithet “mate” is given in reference to the herb used as an infusion in a traditional drink (“chimarrão”) from South Brazil, Argentina, and Uruguay. Additionally, the type locality of the new species, “Venâncio Aires”, is known as the “Terra do Chimarrão”. A noun in apposition.
Conservation status. Scleronema mate has an Extent of Occurrence (EOO) less than 5,000 km2. The arroio Castelhano in Venâncio Aires has suffered from siltation and is strongly impacted by domestic and industrial sewage, as well as excess of fertilisers and pesticides in the surrounding crops. A similar decline in the quality of habitat due to dense urbanisation and agricultural activity can be observed in other areas of occupancy of S. mate. Therefore, the species can be classified as Near Threatened (NT), approaching EN B1b(iii) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Additional material examined. 15 specimens from Brazil, Rio Grande do Sul State: MCN 6252, 1, 38.7 mm SL, Três Coroas, arroio Quilombo, rio dos Sinos basin. MCN 6888, 3, 24.9-31.8 mm SL, Três Coroas, arroio Quilombo, rio dos Sinos basin. MCN 10910, 1, 39.5 mm SL, Três Coroas, arroio Moreira, rio dos Sinos basin. MNRJ 41088, 7 (not measured), Rolante, rio Rolantezinho, rio dos Sinos basin. MCP 11173, 1, 23.9 mm SL, São Sebastião do Caí, rio Caí. MCP 17504, 1, 42.9 mm SL, Cruzeiro do Sul, rio Sampaio, tributary of rio Taquari. MCP 19136, 1, 29.2 mm SL, Vera Cruz, arroio Andreas.
Scleronema milonga, new species
urn:lsid:zoobank.org:act:5F928D71-F119-4D49-A407-465C21CE08CA
(Figs. 1b, 5c, 14-15; Tabs. 3, 7)
Scleronema operculatum [nonEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.] -Adriaens et al., 2010Adriaens D, Baskin JN, Coppens H. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in. In: Nelson JS, Schultze HP, Wilson MVH, editors. Origin and Phylogenetic Interrelationships of Teleosts. München: Verlag Dr. Friedrich Pfeil; 2010. p.337-62.: 339 (specimen used for CT-scanning), 349; fig. 5C (CT-scanning from lateral view of head, pectoral fin, and anterior portion of axial skeleton).
Scleronema sp. n. 1 -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 95-100; figs. 47-50 (phylogenetic relationships, taxonomy).
Scleronema aff. minutum -Carvalho, 2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.: 20 (diet, ecomorphology and reproduction).
Scleronema sp. -Cavalheiro, Fialho, 2020Cavalheiro LW, Fialho CB. Fishes community composition and patterns of species distribution in Neotropical streams. Biota Neotrop. 2020; 20(1):1-13. https://doi.org/10.1590/1676-0611-bn-2019-0828
https://doi.org/10.1590/1676-0611-bn-201...
: 7 (listed).
Holotype. MCP 54165, 37.8 mm SL, Brazil, Rio Grande do Sul, Dezesseis de Novembro, arroio Lageado Araçá, rio Ijuí basin, lower rio Uruguay, 28º12’23”S 54º56’58”W, 1 Nov 2004, A. R. Cardoso & V. A. Bertaco.
Paratypes. 43 specimens from lower rio Uruguay. Argentina, Misiones: MCP 13354*, 3, 28.8-30.7 mm SL, Itacaruaré, arroyo Itacaruaré, 27°53’S 55°17’W, 28 Jun 1989, E. Lerner. MCP 13445*, 1, 17.5 mm SL, Itacaruaré, arroyo Itacaruaré, 27°53’S 55°17’W, 24 Abr 1989, E. Lerner et al. Brazil, all from rio Ijuí basin: LIRP 16775*, 5, 25.7-35.3 mm SL, Panambi, rio Palmeira, 28°14’35”S 53°33’12”W, 8 Fev 2014, A. Hirschmann & C. B. Kasper. MCP 16768*, 2, 25.3-33.8 mm SL, Entre-Ijuís, arroio Lageado do Moinho, rio Ijuizinho basin, 28°27’S 54°22’W, 14 Dez 1993, J. F. P. Silva, M. P. Barros & R. E. Reis. MCP 35344*, 7, 21.3-39.2 mm SL, Cerro Largo, arroio Brum, 28º11’10”S 54º49’36”W, 12 Jun 2004, A. R. Cardoso & V. A. Bertaco. MCP 37034*, 4 (1 c&s), 32.9-36.4 mm SL, Salvador das Missões, arroio Alexandrino, 28º10’25”S 54º48’05”W, 31 Out 2004, A. R. Cardoso & V. A. Bertaco. MCP 37052*, 5, 25.8-33.9 mm SL, collected with holotype. UFRGS 7685*, 4, 19.5-32.7 mm SL, São Nicolau, unnamed stream tributary of rio Ijuí, 28°10’32”S 55°04’01”W, 5 Mar 2005, C. Oliveira, J. Ferrer, L. R. Malabarba, M. A. Azevedo. UFRGS 11706*, 3 (2 c&s), 29.2-33.5 mm SL, Panambi, rio Divisa, 28°13’13”S 53°33’46”W, 19 Out 2009, A. Hirschmann & C. B. Kasper. UFRGS 14621, 4, 18.4-21.5 mm SL, Pirapó, rio Ijuí-Mirim near mouth wih rio Ijuí, 28°02’53”S 55°10’59”W, 5 Mar 2005, C. Oliveira, J. Ferrer, L. R. Malabarba, M. A. Azevedo. UFRGS 15287, 1, 34.3 mm SL, Panambi, rio Palmeira, 28°14’35”S 53°33’12”W, 24 Jun 2011, A. Hirschmann & C. B. Kasper. UFRGS 19104, 4, 19.8-32.3 mm SL, Panambi, rio Palmeira, 28°14’35”S 53°33’12”W, 8 Fev 2014, A. Hirschmann & C. B. Kasper.
Diagnosis. Scleronema milonga is distinguished from all congeners, with the exception of S. ibirapuita and S. teiniagua, by the absence of the pore s3 of the supraorbital line of the laterosensory system (vs. pore s3 present). Scleronema milonga differs from S. ibirapuita and S. teiniagua by the presence of the pore s6 of the supraorbital line of the laterosensory system (vs. pore s6 absent). Additionally, Scleronema milonga is distinguished from S. mate and S. guapa by having the rounded brown blotches at the midlateral line larger than opercle (vs. rounded brown blotches as large as or smaller than opercle in S. mate and absent in S. guapa).
Description. Based on specimens ranging from 17.5 to 39.2 mm SL; 3 c&s (one dissected). Morphometric data for 35 types in Tab. 7.
| Morphometric data of Scleronema milonga, new species (data of holotype included in the range). N = number of specimens; SD = standard deviation.
External morphology. Greatest height and width of body in half-length of trunk. Body elongate, trunk roughly cylindrical gradually compressed towards to caudal fin. Dorsal profile of trunk convex and ventral profile straight to slightly convex. Dorsal and ventral profiles of caudal peduncle straight. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin (Fig. 1). Head depressed and wide, trapezoid-shaped from dorsal view, wider posteriorly. Dorsal and ventral profiles of head straight. Anterior snout profile usually rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel long; emerging from posterolateral edge of anterior nostril extending between anterior and posterior margins of eye. Maxillary barbel long; emerging from edge of upper lip and extending between anterior and posterior margins of interopercle. Basal portion of maxillary barbel wide with thin fleshy flap dorsally and distal margin rounded. Maxillary barbel with thinner portion longer in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Ventral surface of lower lip with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap short and rounded. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 7(n = 44; including holotype) rays; first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture usually covered by branchial membrane anteriorly. Some specimens with intumescence above anterior portion of pectoral fin and axillary pore visible. Pelvic fin with distal margin convex when expanded, 4/5(n = 1) or 5(n = 43; including holotype) rays; first one always unbranched. Pelvic-fin origin located at vertical through dorsal-fin origin or briefly anterior extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight to slightly convex when expanded, 9(n = 18), or 10(n = 26; including holotype) rays; usually first two rays unbranched. Dorsal fin with 2(n = 2) procurrent rays. Dorsal-fin origin located at vertical through origin of pelvic fin or briefly posterior. Anal fin with distal margin slightly convex when expanded, 6(n = 40; including holotype) or 7(n = 4); usually first two rays unbranched. Anal fin with 2(n = 2) procurrent rays. Anal-fin origin located at vertical through last third of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 10(n = 1), 11(n = 1) or 12(n = 42; including holotype) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 10(n = 1) or 13(n = 1) procurrent rays dorsally and 10(n = 1) or 11(n = 1) procurrent rays ventrally. Procurrent rays of dorsal, anal, and caudal fins rarely visible.
Osteology. Premaxilla with teeth arranged in three rows. Dentary with 21-27(n = 2). Opercle with 12-16(n = 2) odontodes and interopercle with 14-16(n = 2) odontodes. Hyoid arch with 6(n = 2) branchiostegal rays. Free vertebrae 34(n = 2) or 35(n = 1); abdominal vertebrae 3(n = 2) or 5(n = 1). Ribs 9(n = 2) or 11(n = 1). First complete haemal arch in 4th(n = 2) or 6th(n = 1) free vertebra, first haemal spine in 11th(n = 2) or 12th(n = 1) free vertebra. Dorsal fin with 9(n = 1) or 10(n = 2) pterygiophores; first one inserted anteriorly to neural spine of 14th(n = 3) vertebra. Anal fin 6(n = 3) pterygiophores; first one inserted anteriorly to haemal spine of 18th(n = 1) or 19th(n = 2) vertebra.
Laterosensory system. Data for 44 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with nasal canal and pore s3 of frontal canal invariably absent; pore s6 of frontal canal usually present (Fig. 5C). Infraorbital line with antorbital segment invariably absent and sphenotic canal with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short and usually with one pore each (po1 and po2, respectively). Trunk canal short usually with two pores.
Coloration in alcohol. Lateral surface of body with midlateral line of 6-8 rounded black blotches larger than opercle over light yellow background; blotches of some individuals becoming fade or absent towards caudal peduncle (Figs. 14-15). Some individuals with lateral blotches in contact, almost forming continuous stripe (LIRP 16775). Dorsal surface of body with 5-6 rectangular black blotches extending ventrally to laterodorsal surface of body. Anterior portion of opercle black. Ventral surface of body light yellow with few brown blotches in caudal peduncle. Dorsal and laterodorsal surfaces of head with numerous brown rounded blotches over light yellow background. Ventral surface of head light yellow with few small brown blotches in lower lip, sometimes forming thin stripe. Barbels uniformly yellow or intercalated with brown areas. Pectoral-, pelvic-, and anal-fin rays faintly brown. Dorsal and caudal fins with vertical light brown stripe basally, rays faintly brown, and distal margins hyaline (Figs. 14-15).
| Scleronema milonga, new species, holotype (MCP 54165; 37.8 mm SL), Brazil, Rio Grande do Sul, Dezesseis de Novembro, arroio Lageado Araçá, rio Ijuí drainage, lower rio Uruguay.
| Specimen just after the fixation in formalin of Scleronema milonga, new species, paratype (UFRGS 7685; 32.7 mm SL), Brazil, Rio Grande do Sul, São Nicolau, unnamed stream tributary to rio Ijuí, lower rio Uruguay.
Coloration in life. Coloration in life similar to that of specimens preserved in ethyl alcohol, but more intense and with midlateral blotches of body brown (Fig. 15).
Geographical distribution. Scleronema milonga occurs in the rio Ijuí and rio Santa Rosa basins, two tributaries to the left bank of rio Uruguay, southern Brazil; and small tributaries of the right bank of the rio Uruguay, Misiones, Argentina (Fig. 4).
Ecological notes. Scleronema milonga inhabits rivers and streams with sand- and gravel-bottoms. The species has not been collected syntopically with its congeners. Individuals of S. milonga larger than 31.0 mm SL are capable to spawn and considered adults by Carvalho (2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.). Its diet is composed mainly of immature, autochthonous and benthic insects (Ephemeroptera, Chironomidae, and Trichoptera), in both adults and immatures (Carvalho, 2017).
Etymology. The species epithet “milonga” is given in reference to the musical rhythm popularized in Argentina and Rio Grande do Sul State (Brazil), both regions where the new species can be found. A noun in apposition.
Conservation status.Scleronema milonga has an Extent of Occurrence (EOO) less than 5,000 km2 and a significant part of the original vegetation of the rio Ijuí basin in Brazil was converted to soybean crops. However, no additional threats were detected and the species occurs in well-preserved areas of Argentina. Thus, the species can be classified as Least Concern (LC) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Additional material examined. MCP 13442, 1, 16.3 mm SL, Argentina, Misiones, Itacaruaré, arroyo Santa Maria, lower río Uruguay. UFRGS 27844, 8, 25.9-34.6 mm SL, Brazil, Santa Rosa, rio Santa Rosa at the confluence with a stream, lower rio Uruguay.
Scleronema minutum ( Boulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35. )
Trichomycterus minutusBoulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35.: 235 (original description, type locality: San Lorenzo district), plate 26; fig. 3 (drawing of one syntype from lateral view and drawing of the head and pectoral fins of one syntype from dorsal view). -Ihering, 1893Ihering H von. Die Süßwasser-Fische von Rio Grande do Sul. In: Koseritz’ Deutscher Volkskalender für Brasilien. Rio Grande do Sul; 1893. p.89-119.: 110 (original description). -Burgess, 1989Burgess WE. An atlas of freshwater and marine catfishes: a preliminary survey of the Siluriformes. Neptune City: T.F.H. Publications; 1989.: 322 (listed). -Nion et al., 2002Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres communes. Montevideo: Dinara; 2002.: 15 (listed). -Eschmeyer, 1998Eschmeyer WN. Catalog of fishes. California Academy of Sciences, San Francisco; 1998.: 2123 (listed in catalog). -Nion et al., 2016Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres comunes; segunda edición corregida y ampliada. Montevideo: Dinara ; 2016.: 15 (listed).
Pygidium angustirostrisDevincenzi, in Devincenzi, Teague, 1942Devincenzi GJ, Teague GW. Ictiofauna del Río Uruguay Medio. Anales del Museo de Historia Natural de Montevideo. 1942; 5(4)1-100.new synonym 30-31 (original description, type locality: Cañada de las Piedras), plate 4, fig. 3 (drawing of a type from lateral view and head detail from dorsal view). -Olazarri et al., 1970Olazarri J, Mones A, Ximénez A, Philippi ME. Lista de los ejemplares-tipo depositados en el Museo Nacional de Historia Natural de Montevideo, Uruguay. Comun Zool Mus Hist Nat Montev. 1970; 10(131):1-12.: 4 (type catalog of the MNHM). -Eschmeyer, 1998Eschmeyer WN. Catalog of fishes. California Academy of Sciences, San Francisco; 1998.: 2123 (listed in catalog). -Loureiro et al., 2016Loureiro M, Serra WS, Scarabino F. Colecciones ictiológicas del Uruguay: pasado y presente. In: Del Moral-Flores LFD, Villalobos AJR, Pérez JAM, Acosta AFG, López JF, editors. Colecciones ictiológicas de Latinoamérica. Ciudad de México: Facultad de Estudios Superiores Iztacala, UNAM Sociedad Ictiológica Mexicana; 2016. p.400-14.: 412 (notes on types).
Pygidium minutum -Ihering, 1898Ihering H von. Os peixes de água doce do Rio Grande do Sul. In: Annuario do estado do Rio Grande do Sul. Porto Alegre; 1898. p.161-90.: 172-173 (notes on original description). -Eigenmann, 1910Eigenmann CH. Catalogue of the fresh-water fishes of tropical and south temperate America. In: Scott WB, editor. Reports of the Princeton University expeditions to Patagonia, 1896-1899. Stuttgart: Princeton University; 1910. p.375-511.: 399 (listed). -Eigenmann, 1918: 340, fig. 20 (drawings of original description and original description with few modifications). -Gosline, 1945Gosline WA. Catálogo dos nematognatos de água-doce da América do sul e central. Bol Mus Nac Rio de Janeiro Zool. 1945; 33:1-138.: 61 (listed).
Scleronema angustirostris -Tchernavin, 1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
: 238 (brief redescription). -Costa, Bockmann, 1993Costa WJEM, Bockmann FA. Un nouveau genre néotropical de la famille des Trichomycteridae (Siluriformes: Loricarioidei). Rev Fr Aquariol. 1993; 20(2): 43-46.: 44 (comparative material). -López et al., 2003López HL, Miquelarena AM, Menni RC. Lista comentada de los peces continentales de la Argentina. Buenos Aires: ProBiota, FCNyM, UNLP ; 2003. (Serie Técnica y Didáctica; 5): 38 (listed). -de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.: 278 (listed). -Menni, 2004Menni RC. Peces y ambientes en la Argentina continental. Monogr Mus Argentino Cienc Nat. 2004; 5:1-316.: 82, 96 (listed). -Liotta, 2005Liotta J. Distribución geográfica de los peces de aguas continentales de la República Argentina. Buenos Aires: ProBiota, FCNyM, UNLP; 2005. (Serie Documentos; 3): 623 (distribution in Argentina)
Scleronema operculatum [nonEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.] -Castello et al., 1978Castello HP, Ehrlich MD, Wais IR, Puig A. Adiciones a la fauna de los peces de los rios Paraná medio y Bermejo. Rev Mus Argent Cienc Nat “Bernardino Rivadavia”. 1978; 12(9):119-35.: 125-127 (brief description), figs. 2-3 (drawings of a specimen from dorsal, lateral and ventral views and detail of the opercle and interopercle). -López et al., 2003López HL, Miquelarena AM, Menni RC. Lista comentada de los peces continentales de la Argentina. Buenos Aires: ProBiota, FCNyM, UNLP ; 2003. (Serie Técnica y Didáctica; 5): 38 (listed). -López et al., 2005López HL, Miquelarena AM, Ponte Gómez J. Biodiversidad y distribución de la ictiofauna mesopotámica. Miscelánea. 2005; 14:311-54.: 326, 336 (listed). -Menni, 2004Menni RC. Peces y ambientes en la Argentina continental. Monogr Mus Argentino Cienc Nat. 2004; 5:1-316.: 82 (listed). -Liotta, 2005Liotta J. Distribución geográfica de los peces de aguas continentales de la República Argentina. Buenos Aires: ProBiota, FCNyM, UNLP; 2005. (Serie Documentos; 3): 280 (distribution in Argentina). -Arias et al., 2013Arias JD, Demonte LD, Miquelarena AM, Protogino LC, López H. Lista de peces de la provincia de Entre Ríos. ProBiota, FCNyM, UNLP, Serie Técnica y Didáctica. 2013; 22:1-19.: 13 (listed).
Scleronema angustirostre -Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
: 413 (listed). -Datovo, Bockmann, 2010Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol . 2010; 8(2):193-246. https://doi.org/10.1590/S1679-62252010000200001: 198, 203, 219, 220; fig. 31, 221; fig. 32, 234, 235, 236 (phylogenetic relationships, data matrix, phylogenetic tree). -Serra et al., 2014Serra S, Bessonart J, Melo FT, Duarte A, Malabarba LR, Loureiro M. Peces del Río Negro. Montevideo: MGAP-DINARA; 2014.: 73 (photo, notes on distribution, habitat, and morphological traits). -Litz, Koerber, 2014Litz TO, Koerber S. Check list of the freshwater fishes of Uruguay (CLOFF-UY). ICP. 2014; 28:1-40.: 22 (listed). -Paullier et al., 2019Paullier S, Bessonart J, Brum E, Loureiro M. Lista de especies de peces de la cuenca del río Queguay, río Uruguay Bajo. Bol Soc Zool Uruguay. 2019; 28(2):66-78.: 69, 74; fig. 6b (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 122-131; figs. 47-50 (phylogenetic relationships, taxonomy)
Scleronema cf. angustirostre -Ochoa et al., 2017aOchoa LE, Roxo FF, DoNascimiento C, Sabaj MH, Datovo A, Alfaro M, Oliveira C. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae. Mol phylogenet evol. 2017a; 115:71-81. http://dx.doi.org/10.1016/j.ympev.2017.07.007
http://dx.doi.org/10.1016/j.ympev.2017.0...
: 75, 76; fig. 2, 77; fig. 3, 79 (phylogenetic relationships, phylogenetic tree).
Scleronema minutum -Tchernavin, 1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
: 236-237 (redescription). -Fowler, 1954Fowler HW. Os peixes de água doce do Brasil. São Paulo: Arq Zool Estado São Paulo. 1954; (vol 2).: 37-38, fig. 639 (listed, drawing of one syntype from lateral view). -Burgess, 1989Burgess WE. An atlas of freshwater and marine catfishes: a preliminary survey of the Siluriformes. Neptune City: T.F.H. Publications; 1989.: 313 (drawing of Fowler, 1954). -Malabarba, 1989Malabarba LR. Histórico sistemático e lista comentada das espécies de peixes de água doce do sistema da Laguna dos Patos, Rio Grande do Sul, Brasil. Comun Mus Ciênc Tecnol PUCRS. 1989; 2(8):107-79. (Série Zoologia): 145 (listed). -Casciotta, Almirón, 1996Casciotta JR, Almirón AE. Scleronema minutum (Boulenger) y Ochmacanthus batrachosloma (M. Ribeiro) (Siluriformes: Trichomycteridae), dos citas nuevas para la cuenca del Plata en Argentina. Neotrópica. 1996; 42(107-108):51-54.: 51-53 (record to Argentina). -de Pinna, 1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.: 300 (notes on vertebrae). -Wosiacki, 2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.: 259; fig. 2, 260; fig. 3, 261; fig. 4, 262; fig. 5, 263; fig. 6 (phylogenetic relationships). -de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.: 278 (listed). -López et al., 2003López HL, Miquelarena AM, Menni RC. Lista comentada de los peces continentales de la Argentina. Buenos Aires: ProBiota, FCNyM, UNLP ; 2003. (Serie Técnica y Didáctica; 5): 38 (listed). -Menni, 2004Menni RC. Peces y ambientes en la Argentina continental. Monogr Mus Argentino Cienc Nat. 2004; 5:1-316.: 82, 96 (listed). -López et al., 2005López HL, Miquelarena AM, Ponte Gómez J. Biodiversidad y distribución de la ictiofauna mesopotámica. Miscelánea. 2005; 14:311-54.: 336 (listed). -Liotta, 2005Liotta J. Distribución geográfica de los peces de aguas continentales de la República Argentina. Buenos Aires: ProBiota, FCNyM, UNLP; 2005. (Serie Documentos; 3): 279 (distribution in Argentina). -Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
: 413 (listed). -Wosiacki, de Pinna, 2007Wosiacki WB, de Pinna MCC. Família Trichomycteridae. In: Buckup PA, Menezes NA, Ghazzi MAS, editors. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional; 2007. p.67-75.:69 (listed). -Arias et al., 2013Arias JD, Demonte LD, Miquelarena AM, Protogino LC, López H. Lista de peces de la provincia de Entre Ríos. ProBiota, FCNyM, UNLP, Serie Técnica y Didáctica. 2013; 22:1-19.: 13 (listed). -Fernández et al., 2015: 14 (catalog of the MLP). -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 111-121; figs. 47-50 (phylogenetic relationships, taxonomy). -Ochoa et al., 2017aOchoa LE, Roxo FF, DoNascimiento C, Sabaj MH, Datovo A, Alfaro M, Oliveira C. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae. Mol phylogenet evol. 2017a; 115:71-81. http://dx.doi.org/10.1016/j.ympev.2017.07.007
http://dx.doi.org/10.1016/j.ympev.2017.0...
: 75, 76; fig. 2, 77; fig. 3, 79 (phylogenetic relationships). -Ochoa et al., 2017bOchoa LE, Silva GSC, Costa e Silva GJ, Oliveira C, Datovo A. New species of Trichomycterus (Siluriformes: Trichomycteridae) lacking pelvic fins from Paranapanema basin, southeastern Brazil. Zootaxa. 2017b; 4319(3):550-60. http://dx.doi.org/10.11646/zootaxa.4319.3.7
http://dx.doi.org/10.11646/zootaxa.4319....
: 558, fig. 5 (phylogenetic tree). -Carvalho, 2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.: 14 (diet, ecomorphology, and reproduction). -Ochoa et al., 2020Ochoa LE, Datovo A, DoNascimiento C, Roxo FF, Sabaj MH, Chang J et al
. Phylogenomic analysis of trichomycterid catfishes (Teleostei: Siluriformes) inferred from ultraconserved elements. Sci Rep. 2020; 10:2697. http://doi.org/ 10.1038/s41598-020-59519-w
http://doi.org/ 10.1038/s41598-020-59519...
: fig. 3 (phylogenetic relationships).
Trichomycterus angustirostris -Nion et al., 2002Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres communes. Montevideo: Dinara; 2002.: 15 (listed). -Nion et al., 2016Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres comunes; segunda edición corregida y ampliada. Montevideo: Dinara ; 2016.: 15 (listed).
Lectotype by present designation. BMNH 1891.3.16.84, 32.4 mm SL, Brazil, Rio Grande do Sul, São Lourenço do Sul, stream tributary of laguna dos Patos, ca 31º21’55”S 51º58’42”W, H. Ihering (Fig. 16).
Paralectotypes. BMNH 1891.3.16.85-86, 2, 16.6-18.7 mm SL, Brazil, Rio Grande do Sul, São Lourenço do Sul, stream tributary of laguna dos Patos, ca 31º21’55”S 51º58’42”W, H. Ihering.
| Scleronema minutum, lectotype of Trichomycterus minutus (BMNH 1891.3.16.84; 32.4 mm SL), Brazil, Rio Grande do Sul, São Lourenço do Sul, stream tributary to laguna dos Patos. Photograph taken by Sandra Raredon.
| Scleronema minutum (MCP 17514; 39.8 mm SL), Brazil, Rio Grande do Sul, São Lourenço do Sul, arroio São Lourenço, laguna dos Patos system.
Diagnosis. Scleronema minutum is distinguished from S. macanuda and S. operculatum by the following external characters: maxillary barbel longer than half-length of the head (vs. shorter than half-length of the head); tips of pectoral-fin rays not extending beyond the interadial membrane (vs. extending beyond the interadial membrane), skin flap in the posterior margin of the opercle rounded and short (vs. skin flap pointed and long); fleshy flap at the base of the maxillary barbel located posteriorly, thin, restricted to the maxillary and distal margin rounded (vs. fleshy flap located anteriorly, thick, prolonged up to the snout and with distal margin straight); and by the caudal fin uniformly brown (vs. caudal fin with a transversal black bar distally). Scleronema minutum differs from S. ibirapuita, S. milonga, and S. teiniagua by having the pore s3 of the supraorbital line of the laterosensory system (vs. pores s3 absent). Scleronema minutum is distinguished from S. mate and S. guapa by having the rounded brown blotches at the midlateral line larger than opercle (vs. rounded brown blotches as large as or smaller than opercle in S. mate and absent in S. guapa).
Description. Based on specimens ranging from 14.5 to 53.9 mm SL; 7 c&s (2 dissected) and 4 xr (including lectotype and two paralectotypes). Morphometric data for types and non-types in Tab. 8.
| Morphometric data of Scleronema minutum (data of type specimens not included in the range of non-types). N = number of non-types; SD = standard deviation.
External morphology. Greatest height and width of body in half-length of trunk. Body elongate, trunk roughly cylindrical gradually compressed towards to caudal fin. Dorsal profile of trunk convex and ventral profile straight to slightly convex. Dorsal and ventral profiles of caudal peduncle straight. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin. Head depressed and wide, trapezoid-shaped from dorsal view, wider posteriorly; square-shaped in specimens with muscles of cheeks well developed. Dorsal and ventral profiles of head straight to slightly convex. Anterior snout profile usually rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel long; emerging from posterolateral edge of anterior nostril extending between anterior and posterior margins of eye. Maxillary barbel long; emerging from edge of upper lip and extending between anterior and posterior margins of interopercle. Basal portion of maxillary barbel wide with thin fleshy flap dorsally and distal margin rounded. Maxillary barbel with thinner portion longer in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Ventral surface of lips with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap short and rounded. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 6(n = 2), 6/7(n = 1) or 7(n = 69; including lectotype and two paralectotypes) principal rays; first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture usually covered by branchial membrane anteriorly. Some specimens with intumescence above anterior portion of pectoral fin and axillary pore visible. Pelvic fin with distal margin convex when expanded, 5(n = 71; including lectotype and one paralectotype) rays; first one always unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight to slightly convex when expanded, 9(n = 36), 10(n = 33; including one paralectotype) or 11(n = 1) rays; first two rays unbranched. Dorsal fin with 2(n = 3) or 3(n = 3) procurrent rays. Dorsal-fin origin located at vertical through half-length of pelvic fin. Anal fin with distal margin slightly convex when expanded, 6(n = 69; including one paralectotype) rays (one anomalous specimen with 4 rays); first two rays unbranched. Anal fin with 2(n = 6) procurrent rays. Anal-fin origin located at vertical through last third of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 11(n = 2), 12(n = 69; including lectotype and two paralectotypes) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 10(n = 1), 11(n = 1), 12(n = 2) or 14(n = 2) procurrent rays dorsally and 8(n = 2), 9(n = 2) or 10(n = 2) procurrent rays ventrally. Procurrent rays of dorsal, anal, and caudal fins rarely visible.
Osteology. Premaxilla with 19-25(n = 5) arranged in two or three rows. Dentary with 23-24(n = 1) arranged in one to three rows. Opercle with 10-15(n = 7) odontodes and interopercle with 13-23(n = 7) odontodes. Hyoid arch with 6(n = 6) branchiostegal rays. Free vertebrae 34(n = 1), 35(n = 3) or 36(n = 7; including paralectotype and two paralectotypes); abdominal vertebrae 2(n = 1) or 3(n = 6). Ribs 10(n = 3), 11(n = 5; including one paralectotype) or 12(n = 3; including one lectotype and one paralectotype). First complete haemal arch in 3th(n = 1) or 4th(n = 6) free vertebra, first haemal spine in 12th(n = 5) or 13th(n = 2) free vertebra. Dorsal fin with 8(n = 1), 9(n = 3) or 10(n = 4) pterygiophores; first one inserted anteriorly to neural spine of 13th(n = 1) or 14th(n = 6; including lectotype) or 16th(n = 4; including two paralectotypes) vertebra. Anal fin with 6(n = 8; including lectotype) pterygiophores; first one inserted anteriorly to haemal spine of 18th(n = 1), 19th(n = 4) or 20th(n = 6; including lectotype and two paralectotypes) vertebra.
Laterosensory system. Data for 291 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with nasal canal invariably absent and frontal canal usually with pores s3 and s6 (Fig. 5D). Infraorbital line with antorbital segment invariably absent and sphenotic canal usually with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short with one pore each (po1 and po2, respectively). Trunk canal short usually with two pores.
Coloration in alcohol. Lateral surface of body with midlateral line of 5-9 (rarely 4 or 10) rounded brown blotches larger than opercle over light yellow background; blotches of some individuals becoming fade or absent towards caudal peduncle (Figs. 16-20). Lateral surface of body with additional smaller brown blotches. Some smaller individuals (< 28.0 mm SL) with lateral blotches in contact, almost forming continuous stripe (UFRGS 8622 and UFRGS 13665). Dorsal surface of body with 5-9 rectangular brown blotches extending ventrally to laterodorsal surface of body; some individuals with blotches of dorsal surface discontinuous (UFRGS 19647). Ventral surface of body light yellow with few brown blotches in caudal peduncle. Dorsal and laterodorsal surfaces of head with numerous brown rounded blotches over light yellow background. Anterior portion of opercle black. Ventral surface of head light yellow with few small brown blotches in lower lip, sometimes forming thin stripe. Barbels uniformly yellow or intercalated with brown areas. Pectoral-, pelvic-, and anal-fin rays weakly brown or completely hyalines. Dorsal- and caudal-fin rays brown. Caudal fin with vertical light brown stripe basally.
Coloration in life. Coloration in life similar to that of specimens preserved in ethyl alcohol, but more intense (Fig. 18).
| Specimens in life of Scleronema minutum (A. UFRGS 22100; 37.4 mm SL, Santana da Boa Vista, arroio das Neves, B. UFRGS 22128; 39.4 mm SL, arroio Igrejinha), Brazil, Rio Grande do Sul.
Geographical distribution. Scleronema minutum occurs in the laguna dos Patos system, lower rio Uruguay basin and lower rio Paraná basin. In the former, the species is widely distributed in rivers and streams that drain directly to laguna dos Patos (Brazil) and lagoa Mirim (Brazil and Uruguay). The species is known from only one stream of the right bank of the lower rio Uruguay, the arroyo Yuquerí Chico (Argentina), and widespread in following drainages of its other side: rio Negro (Brazil and Uruguay), rio Ibicuí (Brazil), río Dayman (Uruguay), río Queguay (Uruguay), and río San Salvador (Uruguay). In the lower rio Paraná basin, the species has few records to two tributaries of its left bank, the arroyo Feliciano and río Guayquiraro, Argentina (Fig. 4). Other two possible records of S. minutum for Argentina (MLP 10557; arroyo Mármol, tributary of río Uruguay, and MLP 10649; río Gualeguay, tributary of río Paraná) could not be accessed and examined.
Ecological notes.Scleronema minutum inhabits rivers and streams usually with sand or gravel-bottoms. The species is found syntopically with S. macanuda in the laguna dos Patos system and rio Negro basin and syntopically with S. operculatum in the headwaters of the rio Ibicuí. The stomachs of eight specimens were analyzed and seven had immature aquatic Diptera (Chironomidae), Ephemeroptera, and Plecoptera. Individuals of S. minutum larger than 29.0 mm SL are capable to spawn and considered adults by Carvalho (2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.). Its diet is composed mainly by “organic matter indeterminate” and immature, autochthonous and benthic insects (Chironomidae, Ephemeroptera and Trichoptera) in adults and Chironomidae in immatures (Carvalho, 2017).
Conservation status. Scleronema minutum is frequent and abundant in the lower rio Uruguay and Laguna dos Patos system, also occuring in the lower rio Paraná. No specific threats were detected and the species can be classified as Least Concern (LC) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Remarks on the type locality of Scleronema minutum. Boulenger (1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35.) quoted the type locality of Scleronema minutum as San Lorenzo District, in the state of Rio Grande do Sul, Brazil. Such a locality was erroneously interpreted by de Pinna, Wosiacki (2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.) as “São Lourenço das Missões = 28°30’S 54°40’W”, also in Rio Grande do Sul State, Brazil, in the rio Uruguay drainage. Such mistake has been later repeated in subquently published systematic fish catalogs (Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
; Wosiacki, de Pinna, 2007).
The actual type locality, however, has long been correctly referred by Ihering (1898Ihering H von. Os peixes de água doce do Rio Grande do Sul. In: Annuario do estado do Rio Grande do Sul. Porto Alegre; 1898. p.161-90.: 172, 173), who collected those fishes and sent them to Boulenger. Ihering mentions the type locality of S. minutum as “arroios da colônia de S. Lourenço” (= creeks of the colony of S. Lourenço). The German colony called San Lorenzo by Boulenger (1891) or S. Lourenço by Ihering (1898) is currently the city of São Lourenço do Sul (31º21’55”S 51º58’42”W), which encompass two streams tributaries of the laguna dos Patos, the arroio São Lourenço and the arroio Cahará, possibly the localities where the type specimens were collected (Fig. 4).
Malabarba (1989Malabarba LR. Histórico sistemático e lista comentada das espécies de peixes de água doce do sistema da Laguna dos Patos, Rio Grande do Sul, Brasil. Comun Mus Ciênc Tecnol PUCRS. 1989; 2(8):107-79. (Série Zoologia)) discussed the origin of several fish specimens collected in Rio Grande do Sul by Herman von Ihering that were sent to the collections of the British Museum (Natural History) and Indiana University (now at California Academy of Sciences), referring to Ihering (1898) who clearly stated that all fishes he collected come from laguna dos Patos drainage, and not from the rio Uruguay drainage: “It [the Rio Grande do Sul State] is divided, considering the freshwater fauna, into two perfectly distinct regions, that of the Uruguay River and that of the Lagoa dos Patos, one and the other comprising the respective slopes. Regarding the first, nothing is known about the tributaries and slopes of the Uruguay River born in the State of Rio Grande do Sul. (...) Only the works of Eigenmann bring some fish from Rio Grande do Sul that were not collected by Hensel, nor by me and that evidently belong to the collection made in Uruguayana [this is a reference to the collection made by Brazilian Emperor Dom Pedro II and donated to the Thayer expedition]. (...) In abstraction from them there remain fifty-nine species which were signaled by Hensel, by myself and others in the latter region [the laguna dos Patos drainage].”
Finally, it is worthy mentioning that the locality given by de Pinna, Wosiacki (2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.) is not included in the area of distribution of Scleronema minutum. Instead, it is whitin the distribution range of a distinct species, S. milonga.
Remarks on type specimens of Trichomycterus minutus. The three syntypes of T. minutus (BMNH 1891.3.16.84-86) are small in comparison with the maximum SL of the species (16.3-32.4 vs. 55.5 mm SL). The largest and better-preserved specimen among the three syntypes of T. minutus (BMNH 1891.3.16.84) is designated herein as lectotype according to the Article 74 of the ICZN (1999International Commission on Zoological Nomenclature (ICZN). International code of zoological nomenclature. 4th ed. London: International trust for zoological nomenclature Natural History Museum [Internet]. London; 1999. Available from: https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/
https://www.iczn.org/the-code/the-intern...
) (Fig. 16) and because S. minutum occurs sympatrically with two congeners, S. macanuda and S. operculatum. Rounded blotches in the dorsal and lateral surfaces of body are visible in the lectotype herein designated, but not clearly visible in the two paralectotypes (BMNH 1891.3.16.85-86). The lectotype is also uncharacterized by the broken fin rays (more evident in the dorsal and anal fins) and the cutted nasal barbels. However, the fleshy flap in the maxillary barbel, the skin flap posterior to opercle, and the membrane in the dorsal margin of caudal peduncle are easily visible in all type specimens. The lectotype has 5-6 odontodes in the opercle and 9-12 in the interopercle, but at least three odontodes are lost in both bones.
Remarks on type specimens and type locality of Scleronema angustirostre. The holotype and three paratypes of Pygidium angustirostris housed at MHNM (Montevideo) are lost (Olazarri et al., 1970Olazarri J, Mones A, Ximénez A, Philippi ME. Lista de los ejemplares-tipo depositados en el Museo Nacional de Historia Natural de Montevideo, Uruguay. Comun Zool Mus Hist Nat Montev. 1970; 10(131):1-12.; de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.; Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
; Loureiro et al., 2016Loureiro M, Serra WS, Scarabino F. Colecciones ictiológicas del Uruguay: pasado y presente. In: Del Moral-Flores LFD, Villalobos AJR, Pérez JAM, Acosta AFG, López JF, editors. Colecciones ictiológicas de Latinoamérica. Ciudad de México: Facultad de Estudios Superiores Iztacala, UNAM Sociedad Ictiológica Mexicana; 2016. p.400-14.). The paratype sent to London (BMNH 1944.6.20.1; 34.5 mm SL) has the fins damaged (with rays broken and dyed of blue); the skin of the lower jaw was removed, possibly to observe the teeth, which are conical and directed inward; and odontodes dyed of blue and some missing (Fig. 19). However, the coloration of body is not faded completely and it is possible to observe the rounded blotches in the dorsal and lateral surfaces of body. Other important traits observed in the paratype are the papillae on lips, the posterior flap in the opercle, the thin membrane above caudal peduncle, and the pattern of pores in the laterosensory system compatible with that of S. minutum. The state of preservation of the two MNRJ 3605 paratypes of P. angustirostris (19.9-32.4 mm SL) is quite worse (Fig. 20) and few information could be obtained from them, such as in the laterosensory system. Coloration of specimens faded completely, barbels are broken, fins rays are broken or bent, and the skin over the head in the smallest specimen is damaged.
| Scleronema minutum, paratype of Pygidium angustirostris (BMNH 1944.6.20.1; 34.5 mm SL), Uruguay, Paysandú, cañada de las Piedras Blancas, tributary to río Queguay, lower rio Uruguay. Photograph taken by Sandra Raredon.
| Scleronema minutum, paratype of Pygidium angustirostris (MNRJ 3605; 32.4 mm SL), Uruguay, Paysandú, cañada de las Piedras Blancas, tributary to río Queguay, lower rio Uruguay.
Devincenzi, Teague (1942Devincenzi GJ, Teague GW. Ictiofauna del Río Uruguay Medio. Anales del Museo de Historia Natural de Montevideo. 1942; 5(4)1-100.) described the type locality of S. angustirostre, Cañada de las Piedras, in detail including photos and a map with the itinerary of its collector, Mr. Teague. The Cañada de las Piedras, also called by these authors as “Cañada de las Piedras Blancas”, is a stream tributary to the right bank of the río Queguay in Paysandú, lower rio Uruguay (Fig. 4), near to a railroad bridge (ca 32º05’09”S 57º53’13”W).
Material examined. 899 specimens. Argentina, lower río Parana: MACN 7130, 1, 27.5 mm SL, Corrientes, río Guayquiraró. MACN 7131, 1, 26.9 mm SL, Entre Ríos, arroyo Feliciano. MACN 7132, 3, 30.4-35.3 mm SL, Entre Ríos, puddles 1 km from arroyo Feliciano. Brazil, Rio Grande do Sul State, laguna dos Patos system: BMNH 1891.3.16.84, 32.4 mm SL, lectotype of Trichomycterus minutusBoulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35., stream tributary of laguna dos Patos, São Lourenço do Sul. BMNH 1891.3.16.85-86, 2, 16.6-18.7 mm SL, paralectotypes of Trichomycterus minutus Boulenger, 1891, collected with lectotype. FMNH 82728, 2, 12.5-13.5 mm SL, Santa Maria, rio Vacacaí-Mirim. MCN 14792*, 1, 39.0 mm SL, Mariana Pimentel, arroio Ribeiro Pequeno. MCN 16523, 6, 15.3-17.6 mm SL, São Jerônimo, arroio dos Cachorros. MCN 19007, 3, 25.1-44.4 mm SL, Sentinela do Sul, irrigation water channel draining to arroio Velhaco. MCP 11169, 12 (4 c&s), 17.7-36.5 mm SL, Jaguarão, rio do Telho in the mouth with rio Jaguarão. MCP 17260, 3, 24.6-29.1 mm SL, Caçapava do Sul, arroio Santa Bárbara. MCP 17341, 2, 34.0-35.5 mm SL, Minas do Leão, arroio Taquara. MCP 17506*, 1, 33.3 mm SL, São Lourenço do Sul, arroio São Lourenço. MCP 17509*, 8, 27.1-43.9 mm SL, Pelotas, arroio dos Porcos, tributary of rio Cadeia. MCP 17512*, 39, 16.4-37.3 mm SL, Vila Nova do Sul, arroio Cambaí, tributary of rio Vacacaí. MCP 17514*, 1 (xr), 39.8 mm SL, São Lourenço do Sul, arroio São Lourenço. MCP 19108, 1, 48.2 mm SL, Barra do Ribeiro, arroio do Ribeiro. MCP 25761*, 4, 47.5-51.5 mm SL, Santana da Boa Vista, unnamed stream tributary of arroio das Neves. MCP 25911*, 6, 18.5-45.1 mm SL, Lavras do Sul, unnamed stream tributary of rio Camaquã Chico. MCP 25927, 5, 19.6-45.9 mm SL, Lavras do Sul, arroio da Mantiqueira. MCP 34758, 1, 50.7 mm SL, Pedro Osório, arroio Mata Olho. MCP 36805, 1, 29.7 mm SL, Viamão, stream at Praia da Pedreira, Parque Estadual de Itapuã Conservation Unit. MCP 36808, 10, 27.0-45.3 mm SL, Pedro Osório, arroio Mata Olho. MCP 36810*, 8, 31.6-44.9 mm SL, Piratini, arroio Piratini. MCP 41668, 2, 20.8-21.7 mm SL, Arroio Grande, unnamed stream tributary of arroio Basílio. MCP 45808*, 5, 28.7-49.0 mm SL, Pelotas, arroio Corrientes. MPEG 34067, 5, 30.9-45.7 mm SL, Jaguarão, unnamed stream tributary of arroio Telho Chico. UFRGS 3968, 1, 28.4 mm SL, Tapes, arroio Velhaco. UFRGS 7617, 11, 23.0-44.4 mm SL, Herval, unnamed stream tributary of arroio Grande. UFRGS 7639*, 31 (2 c&s), 18.5-43.6 mm SL, Herval, unnamed stream tributary of rio Jaguarão Chico. UFRGS 7640, 4, 20.2-36.9 mm SL, Pinheiro Machado, arroio dos Pires. UFRGS 7641, 2, 39.5-45.0 mm SL, Herval, sanga Arroito, tributary of rio Jaguarão. UFRGS 8626, 6, 24.0-38.1 mm SL, Pedro Osório, rio Piratini. UFRGS 10625, 12, 21.3-46.1 mm SL, Candiota, rio Jaguarão. UFRGS 10841*, 1, 25.3 mm SL, Encruzilhada do Sul, unnamed stream tributary of arroio da Caneleira. UFRGS 13612, 15, 23.6-36.5 mm SL, Turuçu, arroio Corrientes. UFRGS 13665, 10, 24.1-42.6 mm SL, Pinheiro Machado, unnamed stream tributary of rio Piratini. UFRGS 13709, 19, 27.2-39.1 mm SL, Pinheiro Machado, unnamed stream tributary of arroio Boici. UFRGS 13962, 10, 22.0-44.2 mm SL, Bagé, arroio Camaquã Chico. UFRGS 14097*, 2 (1 c&s), 32.6-33.1 mm SL, Pelotas, arroio Basílio. UFRGS 14100*, 2 (1 c&s), 37.9-39.1 mm SL, Pelotas, unnamed stream tributary of rio Piratinizinho. UFRGS 14105*, 1, 40.7 mm SL, Pelotas, unnamed stream tributary of arroio Basílio. UFRGS 17281, 1, 45.0 mm SL, Pinheiro Machado, unnamed stream tributary of arroio Banhado Grande. UFRGS 17282, 1, 38.1 mm SL, Pinheiro Machado, unnamed stream tributary of Banhado Grande. UFRGS 19384, 16, 33.7-53.9 mm SL, arroio Telho Chico. UFRGS 19388, 45, 21.5-53.4 mm SL, Jaguarão, unnamed stream tributary of arroio Telho Chico. UFRGS 21333, 9, 28.5-43.0 mm SL, Jaguarão, unnamed stream tributary of rio Jaguarão. UFRGS 22100, 14, 15.8-41.1 mm SL, Santana da Boa Vista, arroio das Neves, tributary of rio Camaquã. Rio Ibicuí basin, lower rio Uruguay. MCP 14139, 2 (1 c&s), 24.6-35.6 mm SL, Dom Pedrito, arroio Santa Maria Chico. MCP 54171, 3, 22.4-34.5 mm SL, São Gabriel, arroio Jaguari. MCP 43919, 38, 16.1-46.8 mm SL, Lavras do Sul, arroio Jaguari. MCP 43925, 31, 18.6-46.6 mm SL, São Gabriel, arroio Jaguari. MCP 46740, 1, 31.9 mm SL, Dom Pedrito, rio Jaguari. UFRGS 8622*, 15, 21.2-47.1 mm SL, Bagé, rio Santa Maria. UFRGS 8623*, 7, 30.4-36.5 mm SL, Bagé, arroio Santa Maria Chico. UFRGS 21925*, 1, 40.2 mm SL, arroio Santa Maria Chico. UFRGS 9380*, 1, 45.0 mm SL, Lavras do Sul, arroio Taquarembó. UFRGS 9381, 3, 25.4-29.9 mm SL, Lavras do Sul, arroio Taquarembó. UFRGS 9382, 2, 30.0-35.6 mm SL, Lavras do Sul, arroio Taquarembó. UFRGS 9383, 1, 34.1 mm SL, Lavras do Sul, arroio Taquarembó. UFRGS 9384, 2, 31.1-37.2 mm SL, Lavras do Sul, arroio Taquarembó. UFRGS 9385*, 1, 37.1 mm SL, Lavras do Sul, arroio Taquarembó. UFRGS 11761*, 14, 21.5-36.3 mm SL, Dom Pedrito, arroio Taquarembó. UFRGS 11762, 2, 20.8-28.6 mm SL, Dom Pedrito, arroio Taquarembó. UFRGS 19647, 12, 25.0-52.3 mm SL, Santana do Livramento, arroio Porteirinha. UFRGS 19648, 4, 24.0-48.3 mm SL, Santana do Livramento, Sanga do Cerro Verde. UFRGS 19650, 1, 38.7 mm SL, Lavras do Sul, arroio Santo Antônio. UFRGS 19651, 28, 21.9-46.3 mm SL, Lavras do Sul, stream tributary of rio Santo Antônio. UFRGS 19652, 18, 17.2-46.4 mm SL, Lavras do Sul, arroio Santo Antônio. Rio Negro basin, lower rio Uruguay. MCP 35127, 8, 22.9-46.9 mm SL, Bagé, arroio Santa Tecla. UFRGS 20740, 2, 33.5-37.4 mm SL, Bagé, rio Negro. MCP 22874, 1, 21.3 mm SL, Bagé, rio Piraizinho. UFRGS 8625*, 2, 30.8-43.5 mm SL, Bagé, arroio Piraí. UFRGS 20741, 36, 30.9-45.1 mm SL, Bagé, Sanga do Acampamento, stream tributarty of rio Piraí. UFRGS 20742, 28, 23.1-50.0 mm SL, Bagé, unnamed stream tributaty to arroio Quebrado. UFRGS 21636, 59 (10 c&s), 14.5-41.5 mm SL, Bagé, rio Piraí. UFRGS 22128, 38, 16.6-39.4 mm SL, arroio Igrejinha. UMMZ 225384, 3, 30.4-35.6 mm SL, Bagé, rio Piraizinho. Uruguay, laguna de los Patos system. FMNH 125691, 2, 34.8-35.3 mm SL, Lavalleja, arroyo Polanco, río Cebollatí basin. ZVC-P 1933, 4, 23.7-39.7 mm SL, Lavalleja, arroyo de los Chanchos, río Cebollatí basin. ZVC-P 3377, 2, 46.7-48.8 mm SL, Maldonado, arroyo Aiguá, río Cebollatí basin. ZVC-P 5799, 2, 35.4-49.7 mm SL, Maldonado, arroyo Calera, río Cebollatí basin. ZVC-P, 6590*, 3, 1.73-36.4 mm SL, Treinta y Tres, río Tacuarí at Paso del Dragón. ZVC-P 6740, 1, 35.6 mm SL, Cerro Largo, arroyo de la Mina at Paso del Duraznero, río Yaguarón basin. ZVC-P 6782, 9, 18.8-45.3 mm SL, Lavalleja, río Cebollatí at Paso del Rey. ZVC-P 6819, 3, 38,9-42,3 mm SL, Lavalleja, arroyo de los Chanchos, bacia do río Cebollatí. ZVC-P 6910*, 2, 38.3-39.7 mm SL, Treinta y Tres, río Olimar, río Cebollatí basin. ZVC-P 7177, 3, 33.0-41.4 mm SL, Treinta y Tres, río Olimar at Paso de las Piedras, río Cebollatí basin. ZVC-P 7178, 2, 32.3-42.8 mm SL, Treinta y Tres, arroyo de las Averias at Paso del Aguila, río Cebollatí basin. ZVC-P 7180*, 1, 39.5 mm SL, Lavalleja, río Cebollatí at Paso del Rey. ZVC-P 8571, 4, 19.0-39.9 mm SL, Lavalleja, arroyo de los Chanchos, río Cebollatí basin. ZVC-P 12765, 2, 34.3-38.4 mm SL, Maldonado, arroyo Valdivia, río Cebollatí basin. Río Dayman basin, lower río Uruguay. ZVC-P 2847, 3, 25.0-26.4 mm SL, Paysandú, río Dayman at Paso de las Piedras. ZVC-P 7516, 1, 48.0 mm SL, Salto, río Dayman. Río Queguay basin, lower río Uruguay. MNRJ 3605, 2 (xr), 19.9-32.4 mm SL, paratypes of Pygidium angustirostris, Paysandú, Cañada de las Piedras Blancas, tributary of río Queguay. BMNH 1944.6.20.1*, 1 (xr), 34.5 mm SL, paratype of Pygidium angustirostris, Paysandú, Cañada de las Piedras Blancas, tributary of río Queguay. MCP 17513*, 3, 30.2-34.6 mm SL, Cañada del Pantano, tributary of río Queguay. MHNM 3475, 2, 32.6-42.9 mm SL, Paysandú, Cañada de las Piedras Blancas, tributary of río Queguay. MZUSP 3428*, 1, 39.0 mm SL, Cañada de las Piedras, tributary of río Queguay. ZVC-P 1058, 1, 50.1 mm SL, Paysandú, Cañada de las Piedras Blancas, tributary of río Queguay. ZVC-P 7480, 3, 26.9-38.2 mm SL, Paysandú, río Queguay Chico. ZVC-P 11606, 5, 29.6-37.0 mm SL, Paysandú, río Queguay Grande. ZVC-P 12491, 14 (1 c&s), 21.2-46.2 mm SL, Paysandú, Cañada de las Piedras Blancas, tributary of río Queguay. Río Negro basin, lower río Uruguay. MZUSP 81018, 1, 47.0 mm SL, Tacuarembó, Paso Lambaré. UFRGS 7238, 19, 20.1-47.0 mm SL, Rivera, arroyo Corrales. UFRGS 7244*, 2, 36.9-40.0 mm SL, Rivera, arroyo Yaguarí. UFRGS 7333*, 3, 31.6-42.3 mm SL, Rivera, arroyo Batovi. UFRGS 7367*, 2, 47.3-49.7 mm SL, Durazno, río Yí. UFRGS 13798, 1, 25.9 mm SL, Rivera, arroyo Batovi. UFRGS 14534, 2, 28.0 mm SL, Rivera, arroyo Corrales. ZVC-P 1139, 1, 22.7 mm SL, Rivera, arroyo Cuñapirú. ZVC-P 1148, 1, 31.4 mm SL, Rivera, arroyo Cuñapirú at Paso de la Calera. ZVC-P 1964, 1, 33.2 mm SL, Tacuarembó, arroyo Yaguarí at Paso del Sauce. ZVC-P 2607, 1, 25.8 mm SL, Durazno, arroyo Córdobes at Paso del Gordo. ZVC-P 2815, 5, 29.5-45.6 mm SL, Rivera, arroyo Cuñapiru. ZVC-P 3414, 4, 34.3-43.6 mm SL, Durazno, arroyo Córdobes, Paso de la Cruz. ZVC-P 5181*, 9, 29.4-46.8 mm SL, Tacuarembó, arroyo Pororo. ZVC-P 7338, 1, 55.5 mm SL, Tacuarembó, arroyo Carpintería. ZVC-P 7404, 3, 30.6-35.3 mm SL, Durazno, río Negro. ZVC-P 7532, 8, 25.6-41.3 mm SL, Flores, arroyo Grande. ZVC-P 8568, 9, 27.9-47.8 mm SL, Durazno, río Yí at Paso San Borja. ZVC-P 8575, 2, 27.2-29.3 mm SL, Tacuarembó, arroyo Yaguarí at Paso del Sauce. ZVC-P 8577, 2, 32.9-34.9 mm SL, Tacuarembó, arroyo Yaguarí, Paso del Sauce. ZVC-P 11125, 3, 26.9-32.5 mm SL, Tacuarembó, río Negro. ZVC-P 11159, 1, 33.0 mm SL, Cerro Largo, arroyo Quebracho. ZVC-P 11287, 3, 27.1-39.1 mm SL, Cerro Largo, arroyo Tupambaé at Paso del Sauce. ZVC-P 11442, 2, 42.4-42.6 mm SL, Florida, arroyo Mansavillagra. ZVC-P 11528, 1, 47.1 mm SL, Florida, arroyo del Monzón. ZVC-P 13640, 1, 32.3 mm SL, Durazno, río Yí, Paso Villasboas. ZVC-P 13641, 1, 45.4 mm SL, Soriano, arroyo del Monzón at Paso de los Carros. Río San Salvador basin, lower rio Uruguay. ZVC-P 3580, 3, 27.6-42.1 mm SL, Soriano, río San Salvador. ZVC-P 3584, 2, 23.1-23.8 mm SL, Soriano, río San Salvador. ZVC-P 7541*, 17 (2 c&s), 39.6-51.7 mm SL, Soriano, río San Salvador at Paso de las Tamberas. ZVC-P 12594, 1, 49.6 mm SL, Soriano, río San Salvador.
Scleronema operculatum Eigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.
(Figs. 10b, 21-24, 25a-b; Tabs. 3, 9)
Scleronema operculatumEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.: 691-692 (original description, type species by original designation, type locality: Cacequy, Uruguay basin). -Eigenmann, 1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.: 381; plate 44; fig. 1 (original description, drawing of the holotype from lateral view), 282; fig. 2d-e (drawing of the neurocranium, suspensorium, opercular apparatus, and inferior jaw from dorsal view of one paratype). -Henn, 1928Henn AW. List of types of fishes in the collection of the Carnegie Museum on September 1, 1928. Ann Carnegie Mus . 1928; 19(4):51-99.: 81 (listed, type catalog of the Carnegie Museum). -Tchernavin, 1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
: 235-236 (redescription based on original description). -Gosline, 1945Gosline WA. Catálogo dos nematognatos de água-doce da América do sul e central. Bol Mus Nac Rio de Janeiro Zool. 1945; 33:1-138.: 55 (listed). -Fowler, 1954Fowler HW. Os peixes de água doce do Brasil. São Paulo: Arq Zool Estado São Paulo. 1954; (vol 2).: 38, fig. 640 (listed, distribution, drawing of a type from lateral view). -Ibarra, Stewart, 1987Ibarra M, Stewart DJ. Catalogue of type specimens of Recent fishes in Field Museum of Natural History. Fieldiana Zool (New Series). 1987; 35:1-112.: 78 (type catalog of the FMNH). -Burgess, 1989Burgess WE. An atlas of freshwater and marine catfishes: a preliminary survey of the Siluriformes. Neptune City: T.F.H. Publications; 1989.: 321 (listed). -Arratia, 1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.: 399, fig. 2d (drawing of a specimen from lateral view). -de Pinna, 1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.: 300 (observation on vertebrae). -Eschmeyer, 1998Eschmeyer WN. Catalog of fishes. California Academy of Sciences, San Francisco; 1998.: 1243 (listed in catalog). -Wosiacki, 2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.: 259; fig. 2, 260; fig. 3, 261; fig. 4, 262; fig. 5, 263; fig. 6, 291; fig. 38, 309; fig. 69, 312, fig. 73, 323; fig. 86 (phylogenetic relationships, drawings of characters). -de Pinna, Wosiacki, 2003: 278 (listed). -Ferraris, 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
http://dx.doi.org/10.11646/zootaxa.1418....
: 413 (listed). -Wosiacki, de Pinna, 2007Wosiacki WB, de Pinna MCC. Família Trichomycteridae. In: Buckup PA, Menezes NA, Ghazzi MAS, editors. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional; 2007. p.67-75.: 69 (listed). -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 66-76; figs. 47-50 (phylogenetic relationships, taxonomy).
Scleronema minutum [nonBoulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35.] -de Pinna, 1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.: 4, fig. 22a, 23a (comparative material, drawings of the maxilla, premaxilla, and anterior portion of the mesethmoid from dorsal view).
Diagnosis. Scleronema operculatum is distinguished from all congeners with the exception of S. macanuda by the following external characters: maxillary barbel shorter than half-length of the head (vs. longer than half-length of the head); tips of the pectoral-fin rays extending beyond the interadial membrane (vs. not extending beyond the interadial membrane), skin flap in the posterior margin of the opercle pointed and long (vs. skin flap rounded and short); fleshy flap at the base of the maxillary barbel located anteriorly, thick, prolonged up to the snout and with distal margin straight (vs. fleshy flap located posteriorly, thin, restricted to the maxilla and with distal margin rounded); and by the caudal fin with a transversal black bar distally (vs. caudal fin uniformly brown). Scleronema operculatum differs from S. macanuda by having a midlateral line of 10-14 rounded black blotches as large as or smaller than opercle (vs. midlateral line of 6-9 rounded black blotches larger than opercle); tip of nasal barbel never reaching anterior margin of eye (vs. tip of nasal barbel usually extending beyond anterior margin of eye), and tip of maxillary barbel never surpassing anterior margin of interopercle (vs. tip of maxillary barbel extending between anterior and posterior margins of interopercle).
Description. Based on specimens ranging from 22.4 to 72.8 mm SL; 4 c&s (2 dissected) and 3 xr (holotype and two paratypes). Morphometric data for types and non-types in Tab 9.
| Morphometric data of Scleronema operculatum (data of type specimens not included in the range of non-types). N = number of non-types; SD = standard deviation.
External morphology. Greatest height of body in trunk and greatest width of body in anterior portion of trunk. Body elongate and compressed. Dorsal and ventral profiles of trunk straight to slightly convex. Dorsal and ventral profiles of caudal peduncle straight to slightly convex up to anteriormost procurrent ray insertion. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin. Head depressed and wide, trapezoidal from dorsal view, wider posteriorly. Dorsal and ventral profiles of head straight. Anterior snout profile rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel short; emerging from posterolateral edge of anterior nostril usually surpassing posterior nostril and never reaching anterior margin of eye. Maxillary barbel short; emerging from edge of upper lip and extending near to or reaching anterior margin of interopercle. Basal portion of maxillary barbel wide with thick fleshy flap dorsally and distal margin straight. Maxillary barbel with thinner portion smaller in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Lower lip and corners of upper lip with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap, thin and pointed; some specimens (as the holotype) with groove in skin flap. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 7 principal rays (n = 51; including holotype and three paratypes); first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture usually covered by branchial membrane anteriorly. Rays of pectoral fin extending slightly beyond interadial membrane. Some specimens with wilted skin above anterior portion of pectoral fin (including holotype and one paratype) or with intumescence, axillary pore not visible. Pelvic fin with distal margin convex when expanded, 5(n = 50; including holotype and three paratypes) or rarely 6(n = 1) rays; first one always unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight when expanded, 11(n = 28; including holotype and one paratype), 12(n = 20; including one paratype) or 13(n = 3; including one paratype) rays; usually first two or three rays unbranched. Eight small specimens (< 33.4 mm) of 51 analyzed without branched rays in dorsal fin. Dorsal fin with 2(n = 5; including holotype and two paratypes) or 3(n = 2) procurrent rays. Dorsal-fin origin located at vertical through first half of pelvic fin. Anal fin with distal margin slightly convex when expanded, 6(n = 29; including one paratype) or 7(n = 22; including holotype and two paratypes) rays; usually first one or two rays unbranched. Eight small specimens (< 33.4 mm) of 51 analyzed without branched rays in anal fin. Anal fin with 1(n = 1; paratype) or 2(n = 6; including holotype and one paratype) procurrent rays. Anal-fin origin located at vertical through last third of dorsal-fin base. Caudal fin with distal margin straight and corners slightly rounded, 12(n = 51; including holotype and three paratypes) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 12(n = 2) or 13(n = 5; including holotype and two paratypes) procurrent rays dorsally and 8(n = 1), 9(n = 1; holotype), 10(n = 1) or 11(n = 4; including two paratypes) procurrent rays ventrally. Procurrent rays of dorsal, anal, and caudal fins rarely visible.
Osteology. Premaxilla with 25-42(n = 2) arranged in three to five rows. Dentary with 46-88(n = 2) teeth arranged in one to five rows. Opercle with 8-13(n = 4) odontodes and interopercle with 10-15(n = 4) odontodes. Hyoid arch with 6(n = 4) branchiostegal rays. Free vertebrae 35(n = 2) or 36(n = 5; including holotype and two paratypes); abdominal vertebrae 3(n = 4). Ribs 8(n = 1; paratype), 9(n = 3; including one paratype) or 10(n = 3; including holotype). First complete haemal arch in 4th(n = 4) free vertebra, first haemal spine in 7th(n = 3) or 9th(n = 1) free vertebra. Dorsal fin with 10(n = 2; including one paratype), 11(n = 4; including holotype and one paratype) or 12(n = 1) pterygiophores; first one inserted anteriorly to neural spine of 15th(n = 3; including one paratype) or 16th(n = 4; including holotype and one paratype) vertebra. Anal fin with 6(n = 7; including holotype and two paratypes) pterygiophores; first one inserted anteriorly to haemal spine of 20th(n = 5; including two paratypes) or 21st(n = 2; including holotype) vertebra.
Laterosensory system. Data for 43 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with presence of nasal canals variable: with two paired canals (right and left), only one canal present or both canals absent (Fig. 10B). Nasal canal, if present, interrupted (not connecting with frontal canal) with pores s1 and s2. Frontal canal usually with pores s3 and s6. Infraorbital line with antorbital segment invariably absent and sphenotic canal with pores i10 and i11. Posterior segment of frontal, sphenotic and otic canals fused each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short usually with one pore each (po1 and po2, respectively). Trunk canal short usually with two pores.
Coloration in alcohol. Lateral surface of body with two longitudinal lines (midlateral and laterodorsal) of 10-14 black rounded blotches (as large as or smaller than opercle) over light yellow background; blotches in trunk of some individuals become fade or absent towards caudal peduncle (Figs. 21-22). Some large individuals with smaller blotches among larger ones. Longitudinal line of laterodorsal blotches visible from dorsal view. Ventral surface of body uniformly light yellow. Dorsal and lateral surfaces of head and nape area with numerous black rounded blotches or uniformly black. Ventral surface of head light yellow. Dorsal surface of maxillary and rictal barbels weakly black and ventral surface light yellow. Nasal barbel weakly black. Opercle with anterior, dorsal and ventral margins black; posterior fold light yellow. Pectoral, pelvic, dorsal and anal fins hyaline; one large individual (UFRGS 19654; 70.1 mm SL) with black bar in distal margin of anal fin. Caudal fin hyaline with transversal stripe basally and transversal black bar distally, which could extend up to half-length of fin in larger individuals.
| Scleronema operculatum (UFRGS 18086; 40.0 mm SL), Brazil, Rio Grande do Sul, Rosário do Sul, sanga Santo Antônio, tributary to rio Ibicuí da Armada, lower rio Uruguay.
| Specimen in life of Scleronema operculatum (UFRGS 18086; 42.3 mm SL), Brazil, Rio Grande do Sul, Rosário do Sul, sanga Santo Antônio, tributary to rio Ibicuí da Armada, lower rio Uruguay.
Coloration in life. Coloration in life similar to that of specimens preserved in ethyl alcohol, but more intense (Fig. 22).
Geographical distribution. Scleronema operculatum is endemic to the rio Ibicuí basin, a tributary to the left bank of rio Uruguay, State of Rio Grande do Sul, southern Brazil (Fig. 4).
Ecological notes.Scleronema operculatum inhabits rivers and streams usually with fine sand-bottoms. Scleronema guapa and S. minutum may be collected in the same microhabitat of S. operculatum. The stomachs of three specimens were analyzed and two had immature aquatic Diptera (Chironomidae), Odonata and grains of sand.
Conservation status.Scleronema operculatum has an Extent of Occurrence (EOO) less than 20,000 km2 being found in specific habitats. However, no threats were detected and the species can be classified as Least Concern (LC) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Remarks on type specimens and type locality of Scleronema operculatum. John Haseman collected the holotype and three paratypes of Scleronema operculatum in February 1909 in an expedition to South America. Haseman returned to USA one year later and the fishes were stored in the Carnegie Museum, Pittsburgh (Haseman, Eigenmann, 1911Haseman JD, Eigenmann CH. A brief report upon the expedition of the Carnegie Museum to Central South America, together with a list of localities at which Mr. Haseman collected. Ann Carnegie Mus. 1911; 7:287-314.). Eigenmann described S. operculatum in 1917 and the FMNH purchased all fishes from the former Carnegie Museum collection in 1951 (Ibarra, Stewart, 1987Ibarra M, Stewart DJ. Catalogue of type specimens of Recent fishes in Field Museum of Natural History. Fieldiana Zool (New Series). 1987; 35:1-112.) and, currently, the type specimens of S. operculatum are catalogued under the numbers FMNH 58080 (holotype; ex CM 7077) and FMNH 58520 (paratypes; ex CM 7539).
The coloration of the type specimens of S. operculatum faded and only one paratype has the black round blotches in the lateral surface of body (FMNH 58520; 58.6 mm SL) (Fig. 23). The caudal-fin rays of the holotype are slightly bent in their insertion and mid-length (Fig. 24). Additionally, the skin of the right maxilla in the holotype was ruptured making the bone exposed (probably by Eigenmann to analyze internally the structure of the conspicuous maxilla). Paratypes present faint wrapping marks in their bodies (Fig. 23). One of paratypes (FMNH 58520; 58.6 mm SL) has a longitudinal slit ventrally from abdomen extending to the lateral surface of trunk. The head of another paratype (FMNH 58520; not measured), osteologically studied by Eigenmann, was cut off and dissected dorsally making the bones exposed, which were drew in Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.; p. 282, fig. 2d-e; reproduced herein in the Fig. 25). In this same paratype, the anal-fin rays are broken.
| Scleronema operculatum, paratype (FMNH 58520; 58.6 mm SL), Brazil, Rio Grande do Sul, Cacequi, rio Cacequi, tributary to rio Santa Maria, rio Ibicuí basin, lower rio Uruguay. Photograph taken by Sandra Raredon.
| Scleronema operculatum, holotype (FMNH 58080; 65.9 mm SL), Brazil, Rio Grande do Sul, Cacequi, rio Cacequi, tributary to rio Santa Maria, rio Ibicuí basin, lower rio Uruguay. Photograph taken by Sandra Raredon.
External synapomorphies of the genus could be observed in the type specimens of S. operculatum, such as the skin folds in the maxillary barbel and posterior to opercle. The membrane on the dorsal margin of caudal peduncle is present in all types. Diagnostic characters for the Scleronema operculatum species group (see discussion) are also observed in the types, such as the maxillary barbel shorter than half-length of the head. Additionally, all types present the tip of nasal barbel not reaching the anterior margin of eye, a diagnostic character for S. operculatum.
It is possible to count the principal rays of fins (see description). Through the skin is visible 3-5 branchiostegal rays and were counted 5-7 and 6-11 odontodes in the opercular and interopercular, respectively; with posterior ones longer and curved. However, these counts should be interpreted with caution considering the losses of some odontodes along the time and those completely covering by skin.
The type locality, based on notes accompanying the type material, “Cacequy, Feb 2 09, 2383” [sic], fits with John Haseman´s itinerary of expedition (cf. Haseman, Eigenmann, 1911Haseman JD, Eigenmann CH. A brief report upon the expedition of the Carnegie Museum to Central South America, together with a list of localities at which Mr. Haseman collected. Ann Carnegie Mus. 1911; 7:287-314.: 308), and can be specified as Brazil, Rio Grande do Sul State, Cacequi, rio Cacequi, one mile from railroad station, rio Ibicuí basin, lower rio Uruguay, [ca 29°53’58”S 54°50’24”W].
Remarks on drawings of Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.). Even though Eigenmann (1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.) did not provide illustrations of Scleronema operculatum in his original description, one year later drawings of the holotype from lateral view and of the neurocranium, suspensorium, opercular apparatus, and lower jaw from dorsal view of one paratype were include in a revision of Trichomycteridae (Fig. 25; reproduced from Eigenmann, 1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.: 381; plate 44, fig. 1 and 282; fig. 2d-e). However, some details of those figures do not correspond with the redescription above and deserve comments. The distal margins of the dorsal and caudal fins are straight in all types and non-types analyzed in opposition with the convex profile exhibited in the drawing of the holotype in Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.). Similarly, the anterior margin of the mesethmoid Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.) is convex whereas the c&s specimens observed have this margin straight to slightly concave. Lastly, the posterior process of the autopalatine in S. operculatum is long, overlapping almost the entire area of the metapterygoid, distinctly longer than the illustration in Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.). However, some characters diagnostic for S. operculatum or synapomorphic for the genus can be recognized in the drawings: the lateral surface with 14 round black blotches smaller than opercle, the long and pointed skin flap posterior to opercle and the conspicuous ventral flap in the ventral margin of the opercle.
| Original drawings published by Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.): A. lateral view of the holotype of Scleronema operculatum, FMNH 58080, (plate 44; fig. 1); B. neurocranium, suspensorium, opercular apparatus, and inferior jaw from dorsal view of one paratype of S. operculatum, FMNH 58520, (p. 282; fig. 2d-e); C. part of the distribution map (modified from original drawing) showing the occurrence area of Scleronema (dashed square) at that time (plate 36).
Material examined. 54 specimens from Brazil, rio Ibicuí basin, lower rio Uruguay: FMNH 58080, 65.9 mm SL, holotype of Scleronema operculatumEigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703., Cacequi, rio Cacequi. FMNH 58520, 3, 53.3-58.6 mm SL (one specimen not measured), paratypes of Scleronema operculatum Eigenmann, 1917, collected with holotype. MCP 9315*, 2 (1 c&s), 38.2-41.8 mm SL, São Francisco de Assis, rio Jaguari. MCP 27457*, 4 (2 c&s), 24.2-41.3 mm SL, São Francisco de Assis, stream tributary of rio Inhacundá. MCP 27543, 5, 24.0-28.6 mm SL, Jaguari, arroio do Tigre, tributary of rio Jaguari. MCP 43904*, 4, 22.5-34.2 mm SL, São Gabriel, arroio Jaguari, tributary of rio Santa Maria. UFRGS 5292*, 3, 28.6-33.4 mm SL, Rosário do Sul, arroio do Salso. UFRGS 5349*, 1, 30.7 mm SL, Santana do Livramento, rio Ibicuí da Faxina. UFRGS 8624*, 1, 51.4 mm SL, Bagé, arroio Santa Maria Chico. UFRGS 18086*, 18 (1 c&s), 25.5-42.3 mm SL, Rosário do Sul, arroio Santo Antônio, tributary of rio Ibicuí da Armada. UFRGS 19308, 2, 29.9-45.6 mm SL, Alegrete, unnamed stream tributary of arroio São João. UFRGS 19309, 1, 38.5 mm SL, Alegrete, arroio São João. UFRGS 19646, 4, 41.9-57.7 mm SL, Santana do Livramento, unnamed stream tributary of arroio do Salso. UFRGS 19654*, 3 (1 c&s), 54.3-72.8 mm SL, Alegrete, arroio Jacaquá.
Scleronema teiniagua, new species
urn:lsid:zoobank.org:act:C32F77D3-67BA-40F5-8AAE-C00C0DF6A6A8
(Figs. 13b, 26-27; Tabs. 3, 10)
Scleronema sp. n. 3 -Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
: 421 (listed). -Ferrer, 2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.: 106-110; figs. 47-50 (phylogenetic relationships, taxonomy).
Holotype. ZVC-P 14522, 45.2 mm SL, Uruguay, Artigas, arroyo Tres Cruces, río Cuareím basin, lower río Uruguay, 30°35’31”S 56°37’34”W, 8 Sep 2005, F. Canteira, J. Ferrer, L. R. Malabarba & V. A. Bertaco.
Paratypes. 40 specimens. Brazil, Rio Grande do Sul State, rio Quaraí basin, lower rio Uruguay: LIRP 16776, 1, 29.3 mm SL, Quaraí, arroio Salsal, tributary of arroio Areal, 30°25’24”S 56°15’58”W, B. Menezes, C. Hartmann & T. Guimarães, 14 May 2014. MCP 10963*, 2, 30.7-35.0 mm SL, Santana do Livramento, arroio Cati, 30°33’21”S 56°06’24”W, 23 Jul 1986, L. A. Bergmann, P. V. Azevedo & R. E. Reis. MCP 16179*, 4, 15.5-18.2 mm SL, Santana do Livramento, rio Sarandi III, 30°34’23”S 56°04’02”W, 10 Dec 1992, J. P. F. Silva, P. H. Wimberger, R. E. Reis. UFRGS 20737, 2, 18.9-32.6 mm SL Quaraí, arroio Areal, 30°25’56”S 56°18’44”W, B. Menezes, C. Hartmann & T. Guimarães, 14 May 2014. Uruguay, Artigas, río Cuareím basin, lower río Uruguay: UFRGS 7755*, 1, 38.5 mm SL, arroyo Cuaró Grande, 30°47’03”S 56°46’53”W, 8 Sep 2005, F. Cantera, J. Ferrer, L. R. Malabarba & V. A. Bertaco. UFRGS 7811*, 10, 26.9-37.6 mm SL (2 c&s), collected with holotype. UFRGS 14636, 2, 36.0-37.3 mm SL, collected with holotype. ZVC-P 2497, 2, 35.6-41.3 mm SL, arroyo los Molles, 3 Feb 1970, 30°12’24”S 56°44’31”W, staff of Facultad de Humanidades Y Ciencias. ZVC-P 7669, 1, 22.3 mm SL, arroyo Catalán Chico, 30°42’28”S 56°19’21”W, 25 Jan 1958, J. Soriano. ZVC-P 9910*, 1, 37.4 mm SL, Cañada Mataojo, 30°47’47”S 56°56’52”W, Aug 2006, F. Quintans, F. Teixeira, I. González, M. Loureiro. ZVC-P 10122, 1, 38.9 mm SL, Cañada Honda, 30°29’07”S 56°50’16”W, Aug 2006, F. Quintans, F. Teixeira, I. González, M. Loureiro. ZVC-P 10160, 1, 41.8 mm SL, Cañada de Brum, 30°35’40”S 56°25’23”W, Aug 2006, F. Quintans, F. Teixeira, I. González, M. Loureiro. ZVC-P 10231*, 1, 41.8 mm SL, Cañada de la Cruz, 30°42’04”S 57°02’26”W, Aug 2006, F. Quintans, F. Teixeira, I. González, M. Loureiro. ZVC-P 10589, 6, 22.8-37.5 mm SL, Artigas, unspecific locality in río Cuareím basin, Feb 2006. ZVC-P 10617, 1, 35.2 mm SL, arroyo Tres Cruzes Grande, 30°26’20”S 56°48’13”W, Feb 2006, F. Quintans, F. Teixeira, I. González, M. Loureiro. ZVC-P 10618, 4, 27.7-35.1 mm SL, Artigas, unspecific locality in río Cuareím basin, Feb 2006.
Diagnosis. Scleronema teiniagua is distinguished from all congeners by a remarkable reduction in the laterosensory system lacking the pores s1, s2, s3 e s6 in the supraorbital line and pore i10 in the infraorbital line (vs. presence of, at least, the pore i10) and in the lower number of pterygiophores of dorsal fin (8 vs. 9-14).
Description. Based on specimens ranging from 15.5 to 45.2 mm SL; 2 c&s (one dissected). Morphometric data for 20 types in Tab. 10.
| Morphometric data of Scleronema teiniagua, new species (data of holotype included in the range). N = number of specimens; SD = standard deviation.
External morphology. Greatest height and width of body in half-length of trunk. Body elongate, trunk roughly cylindrical gradually compressed towards to caudal fin. Dorsal and ventral profiles of trunk slightly convex. Dorsal and ventral profiles of caudal peduncle straight. Dorsal margin of caudal peduncle with thin membrane, resembling adipose fin. Head depressed and wide, trapezoid-shaped from dorsal view, wider posteriorly; square-shaped in specimens with muscles of cheeks well developed. Dorsal and ventral profiles of head straight to slightly convex. Anterior snout profile usually rounded from dorsal view. Nostrils of equivalent size, smaller than eye diameter. Anterior nostril surrounded by fleshy flap of integument, posterolaterally continuous with nasal barbel. Posterior nostril surrounded anterolaterally by thin flap of integument. Eyes rounded, dorsally oriented but also visible from lateral view; located behind posterior nostrils; orbital rim not free; eyes covered by thin and transparent skin.
Barbels with large bases and tapering gradually towards tips. Nasal barbel long; emerging from posterolateral edge of anterior nostril extending up to posterior margin of eye or briefly surpassing. Maxillary barbel long; emerging from edge of upper lip and extending up to posterior margin of interopercle or briefly surpassing. Basal portion of maxillary barbel wide with thin fleshy flap dorsally and distal margin rounded. Maxillary barbel with thinner portion longer in length than wider one. Rictal barbel emerging from lateral lobe of lower lip and slightly shorter than maxillary barbel. Mouth subterminal with edges posteriorly oriented. Upper lip wider than lower lip. Lower lip with round fleshy lobes in corners. Ventral surface of lower lip with small papillae. Gill openings not constricted united with isthmus anteriorly forming free fold. Opercular patch of odontodes rounded, inserted in posterior region of head visible from dorsal and lateral views. Posterior margin of opercle with distinct skin flap short and rounded. Interopercular patch of odontodes elongate inserted on posteroventral region of head visible from lateral and ventral views. Odontodes of opercle and interopercle barely visible, completely involved by flesh.
Pectoral fin with distal margin convex when expanded, 6/7(n = 2), or 7(n = 21; including holotype) rays; first one always unbranched and not prolonged as filament; fourth and fifth longest. Pectoral-fin insertion posterior to branchial aperture covered by branchial membrane anteriorly. Some specimens with intumescence above anterior portion of pectoral fin and axillary pore visible. Pelvic fin with distal margin convex when expanded, 4/5(n = 1) or 5(n = 22; including holotype) rays; first one always unbranched. Pelvic-fin origin located at half-length of SL extending between urogenital papilla and anal-fin anterior insertion; tangentially inserted with inner margins separated by large interspace. Urogenital papilla located between last third of pelvic fins.
Dorsal fin with distal margin straight to slightly convex when expanded, 8(n = 5), 9(n = 30; including holotype), or 10(n = 2) rays; usually first two rays unbranched. Dorsal fin with 2(n = 1) or 3(n = 1) procurrent rays. Dorsal-fin origin located at half-length of pelvic fin. Anal fin with distal margin slightly convex when expanded, 6(n = 23; including holotype) rays; usually first two rays unbranched. Anal fin with 2(n = 2) procurrent rays. Anal-fin origin located at vertical through last third of dorsal-fin base. Caudal fin with distal margin straight and corners slightly convex, 11(n = 2) or 12(n = 21; including holotype) rays; most-external rays of dorsal and ventral plates of caudal fin always unbranched and smaller than branched rays. Branched rays of caudal fin splitting up to twice. Caudal fin with 12(n = 1) or 13(n = 1) procurrent rays dorsally and 9(n = 1) or 10(n = 1) procurrent rays ventrally. Procurrent rays of dorsal, anal, and caudal fins rarely visible.
Osteology. Premaxilla with 25-26(n = 1) teeth arranged in three rows. Dentary with 40(n = 1) teeth. Opercle with 14-15(n = 2) odontodes and interopercle with 17-21(n = 2) odontodes. Hyoid arch with 6(n = 2) branchiostegal rays. Free vertebrae 34(n = 1) or 35(n = 1); abdominal vertebrae 3(n = 1) or 4(n = 1). Ribs 10(n = 1) or 12(n = 1). First complete haemal arch in 4th(n = 1) or 5th(n = 1) free vertebra, first haemal spine in 11th(n = 1) or 12th(n = 1) free vertebra. Dorsal fin with 8(n = 2) pterygiophores; first one inserted anteriorly to neural spine of 14th(n = 1) or 16th(n = 1) vertebra. Anal fin with 6(n = 2) pterygiophores; first one inserted anteriorly to haemal spine of 18th(n = 1) or 20th(n = 1) vertebra.
Laterosensory system. Data for 40 specimens summarized in Tab. 3. Canals of laterosensory system with simple (non-dendritic) tubes and external pores. Supraorbital line with nasal and frontal canals invariably absent. Infraorbital line with antorbital segment and pore i10 of sphenotic canal invariably absent; pore i11 of sphenotic canal usually present. Posterior segment of frontal, sphenotic and otic canals fused each other. Otic, posotic and scapular canals present with preoperculo-mandibular and pterotic branches short with one pore each (po1 and po2, respectively). Trunk canal short with two pores.
Coloration in alcohol. Lateral surface of body with midlateral line of 5-9 rounded brown blotches larger than opercle over light yellow background; blotches of some individuals becoming fade or absent towards caudal peduncle (Figs. 13B, 26). Dorsal surface of body with 4-6 rectangular brown blotches extending ventrally to laterodorsal surface of body; smaller specimens with these blotches smaller, dorsally discontinuous (MCP 16179). Ventral surface of body light yellow with few brown blotches in caudal peduncle. Dorsal and laterodorsal surfaces of head with numerous brown rounded blotches over light yellow background. Anterior portion of opercle black. Ventral surface of head light yellow with few small brown blotches in lower lip, sometimes forming thin stripe. Barbels uniformly yellow or intercalated with brown areas. Pectoral-, pelvic-, and anal- fin rays weakly brown. Dorsal and caudal fins with vertical light brown stripe basally, rays weakly brown, and distal margins hyaline (Figs. 13B, 26).
Coloration in life. Coloration in life similar to that specimens preserved in ethyl alcohol, but more intense with blochtes better defined (Fig. 27).
| Scleronema teiniagua, holotype (ZVC-P 14522; 45.2 mm SL) Uruguay, Artigas, arroyo Tres Cruzes, río Cuareím basin, lower río Uruguay.
| Specimen in life out of the water of Scleronema teiniagua (ZVCP 13763), Uruguay, Artigas, arroyo Catalán Grande at Paso Santiño, río Cuareim basin lower río Uruguay. Photograph taken by Sebastián Serra.
Geographical distribution. Scleronema teiniagua is endemic to the drainage of the río Cuareím (Uruguay), also named rio Quaraí (Brazil), a tributary to the left bank of lower rio Uruguay (Fig. 4).
Only two lots identified on the basis of external characters as S. teiniagua were supposedly sampled outside of the río Cuareím basin: ZVC-P 3462 from arroyo Milano, Santa Lucía basin (collection date 1958) and ZVC-P 5592 (collection date 1971) from río Queguay basin. The ichthyofauna of the río Santa Lucía has been densely inventoried and none trichomycterid was recorded until now (Zarucki et al., 2011Zarucki M, González-Bergonzoni I, Teixeira-de-Mello F, Loureiro M. Fish diversity loss in an urban stream of Uruguay throughout the last century. Panam J Aquat Sci . 2011; 6(1):71-75; Marcelo Loureiro, 2014, pers. comm.). The unique specimen of the lot ZVC-P 5592 is originated from the type locality of S. angustirostre, Cañada de las Piedras, and all material analyzed from this locality and the río Queguay basin belong to S. minutum (senior synonym of S. angustirostre). The occurrence of S. teiniagua in the río Santa Lucía or río Queguay basins should be considered with caution and need to be confirmed by new samples.
Ecological notes.Scleronema teiniagua inhabits rivers and streams and has not been collected with its congeners. The stomachs of two specimens were analyzed and had immature aquatic Diptera (Chironomidae and Simuliidae) and Ephemeroptera.
Etymology. The species epithet “teiniagua” is given in reference to the character of a fictional tale entitled “Salamanca do Jarau” popularized in the Rio Grande do Sul State by the writer Simões Lopes Neto (Lopes-Neto, 1913Lopes-Neto JS. Lendas do Sul. Pelotas: Echenique & C.; 1913.). In this story, Teiniaguá was a princess transformed to a witch that lives in a cave at the hill “Cerro do Jarau”, which is inserted in the area of distribution of the new species. A noun in apposition.
Conservation status.Scleronema teiniagua has an Extent of Occurrence (EOO) less than 20,000 km2, but no threats were detected to the species. Thus, the species can be classified as Least Concern (LC) according to IUCN criteria (IUCN, 2019International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
Remarks. Specimens of Scleronema minutum analyzed by Vaz-Ferreira, Soriano (1959Vaz-Ferreira R, Soriano BS. Relación entre las longitudes antedorsal y anteventral y la longitud del cuerpo en dos Trichomycteridae (Pisces, Siluroidei). Arch Soc Biol Montevideo. 1959; 24:76-81.) from Artigas probably belong to S. teiniagua. However, these authors did not provide the voucher numbers to confirm their identification.
Additional material examined. 30 specimens from Uruguay. ZVC-P 3462, 17, 23.6-41.7 mm SL, uncertain locality. ZVC-P 5592, 1, not measured, uncertain locality. Artigas, río Cuareím basin, lower río Uruguay: ZVC-P 412, 3, 18.0-22.3 mm SL, río Cuareím near mouth of arroyo Yacaré. ZVC-P 10588, 8, 20.1-38.4 mm SL, río Cuareím. ZVC-P 10590, 1, 31.9 mm SL, arroyo Catalán Grande. ZVCP 13763, 1, not measured, arroyo Catalán Grande at Paso Santiño.
DISCUSSION
Monophyly of Scleronema. Eigenmann (1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.) defined the genus Scleronema on the basis of following diagnostic characters: ventrals nearer snout than caudal; outer pectoral rays shortest, without a filament; opercle with a long dermal flap; interopercular spines in a much more restricted area than in species of Pygidium (species currently allocate in Cambeva, Ituglanis, and Trichomycterus); accessory rays of the caudal inconspicuous; maxillary barbel with a large osseous base (maxillary bone); teeth very narrow incisors; mouth wide, terminal. Eigenmann (1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.) in a revisionary study of Pygidiidae (= Trichomycteridae) repeated all these traits and provided two new characters to recognize Scleronema in an identification key: maxillary bone longer than the attached barbel and anal short. Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) contested the exclusiveness of these characters in Scleronema and mentioned that the genus should be placed in the synonymy of Trichomycterus. According to him, only two characters present in Scleronema were considered of generic significance: the relatively wider cleft of the mouth and the larger maxilla. Based on current knowledge on the family, the most of these characters (Eigenmann, 1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.; 1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.; Tchernavin, 1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) failed to diagnose unequivocally Scleronema from other trichomycterids, with the single exception of the dermal opercular flap (Fig. 1), as noted by de Pinna (1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.; 1998), Arratia (1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.), and Wosiacki (2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.). Some specimens of Cambeva, Ituglanis, and Trichomycterus seem to have a thin opercular fleshy flap as mentioned by Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) for many species of Trichomycterus: “variously developed but always smaller than Scleronema”. Nevertheless, this condition results from the odontodes strongly embedded in the integument and usually observed in specimens preserved for a long time or fixed directly in alcohol. The vandelliine Plectrochilus machadoi is the only trichomycterid, which has a notable flap of integument in the opercle, such as that of Scleronema. However, this flap has a thinner thickness than Scleronema and surrounds practically the entire circumference of the opercle, condition that resembles an “ear” according to de Pinna (2013de Pinna MCC. Trichomycteridae. In: Queiroz LJ, Torrente-Vilara G, Ohara WM, Pires THS, Zuanon J, Doria CRC, editors. Peixes do Rio Madeira. São Paulo: Dialeto Latin American Documentary; 2013. p.142-79.).
Arratia (1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.) proposed the long fleshy projections extending posterior to opercular teeth and other three characters as synapomorphies supporting the monophyly of Scleronema: skin of upper lip without epidermal papillae, transverse skin fold between anterior nostril and base of maxillary barbel, and three abdominal vertebrae. However, we observed epidermal papillae in the lips for all species of Scleronema rejecting this putative synapomorphy proposed by Arratia (1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.) and posteriorly corroborated by de Pinna (1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.) and Wosiacki (2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.). On the other hand, our findings agree with the second charater (i.e., the skin fold on dorsal surface of the maxillary barbel) as a synapomorphy for the genus (Fig. 1), condition also suggested by de Pinna (1998de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.) and Wosiacki (2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.). Among trichomycterids, Sarcoglanis simplex also has a fleshy flap over the maxillary barbel; however, it is longer (extending to the anterior nare and the eye) and quite different in shape to the condition noted in Scleronema. Consequently, the characters are presently hypothesized as not homologous.
Wosiacki (2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.) included four species of Scleronema in a phylogenetic analysis of the subfamily Trichomycterinae. As a result, Scleronema was supported by the two characters discussed above (i.e., skin flap posterior to the opercle and fleshy flap at the base of the maxillary barbel), plus the maxilla elongated and expanded distally, the autopalatines near each other articulating with the vomer ventrally, and the presence of a dorsal canal in the articulation process between the autopalatine and the lateral ethmoid. As proposed by previous authors (cf.de Pinna, 1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.; Arratia, 1990Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.) and confirmed by our observations, the third feature (concerning on the maxilla) is shared by sarcoglanidines and some trichomycterines. The condition of the autopalatines near each other, articulating with the vomer ventrally, is herein corroborated (Fig. 2) and by DoNascimiento (2012DoNascimiento C. Sistemática y relaciones filogenéticas de la subfamília de bagres parasíticos Stegophilinae (Siluriformes: Trichomycteridae). [PhD Thesis]. Caracas: Universidad Central de Venezuela; 2012.).
Finally, Wosiacki (2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.) observed the presence of a canal or foramen in the dorsal surface of the autopalatine in Scleronema and some species of Trichomycterus from Southern and Southeastern Brazil (some of them currently included in the genus Cambeva). The author, however, noted different conditions in these genera: species of Scleronema have one or two canals oriented obliquely across the autopalatine whereas the species of Trichomycterus have various canals without any noticeable organization. We observed, exclusively in Scleronema, a unique canal forming an interrupted arch dorsally similar to the condition illustrated by Wosiacki (2002Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.: fig. 73) or a conspicuous arch not interrupted (Fig. 3) placed exactly above the aperture of the second nare.
Taxonomy and diversity. So far, the single taxonomic revision of the species of Scleronema after the description of the genus by Eigenmann (1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703., 1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.) was that of Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
), which was based on the analysis of type material housed at the BMNH and on Eigenmann’s papers. Tchernavin noticed that the diagnostic features described by Eigenmann (1917Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703., 1918Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.) to the genus and its type species, S. operculatum, were shared by two species formerly described as Trichomycterus minutusBoulenger, 1891Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35. and Pygidium angustirostrisDevincenzi, 1942Devincenzi GJ, Teague GW. Ictiofauna del Río Uruguay Medio. Anales del Museo de Historia Natural de Montevideo. 1942; 5(4)1-100.. Consequently, the author suggested a new combination for these taxa: S. minutum and S. angustirostris.
Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) also noted several features to distinguish S. minutum from S. operculatum, such as the posterior position of the pelvic fin, shorter pectoral-fin rays, larger head, narrower cleft of the mouth, soft part of the maxillary barbel longer, nasal barbel longer, and flap posterior to opercle shorter in the former. However, Tchernavin considered these differences were related to the size of the specimens available for each nominal species (“specimens of S. minutum are very small and probably young” [sic]) and that they belonged to a single species. Additionally, according to Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
), the third nominal species, S. angustirostris, also might been included in the synonymy of S. minutum due to notable similarities. Based on present data, however, all characters mentioned by Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) are valid to diagnose S. minutum and S. operculatum and actually these species reach distinct maximum sizes (55.5 mm SL and 72.8 mm SL, respectively). Posterior to Tchernavin’ view, the comments regarding the taxonomy of Scleronema were focused on the need of a taxonomic revision of the genus (de Pinna, 1989de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.; Casciotta, Almirón, 1996Casciotta JR, Almirón AE. Scleronema minutum (Boulenger) y Ochmacanthus batrachosloma (M. Ribeiro) (Siluriformes: Trichomycteridae), dos citas nuevas para la cuenca del Plata en Argentina. Neotrópica. 1996; 42(107-108):51-54.) and on the reference to undescribed species (de Pinna, Wosiacki, 2003de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.; Becker et al., 2013Becker FG, De Fries LCC, Ferrer J, Bertaco VA, Luz-Agostinho KDG, Silva JFPWW et al
. Fishes of the Taquari-Antas river basin (Patos Lagoon basin), southern Brazil. Braz J Biol. 2013; 73(1):79-90. https://doi.org/10.1590/S1519-69842013000100010
https://doi.org/10.1590/S1519-6984201300...
; Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
).
Scleronema angustirostre is recognized herein as a junior synonym of S. minutum in the absence of morphological differences between their type specimens (Figs. 18-20) and between populations of the lower rio Uruguay drainage versus the laguna dos Patos drainage and coastal rivers from Uruguay, corresponding to the type localities and historically assumed as belonging to S. angustirostre and S. minutum, respectively. The similarity of both species was first noted by Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) and posteriorly by Casciotta, Almirón (1996Casciotta JR, Almirón AE. Scleronema minutum (Boulenger) y Ochmacanthus batrachosloma (M. Ribeiro) (Siluriformes: Trichomycteridae), dos citas nuevas para la cuenca del Plata en Argentina. Neotrópica. 1996; 42(107-108):51-54.). Despite of this synonymy, the diversity of Scleronema increased considerably in the present review, with the recognition of S. minutum and S. operculatum as valid, and the description of six new species: S. guapa, S. ibirapuita, S. macanuda, S. mate, S. milonga, and S. teiniagua. Additionally, two other undescribed species recognized along this study (Fig. 4) are focus of further taxonomic investigation yet incomplete due to the few specimens available.
Interspecific relationships and comparisons. Within the genus, Ferrer (2016Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.) defined two species groups supported by striking morphological features: the S. operculatum species group, which includes S. macanuda and S. operculatum, and the S. minutum species group, which encompasses S. guapa, S. ibirapuita, S. mate, S. milonga, S. minutum, and S. teiniagua. Some diagnostic characters between these two species group are coincidental with those observed by Tchernavin (1944Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
https://doi.org/10.1590/S1679-6225200800...
) when he provided a detailed comparison between S. minutum and S. operculatum, regardless his suggestion that these characters correspond to intraspecific variation due to body size and that the two species would constitute synonyms.
The most conspicuous morphological differences exhibited by these groups are related to those characters that define the genus. For example, the skin flap in the posterior margin of the opercle is pointed and long in the S. operculatum species group and rounded and short in the S. minutum species group (Fig. 1). The fleshy flap at the base of the maxillary barbel is located anteriorly, thick, prolonged up to the snout and with distal margin straight in the S. operculatum species group while it is posteriorly inserted, thin, restricted to the maxillary barbel and with distal margin rounded in the S. minutum species group. Lastly, the articulation of the autopalatine with the vomer is inserted more ventrally with its margins nearer each other in the S. operculatum species in comparison with that condition observed in the species of the S. minutum species group.
Moreover, species of S. operculatum group have the body compressed, the maxillary barbel shorter than half-length of the head, the caudal fin with a vertical black bar distally, the tips of pectoral-fin rays extending beyond the interadial membrane, the coronoid process of the dentary approximately four times wider than the coronoid process of the anguloarticular, one branchiostegal ray articulated with the anterior ceratohyal, and the first haemal spine inserted between the 7th to 9th vertebra.
In contrast, species of the S. minutum group have the body roughly cylindrical, the maxillary barbel longer than half-length of the head, the caudal fin lacking a vertical black bar, the tips of pectoral-fin rays not extending beyond the interadial membrane, the coronoid process of the dentary approximately two times wider than the coronoid process of the anguloarticular, two or three branchiostegal ray attached to the anterior ceratohyal, and the first haemal spine inserted between the 11th to 13th vertebra.
The two species included in the S. operculatum group (Scleronema operculatum and S. macanuda) are diagnosed by the color pattern of the lateral surface of body, composed of 10-14 rounded blotches in the midlateral line as large as or smaller than opercle in the former versus 6-9 round blotches larger than opercle in the latter. The two species also exhibit differences in the length of barbels: tip of nasal barbel never reaching the anterior margin of eye and tip of maxillary barbel never surpassing the anterior margin of interopercle in S. operculatum while the tip of nasal barbel usually extends beyond the anterior margin of eye and the tip of maxillary barbel extends between the anterior and posterior margins of interopercle in S. macanuda.
Interspecific distinction within the S. minutum species group is less noticeable. Scleronema guapa and S. mate have unique color patterns (body with scattered spots and small rounded blotches, respectively) whereas the remaining species possess a similar color pattern, formed by larger rounded blotches.
Additionally, a remarkable reduction in the canals and pores of the laterosensory system observed in Scleronema is quite informative to diagnose its species (Tab. 3; Figs. 5, 10). As emphasized by Ferrer et al. (2015Ferrer J, Donin LM, Malabarba LR. A new species of Ituglanis Costa & Bockmann, 1993 (Siluriformes: Trichomycteridae) endemic to the Tramandaí-Mampituba ecoregion, southern Brazil. Zootaxa. 2015; 4020(2):375-89. http://dx.doi.org/10.11646/zootaxa.4020.2.8
http://dx.doi.org/10.11646/zootaxa.4020....
), characters related to the laterosensory system are useful to diagnose species when based on analyses of large samples. The nasal canal (pores s1 and s2) is usually present in S. macanuda and variably present in S. operculatum (Fig. 10), but always absent in the species of the S. minutum species group (Fig. 5), excepting for one specimen of S. guapa that has its right branch. The reduction in the canals and pores of the laterosensory system is still more conspicuous within the S. minutum species group. In S. milonga the pore s3 of the frontal canal is absent, in S. ibirapuita the entire frontal canal is absent (Fig. 5), and in S. teiniagua the frontal canal and the pore i10 of the sphenotic canal, are missing.
Distribution patterns. Species of the genus Scleronema are distributed in Southern Brazil, Southern Paraguay, Northeastern Argentina and Uruguay (Fig. 4), corresponding to the following freshwater ecoregions sensuAbell et al. (2008Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N et al
. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience. 2008; 58(5):403-14. https://doi.org/10.1641/B580507
https://doi.org/10.1641/B580507...
): Laguna dos Patos, Lower Paraná, Lower Uruguay, Paraguay, and Southeastern Mata Atlântica. Five species are found exclusively in the Lower Uruguay (S. guapa, S. ibirapuita, S. milonga, and S. teiniagua) and S. mate is restricted to the Laguna dos Patos ecoregion. Scleronema minutum is the most widespread species of the genus occurring in the Lower Paraná, Lower Uruguay and Laguna dos Patos ecoregions. Finally, Scleronema macanuda inhabits the Lower Uruguay and Laguna dos Patos ecoregions.
Interestingly, species that make part of each species group are always allopatric whereas it is noticeable several cases of syntopy of species of the S. operculatum group with species of the S. minutum group (Fig. 4). Scleronema operculatum is geographically restricted to the rio Ibicuí basin where it co-occurs with S. minutum in the headwaters and with S. guapa in the middle portion. Likewise, Scleronema macanuda co-occurs with S. minutum in the most localities of its distribution in the rio Negro basin and Laguna dos Patos system. In this latter drainage, S. mate is restricted to its north portion being found with S. macanuda only in the rio Pardo basin. It is likely possible that the two species group of Scleronema, with their different body shapes, represent adaptive morphotypes exploring different niches in sand-bottom environments as observed for S. macanuda and S. minutum in field observations, allowing their coexistence in syntopy. The results obtained by Carvalho (2017Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.) demonstrated a strong relationship between diet and morphology for S. macanuda, S. minutum and S. milonga, which also presented distinct diet compositions although the three species are insenctivores with predominance of immature stages of autochthonous benthic preys.
The allopatric distribution among the species of the S. minutum group is evident in the distinct tributaries of rio Uruguay. Scleronema milonga is restricted to the rio Ijuí basin (Brazil) and arroyo Itacaruaré (Argentina), representing the northernmost occurrence of the genus in the rio Uruguay basin. Scleronema guapa is restrict to the rio Ibicuí basin (Brazil), a downstream tributary to the left bank of the rio Uruguay. Scleronema ibirapuita also occurs in the rio Ibicuí basin, but is found uniquely in the rio Ibirapuitã basin, a small tributary. Scleronema teiniagua is endemic to the rio Quaraí (Brazil) or río Cuareím (Uruguay), the next downstream large tributary to the left bank of the rio Uruguay after the rio Ibicuí. Scleronema minutum is widespread in the following southern affluents of the rio Uruguay, with the exception of the río Arapey. The only record of the genus for this basin is tentatively identified as S. ibirapuita (see remarks on the species).
The distribution pattern of the species of the S. minutum species group in the rio Uruguay drainage (Fig. 4) shows a clear resemblance with that described by Turcati et al. (2018Turcati A, Serra-Alanis WS, Malabarba LR. A new mouth brooder species of Gymnogeophagus with hypertrophied lips (Cichliformes: Cichlidae). Neotrop Ichthyol . 2018; 16(4):e180118. https://doi.org/10.1590/1982-0224-20180118
https://doi.org/10.1590/1982-0224-201801...
: fig. 6) for Gymnogeophagus gymnogenys species group, in the same drainage, with separate species inhabiting the main tributaries (G. constellatus and S. milonga in the rio Ijuí, G. tiraparae, G. mekinos and S. guapa in the rio Ibicuí, G. pseudolabiatus and S. ibirapuita in the rio Quaraí). Turcati et al. (2018) suggested that the main channel of river act as a physical or ecological barrier to dispersal of these species of Gymnogeophagus among rio Uruguay tributaries and it may also be true for the species of Scleronema cited above. It must be considered, however, that the absence of records of these species in the tributaries of rio Uruguay of Argentina may be associated with the lack of sampling in the western tributaries of rio Uruguay, in contrast to the most densely collected areas in the eastern tributaries of rio Uruguay in the Rio Grande do Sul state, Brazil (Bertaco et al., 2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
). It is also remarkable that the rio Ijuí and rio Santa Rosa to the north and rio Quaraí to the South are embebed in a basaltic sediment and the rio ibicui in the middle in a sedimentary bed. Since both Gymnogeophagus and Scleronema habits are closely associated with the substrate for feeding, this is also a possible reason for such disjunct distributions.
Scleronema macanuda and S. minutum inhabit the lower portions of two isolated hydrographic systems, the rio Uruguay and Laguna dos Patos drainages (Fig. 4). Such fact was observed in some killifishes of the genus Austrolebias and Cnesterodon decemmaculatus, being inferred as a consequence of drainage rearrangements (González-Bergonzoni et al., 2009González-Bergonzoni I, Loureiro M, Oviedo S. A new species of Gymnogeophagus from the río Negro and río Tacuarí basins, Uruguay (Teleostei: Perciformes). Neotrop Ichthyol . 2009; 7(1):19-24. https://doi.org/10.1590/S1679-62252009000100003
https://doi.org/10.1590/S1679-6225200900...
; Loureiro et al., 2011Loureiro M, Duarte A, Zarucki M. A new species of Austrolebias Costa (Cyprinodontiformes: Rivulidae) from northeastern Uruguay, with comments on distribution patterns. Neotrop Ichthyol . 2011; 9(2):335-42. https://doi.org/10.1590/S1679-62252011000200010
https://doi.org/10.1590/S1679-6225201100...
; Ramos-Fregonezi et al., 2017Ramos-Fregonezi A, Malabarba LR, Fagundes NJ. Population genetic structure of Cnesterodon decemmaculatus (Poeciliidae): a freshwater look at the Pampa biome in Southern South America. Front Genet. 2017; 8:214. https://doi.org/10.3389/fgene.2017.00214
https://doi.org/10.3389/fgene.2017.00214...
).
Conservation. Species of Scleronema are strongly related to grasslands of the Pampa biome from Southern Brazil (IBGE, 2004Instituto Brasileiro de Geografia e Estatística (IBGE). Mapa de biomas do Brasil [Internet]. Rio de Janeiro; 2004. Available from: http://www.ibge.gov.br/home/presidencia/noticias/21052004biomashtml.shtm
http://www.ibge.gov.br/home/presidencia/...
, 2019Instituto Brasileiro de Geografia e Estatística (IBGE). Biomas do Brasil 1:250 000 [Internet]. Rio de Janeiro; 2019. Available from: https://www.ibge.gov.br/geociencias/informacoes-ambientais/15842-biomas.html?=&t=acesso-ao-produto
https://www.ibge.gov.br/geociencias/info...
) and the Uruguayan savanna ecoregion sensuOlson et al. (2001Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GV, Underwood E et al. Terrestrial ecoregions of the world: a new map of life on earth. A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience. 2001; 51(11):933-38. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) that includes the entire country of Uruguay. Six species of the genus are found exclusively in this region (S. guapa, S. ibirapuita, S. macanuda, S. minutum, S. operculatum, S. teiniagua), whereas Scleronema mate and S. milonga occupy its limits with the Atlantic Forest ecosystem. Loureiro et al. (2013Loureiro M, Zarucki M, González I, Vidal N, Fabiano G. Peces Continentales. In: Soutullo A, Clavijo C, Martínez-Lanfranco JA, editors. Especies prioritarias para la conservación en Uruguay. Vertebrados, moluscos continentales y plantas vasculares. Montevideo: SNAP/DINAMA/MVOTMA y DICYT/MEC; 2013. p.90-112) suggested that the species of Scleronema have priority for conservation in Uruguay in view of some threats, such as habitat destruction and modification, use of fertilizers, pesticides and herbicides.
Similar threats are observed in the grasslands of Southern Brazil, mainly by the currently and rapid expansion of culture soybean in the region. Conservation of the non-forest ecosystems in Brazil have been neglected, although they have biodiversity levels comparable to forests and cover large parts of the country (Overback et al., 2015Overbeck GE, Vélez-Martin E, Scarano FR, Lewinsohn TM, Fonseca CR, Meyer ST et al
. Conservation in Brazil needs to include non-forest ecosystems. Divers Distrib. 2015; 21(12):1455-60. https://doi.org/10.1111/ddi.12380
https://doi.org/10.1111/ddi.12380...
). According to Malabarba et al. (in prep.), approximately 63% of the 422 freshwater fishes from Rio Grande do Sul (Southern Brazil) recorded by Bertaco et al. (2016Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
http://doi.org/10.11646/zootaxa.4138.3.1...
) distribute in the Pampa biome, and around 60 are endemic to this region, which encompass only 2% of the Brazilian territory. The Pampa has the lowest percentage of legally protected areas among the Brazilian biomes (ICMBio, 2016) and S. ibirapuita is the only species of the genus with occurence in a Conservation Unit (“Área de Proteção Ambiental Ibirapuitã”).
Comparative material examined. Nematogenyidae. Nematogenys inermis: MZUSP 107490, 4, 53.5-49.2 mm SL. UMMZ 221574, 2, 57.2-57.8 mm SL. Trichomycteridae. Copionodontinae. Copionodon pecten: MCP 40982, 1 (c&s), 67.2 mm SL. USNM 316023, 2, paratypes of Copionodon pectende Pinna, 1992de Pinna MCC. A new subfamily of Trichomycteridae (Teleostei, Siluriformes), lower loricarioid relationships and a discussion on the impact of additional taxa for phylogenetic analysis. Zool J Linn Soc. 1992; 106(3):175-229. https://doi.org/10.1111/j.1096-3642.1992.tb01247.x
https://doi.org/10.1111/j.1096-3642.1992...
, 38.8-49.6 mm SL. Glanapteryginae. Glanaptyex angilla: USNM 292342, 5 (1 c&s), 31.6-50.6 mm SL. MZUSP 36530, 3, 36.6-60.1 mm SL. Listrura depinnai: UFRGS 19623, 6 (2 c&s), 23.6-31.3 mm SL. Pygidianops cuao: AMNH 240674, 10, 13.6-19.1 mm SL. Typhlobelus guacamaya: AMNH 240673, 1, 23.6 mm SL. Sarcoglanidinae. Malacoglanis gelatinosus: FMNH 98520, 1, 19.0 mm SL. Microcambeva ribeirae: MNRJ 32443, 8 (1 c&s), 29.2-38.7 mm SL. M. barbata: MNRJ, 37572, 3, 23.2-29.7 mm SL. Sarcoglanis simplex: ANSP 180021, 4 (1 c&s), 12.1-15.0 mm SL. Stenolicmus ix: MPEG 15101, holotype of Stenolicmus ix Wosiacki, Coutinho, de Assis Montag 2011, 19.0 mm SL; USNM 409756, 14 (2 c&s), 15.3-22.4 mm SL. S. sarmientoi: USNM 301664, holotype of Stenolicmus sarmientoi de Pinna, Starnes, 1990, 29.5 mm SL. Stegophilinae. Haemomaster venezuelae: UMMZ 216064, 3, 35.9-42.5 mm SL. Henonemus sp.: USNM 399756, 6 (1 c&s), 31.9-39.2 mm SL. H. passarellii: MNRJ 3783, holotype of Stegophilus passarellii (Miranda Ribeiro, 1944), 34.6 mm SL. Ochmacanthus batrachostoma: FMNH 58535, 1, 27.9 mm SL. Ochmacanthus sp.: ANSP 135920, 3 (3 c&s), 38.3-42.1 mm SL. Megalocentor echthrus: USNM 316024, 1, paratype of Megalocentor echthrus de Pinna, Britski, 1991, 48.7 mm SL. Pseudostegophilus maculatus: FMNH 58526, 1, 59.7 mm SL. P. haemomyzon: USNM 260200, 7, 31.8-43.8 mm SL. P. nemurus: USNM 305349, 4 (1 c&s), 26.9-86.1 mm SL. ANSP 116448, 1 (c&s), 54.3 mm SL. FMNH 58528, 5, 53.3-62.3 mm SL. Pseudostegophilus sp.: ANSP 180502, 4 (1 c&s), 33.9-39.0 mm SL. Trichogeninae. Trichogenes claviger: MZUSP 105372, 4, paratypes of Trichogenes claviger de Pinna, Helmer, Britski, Nunes, 2010, 39.7-40.1 mm SL. T. longipinnis: UMMZ 212354, 1, paratype of Trichogenes longipinnis Britski, Ortega, 1983, 70.8 mm SL; MCP 40982, 1 (c&s), not measured. Trichomycterinae. Bullockia
maldonadoi: USNM 84344, 2, paralectotypes of Hatcheria maldonadoi, designated by Eigenmann (1928: Pl. 8), 45.2-49.5 mm SL; USNM 399186, 8 (2 c&s), 31.1-55.9 mm SL. Cambeva balios: UFRGS 16229, holotype of Trichomycterus balios Ferrer, Malabarba, 2013, 82.0 mm SL; UFRGS 6831, 14 (2 c&s), paratypes of Trichomycterus balios, 27.3-87.5 mm SL. C. brachykechenos: MCN 18929, holotype of Trichomycterus brachykechenos Ferrer, Malabarba, 2013, 61.1 mm SL; UFRGS 16244, 1 (c&s), paratype of Trichomycterus brachykechenos, 51.6 mm SL. C. castroi: MZUSP 36964, holotype of Trichomycterus castroi de Pinna, 1992, 118.3 mm SL. C. concolor: MZUSP 43347, holotype of Trichomycterus concolorCosta, 1992Costa WJEM. Description de huit nouvelles espèces du genre Trichomycterus (Siluriformes: Trichomycteridae), du Brésil oriental. Rev Fr Aquariol . 1992; 18(4):101-10., 62.8 mm SL. C. crassicaudata: MZUSP 88518, holotype of Trichomycterus crassicaudatus Wosiacki, de Pinna, 2008, 109.2 mm SL. C. cubataonis: MNRJ 12490, holotype of Trichomycterus cubataonis Bizerril, 1994, 47.1 mm SL; UFRGS 24543, 1 (c&s), 58.1 mm SL. C. davisi: FMNH 60309, holotype of Pygidium davisiHaseman, 1911Haseman JD, Eigenmann CH. A brief report upon the expedition of the Carnegie Museum to Central South America, together with a list of localities at which Mr. Haseman collected. Ann Carnegie Mus. 1911; 7:287-314., 43.8 mm SL (xr); FMNH 52242, 8 (4 xr), paratypes of Pygidium davisi Haseman, 1911, 20.9-44.5 mm SL. C. diabola: MZUSP 78860, holotype of Trichomycterus diabolusBockmann, Casatti, de Pinna, 2004Bockmann FA, Sazima I. Trichomycterus maracaya, a new catfish from the upper rio Paraná, southeastern Brazil (Siluriformes: Trichomycteridae), with notes on the T. brasiliensis species-complex. Neotrop Ichthyol. 2004; 2(2):61-74. https://doi.org/10.1590/S1679-62252004000200003
https://doi.org/10.1590/S1679-6225200400...
, 53.7 mm SL. C. diatropoporos: MCP 46947, holotype of Trichomycterus diatropoporos Ferrer, Malabarba, 2013, 58.8 mm SL. MCP 40933, 2 (c&s), paratypes of Trichomycterus diatropoporus, 38.8-39.7 mm SL. C. guaraquessaba: MPEG 7917, 1 (c&s), paratype of Trichomycterus guaraquessaba Wosiacki, 2005, not measured. C. igobi: MZUSP 94843, 3, paratypes Trichomycterus igobi Wosiacki, de Pinna, 2008, 82.3-88.9 mm SL. C. iheringi: FMNH 58074, 2, 134.3-127.6 mm SL. C. mboycy: MZUSP 94956, 1, 68.6 mm SL. C. naipi: UFRGS 11405, 4, 55.6-65.6 mm SL.C. paolence: MZUSP 108930, 3, 51.8-83.9 mm SL. C. perkos: MCP 46701, 1, paratype of Trichomycterus perkos Datovo, Ferrer, Carvalho, 2012Carvalho FR, Malabarba LR, Lenz AJ, Fukakusa CK, Guimarães TFR, Sanabria JA, Moraes AC. Ictiofauna da Estação Experimental Agronômica da Universidade Federal do Rio Grande do Sul, sul do Brasil: composição e diversidade. Rev Bras Biocien. 2012; 10(1):26-47., 48.9 mm SL. C. plumbea: UFRGS 13947, 1, 62.5 mm SL. C. poikilos: UFRGS 16239, holotype of Trichomycterus poikilos Ferrer, Malabarba, 2013, 63.3 mm SL; UFRGS 16240, 10 (3 c&s), paratypes of Trichomycterus poikilos, 33.1-66.8 mm SL. Cambeva sp.: UFRGS 10651, 1 (c&s) 66.5 mm SL. C. stawiarski: MNRJ 9739, holotype of Pygidium stawiarski Miranda Ribeiro, 1968, 67.1 mm SL; UFRGS 18307, 10 (2 c&s), 31.7-49.4 mm SL.C. tropeiro: MCP 46171, holotype of Trichomycterus tropeiro Ferrer, Malabarba, 2011, 82.3 mm SL; UFRGS 8818, 2 (1 c&s), paratypes of Trichomycterus tropeiro, 46.8-85.1 mm SL. C. tupinamba: MZUSP 61686, 2, 50.3-54.8 mm SL. C. variegata: LIRP 647, 3, 43.1-49.8 mm SL; MZUSP 42316, holotype of Trichomycterus variegatus Costa, 1992, 39.8 mm SL. C. zonata: FMNH 58573, holotype of Pygidium zonatum Eigenmann, 1918 (xr), 53.6 mm SL; FMNH 58574, paratypes of Pygidium zonatum, Eigenmann, 1918, 2 (2 xr), 42.2-48.0 mm SL; UFRGS 24538, 1 (c&s), 48.5 mm SL. Eremophilus mutisii: USNM 79201, 3 (3 xr), 105.5-165.0 mm SL. Hatcheria
macraei: FMNH 58529, 7 (1 c&s), 20.8-96.8 mm SL; UFRGS 17696, 2 (1 c&s), 40.0-50.0 mm SL. Ituglanis apteryx: MZUSP 115048, holotype of Ituglanis apteryx Datovo, 2014. I. australis: UFRGS 13600, 1, paratype of Ituglanis australis Datovo, de Pinna, 2014, 75,2 mm SL. I. boitata: UFRGS 17617, 5 (1 c&s), paratypes of Ituglanis boitata Ferrer, Donin, Malabarba, 2015, 43.0-66.2 mm SL. I. eichhorniarum: AMNH 233244, 1, 64.9 mm SL. I. inusitatus: UFRGS 20201, 8 (1 c&s), paratypes of Ituglanis inusitatus Ferrer, Donin, 2017, 43.3-70.2 mm SL. I. proops: MZUSP 60255, 10 (2 c&s), 57.5-60.4 mm SL. Scleronema sp. from Paraguay: ANSP 176176, 1 (xr), 12.2 mm SL; UMMZ 206905, 4 (4 xr), 19.9-25.6 mm SL. Scleronema sp. from Santa Catarina: MCP 11007, 1, 43.8 mm SL; UFRGS 24562, 2, 31.5-40.0 mm SL; UFRGS 26891, 1, 45.1 mm SL; UFRGS 22873, 4, 25.7-28.3 mm SL. Silvinichthys bortayro: AMNH 233621, 1, paratype of Silvinichthys bortayro Fernández, de Pinna, 2005, 19.9 mm SL. S. mendozensis: USNM 84558, 7, 30.0-65.8 mm SL; USNM 84558, 7 (1 xr), 30.0-65.8 mm SL. Trichomycterus albinotatus: MZUSP 42312, holotype of Trichomycterus albinotatus Costa, 1992, 45.3 mm SL. T. alternatus: FMNH 58083; paratypes of Pygidium alternatum Eigenmann, 1917, 10 (4 xr), 47.5-66.2 mm SL. T. alterus: AMNH 12241, holotype of Pygidium alterum Marini, Nichols, La Monte, 1933, 27.6 mm SL. FML 2085, 1, 49.4 mm SL. T. areolatus: UFRGS 10792, 4, 33.1-54.4 mm SL. USNM 399180, 10 (2 c&s), 41.4-88.4 mm SL. T. argos: MZUSP 106274, 3, paratypes of Trichomycterus argos Lezama, Triques, Santos, 2012, 57.2-92.1 mm SL. T. auroguttatus: MZUSP 43341, holotype of Trichomycterus auroguttatus Costa, 1992, 49.7 mm SL. T. bahianus: MZUSP 43340, holotype of Trichomycterus bahianus Costa, 1992, 68.0 mm SL. T. barbouri: FML 4742, 63.6-75.6 mm SL. T. belensis: FML 2530, holotype of Trichomycterus belensis Fernández, Vari 2002, 63.7 mm SL. T. borellii: MZUSP 2208, 1, 57.8 mm SL. T. brasiliensis: FMNH 58078, 11, 42.6-102.4 mm SL. T. candidus: MNRJ 5209, holotype of Eremophilus candidus, 58.5 mm SL. T. catamarcensis: FML 2507, holotype of Trichomycterus catamarcensis Fernández, Vari, 2000, 36.5 mm SL. T. caudofasciatus: MCP 35030, 2, paratypes of Trichomycterus caudofasciatus Alencar, Costa, 2004, 37.1-38.0 mm SL. T. corduvensis: FML 2463, 2, 57.7-79.5 mm SL. T. dali: MZUSP 106635, 1, paratype of Trichomycterus dali Rizzato, Costa, Trajano, Bichuette, 2011, 47.3 mm SL. T. emanueli: UMMZ 141936, 3, paratypes of Pygidium emanueli emanueli, 101.6-117.5 mm SL. T. giganteus: MCP 35028, 3, paratypes of Trichomycterus giganteus Lima, Costa, 2004, 106.4-130.1 mm SL. T. gorgona: ANSP 149946, holotype of Trichomycterus gorgona Fernández, Schaefer, 2005, 64.8 mm SL. T. guianense: MZUSP 109099, 4, 62.7-85.0 mm SL. T. hualco: FML 2601, holotype of Trichomycterus hualco Fernández, Vari, 2009, 68.3 mm SL. T. immaculatus: FMNH 58079, 2, 70.7-81.5 mm SL; LIRP 285, 2, 82.2-102.3 mm SL. T. itacambirussu: MZUSP 58493, holotype of Trichomycterus itacambirussu Triques, Vono, 2004, 70.9 mm SL. T. itacarambiensis: MZUSP 42649, holotype of Trichomycterus itacarambiensis Trajano, de Pinna, 1996, 47.2 mm SL. T. itatiayae: MNRJ 792, lectotype of Trichomycterus brasiliensis itatiayae Miranda Ribeiro, 1906, designated by Caramaschi, Caramaschi (1991: 223), 67.9 mm SL. T. jacupiranga: UFRGS 24555, 2, 35.1-44.8 mm SL. T. jequitinhonhae: MZUSP 58497, holotype of Trichomycterus jequitinhonhae Triques, Vono, 2004, 70.5 mm SL. T. landinga: MZUSP 58496, holotype of Trichomycterus landinga Triques, Vono, 2004, 43.3 mm SL. T. longibarbatus: MZUSP 43339, holotype of Trichomycterus longibarbatus Costa, 1992, 57.9 mm SL. T. maracaiboensis: UMMZ 142484, 1, paratype of Pygidium banneaui maracaiboensis Schultz, 1944, 37.0 mm SL. T. maracaya: MCP 34575, 2, paratypes of Trichomycterus maracaya Bockmann, Sazima, 2004, 30.7-31.7 mm SL. T. mimonha: MZUSP 43343, holotype of Trichomycterus mimonha Costa, 1992, 56.2 mm SL. T. mirissumba: MZUSP 43345, holotype of Trichomycterus mirissumba Costa, 1992, 57.7 mm SL. T. pantherinus: MCP 35029, 2, paratypes of Trichomycterus pantherinus Alencar, Costa, 2004, 39.6-41.9 mm SL. T. paquequerensis: MZUSP 53755, 4, 25.2-61.5 mm SL. T. payaya: MNRJ 36665, holotype of Trichomycterus payaya Sarmento-Soares, Zanata, Martins-Pinheiro, 2011, 38.5 mm SL. T. potschi: MCP 29061, holotype of Trichomycterus potschi Barbosa, Costa 2003, 78.6 mm SL. T. pradensis: MNRJ 28483, holotype of Trichomycterus pradensis Sarmento-Soares, Martins-Pinheiro, Aranda, Chamon 2005, 64.2 mm SL. T. pseudosilvinichthys: FML 2588, holotype of Trichomycterus pseudosilvinichthys Fernández, Vari 2004, 60.6 mm SL. T. ramosus: FML 2070, holotype of Trichomycterus ramosus Fernández, 2000, 59.0 mm SL. T. reinhardti: FMNH 58081, 1, 53.7 mm SL; MZUSP 94511, 4, 47.7-70.14 mm SL. T. riojanus: MACN 5175, holotype of Pygidium riojanum Berg, 1897, 60.6 mm SL. T. rivulatus: ANSP 22004, holotype of Trichomycterus pardus Cope, 1874, 59.5 mm SL. T. roigi: FML 1503, 2, 51.1-61.4 mm SL. T. romeroi: ANSP 69331, holotype of Pygidium romeroi Fowler, 1941, 54.9 mm SL. T. santaeritae: FMNH 58577, holotype of Pygidium santaeritae Eigenmann, 1918, 19.6 mm SL. T. sketi: ANSP 189652, 1, paratype of Trichomycterus sketi Castellanos-Morales 2011, 60.8 mm SL. T. spegazzinii: FML 4747, 3, 35.8-89.1 mm SL; MACN 4925, 5, syntypes Pygidium spegazzinii Berg, 1897, 33.7-72.8 mm SL. T. striatus: USNM 305351, 3 (c&s), 28.5-69.9 mm SL; USNM 376566, 13, 43.7-78.2 mm SL. T. taczanowskii: MZUSP 26031, 1, 100.6 mm SL. T. taeniops: ANSP 71638, holotype of Pygidium tenue Fowler, 1954. T. tiraquae: ANSP 69126, holotype of Pygidium tiraquae Fowler, 1940, 33.2 mm SL. T. uisae: ANSP 187498, 1, paratype of Trichomycterus uisae Castellanos-Morales 2008, 44.2 mm SL. T. vermiculatus: MZUSP 87189, 4, 36.1-99.1 mm SL. T. weyrauchi: ANSP 71639, holotype of Pygidium weyrauchi Fowler, 1945, 41.2 mm SL. T. yuska: FML 2535, holotype, 88.6 mm SL. Tridentinae. Potamoglanis hasemani: ANSP 175851, 10 (2 c&s), 13.0-13.5 mm SL; FMNH 58579, 1, 13.6 mm SL. P. johnsoni: ANSP 53873, holotype of Pygidium johnsoni Fowler, 1932, 12.3 mm SL. Tridentopsis cahuali: AMNH 223161, 10, 20.5-24.7 mm SL; MACN 9956, 2, 16.0- 17.1 mm SL. Tridentopsis pearsoni: ANSP 170400, 5 (1 c&s), 20.1-21.7 mm SL. T. tocantinsi: AMNH 20926, 2, paratypes of Tridentopsis tocantinsi La Monte, 1939, 22.3-24.8 mm SL. Tridens melanops: USNM 120296, 1, syntype of Tridens melanops Eigenmann, Eigenmann, 1889, 16.2 mm SL. Tridensimilis venezuelae: UMMZ 142492, 1, paratype of Tridensimilis venezuelae Schultz, 1944, 19.2 mm SL. USNM 121291, 2 paratypes of Tridensimilis venezuelae Schultz, 1944, 15.5-17.8 mm SL. Vandelliinae. Paracanthopoma sp.: UMMZ 231729, 1, 57.4 mm SL. Paravandellia phaneronema: ANSP 188915, 5 (1 c&s), 24.4-26.9 mm. Plectrochilus machadoi: UFRGS 22669, 1, 17.8 mm SL. Vandellia beccarii: USNM 404772, 7, 38.5-53.2 mm SL. Vandellia sp.: ANSP 131344, 2 (2 c&s), 61.0-70.3 mm SL.
ACKNOWLEGMENTS
We thank Richard Vari, in memoriam, Carlos David de Santana, Kris Murphy, Jeffrey Clayton, and Sandra Raredon (USNM), Aléssio Datovo (MZUSP), André Esguícero (LIRP), Carlos Lucena and Juliano Romanzini (MCP), Caleb McMahan, Kevin Swagel and Susan Mochel (FMNH), Doug Nelson and Andrea Thomaz (UMMZ), Emanuel Neuhaus, Fábio Pupo, Marcelo Britto and Paulo Buckup (MNRJ), James Maclaine (BMNH), John Lundberg, Mark Sabaj Pérez and Tiago Carvalho (ANSP), Juliana Wingert (UFRGS), Leo Smith and Gloria Arratia (KU), Marcelo Loureiro (ZVC-P), Marco Azevedo and Vinicius Bertaco (MCN), Pablo Lehmann and Thais Mumbach (UNICTIO), Radford Arrindell (AMNH), Ricardo Ferriz (MACN), Sebastián Serra (MHNM) for all support in the fish collections. Mário de Pinna (MZUSP), Tiago Carvalho (MPUJ) and Wolmar Wosiacki (MPEG) for valuable contributions on the PhD Thesis of JF. Flávio Bockmann (LIRP) for a detailed and critical review of the manuscript. We thank to Marcelo Camana (UFRGS) for the Fig. 4 (distribution map) and Sebastián Serra for the Fig. 27 (specimen in life). Sandra Raredon (USNM) gently made photographs of specimens used in this study (Figs. 18, 19, 23, 24). JF was supported in Brazil (Proc.142010/2012-0 and 152354/2016-6) and abroad by CNPq, being funded by the extinct exchange program “Ciências sem Fronteiras” (CNPq; Proc. 201215/2014-4) established in 2011 by the Brazilian federal government. JF also thanks for the “Böhlke Endowment” provided by ANSP. LRM is supported by CNPq (Proc. 307890/2016-3 and 401204/2016-2).
REFERENCES
- Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N et al . Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience. 2008; 58(5):403-14. https://doi.org/10.1641/B580507
» https://doi.org/10.1641/B580507 - Adriaens D, Baskin JN, Coppens H. Evolutionary morphology of trichomycterid catfishes: about hanging on and digging in. In: Nelson JS, Schultze HP, Wilson MVH, editors. Origin and Phylogenetic Interrelationships of Teleosts. München: Verlag Dr. Friedrich Pfeil; 2010. p.337-62.
- Arias JD, Demonte LD, Miquelarena AM, Protogino LC, López H. Lista de peces de la provincia de Entre Ríos. ProBiota, FCNyM, UNLP, Serie Técnica y Didáctica. 2013; 22:1-19.
- Arratia G. The South American Trichomycterinae (Teleostei: Siluriformes), a problematic group. In: Petersand G, Hutterer R, editors. International Symposium on Vertebrate Biogeography and Systematics in the Tropics. Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum; 1990. p.395-403.
- Arratia G. Silvinichthys, a new genus of trichomycterid catfishes from the Argentinian Andes, with redescription of Trichomycterus nigricans Ichthyol Explor Freshw. 1998; 9(4):347-70.
- Arratia G, Huaquín L. Morphology of the lateral line system and of the skin of diplomystids and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonn Zool Monogr. 1995; 36:1-110.
- Baskin JN. Structure and relationships of the Trichomycteridae. [PhD Thesis]. New York: City University of New York; 1973.
- Becker FG, De Fries LCC, Ferrer J, Bertaco VA, Luz-Agostinho KDG, Silva JFPWW et al . Fishes of the Taquari-Antas river basin (Patos Lagoon basin), southern Brazil. Braz J Biol. 2013; 73(1):79-90. https://doi.org/10.1590/S1519-69842013000100010
» https://doi.org/10.1590/S1519-69842013000100010 - Bertaco VA, Azevedo MA. Fishes from Rio Ibirapuita basin, environmental protection area of Ibirapuita, Pampa biome. Check List. 2013; 9(5):966-72. http://dx.doi.org/10.15560/9.5.966
- Bertaco VA, Ferrer J, Carvalho FR, Malabarba LR. Inventory of the freshwater fishes from a densely collected area in South America - a case study of the current knowledge of Neotropical fish diversity. Zootaxa. 2016; 4138(3):401-40. http://doi.org/10.11646/zootaxa.4138.3.1
» http://doi.org/10.11646/zootaxa.4138.3.1 - Bockmann FA, Casatti L, de Pinna MCC. A new species of trichomycterid catfish from the rio Paranapanema basin, southeastern Brazil (Teleostei: Siluriformes), with comments on the phylogeny of the family. Ichthyol Explor Freshw . 2004; 15(3):225-42.
- Bockmann FA, Sazima I. Trichomycterus maracaya, a new catfish from the upper rio Paraná, southeastern Brazil (Siluriformes: Trichomycteridae), with notes on the T. brasiliensis species-complex. Neotrop Ichthyol. 2004; 2(2):61-74. https://doi.org/10.1590/S1679-62252004000200003
» https://doi.org/10.1590/S1679-62252004000200003 - Boulenger GA. An account of the siluroid fishes obtained by Dr. H. von Ihering and Herr Sebastian Wolff in the Province Rio Grande do Sul, Brazil. Proc Zool Soc London. 1891; (pt 2):231-35.
- Burgess WE. An atlas of freshwater and marine catfishes: a preliminary survey of the Siluriformes. Neptune City: T.F.H. Publications; 1989.
- Burns MDM, Corrêa F, Cheffe MM, Foster J, Lopes JB, Santos JDM. The fish fauna of Turuçu river, Patos-Mirim lagoon system, Rio Grande do Sul state, southern Brazil. Panam J Aquat Sci. 2015; 10(4):315-22.
- Carvalho FR, Malabarba LR, Lenz AJ, Fukakusa CK, Guimarães TFR, Sanabria JA, Moraes AC. Ictiofauna da Estação Experimental Agronômica da Universidade Federal do Rio Grande do Sul, sul do Brasil: composição e diversidade. Rev Bras Biocien. 2012; 10(1):26-47.
- Carvalho N. Dieta e ecomorfologia de três espécies de peixes do gênero Scleronema (Siluriformes: Trichomycteridae). [MSc Dissertation]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2017.
- Casciotta JR, Almirón AE. Scleronema minutum (Boulenger) y Ochmacanthus batrachosloma (M. Ribeiro) (Siluriformes: Trichomycteridae), dos citas nuevas para la cuenca del Plata en Argentina. Neotrópica. 1996; 42(107-108):51-54.
- Castello HP, Ehrlich MD, Wais IR, Puig A. Adiciones a la fauna de los peces de los rios Paraná medio y Bermejo. Rev Mus Argent Cienc Nat “Bernardino Rivadavia”. 1978; 12(9):119-35.
- Cavalheiro LW, Fialho CB. Fishes community composition and patterns of species distribution in Neotropical streams. Biota Neotrop. 2020; 20(1):1-13. https://doi.org/10.1590/1676-0611-bn-2019-0828
» https://doi.org/10.1590/1676-0611-bn-2019-0828 - Corrêa F, Oliveira EFD, Tuchtenhagen T, Pouey J, Piedras S. Ichthyofauna of the hydrographic basin of the Chasqueiro Stream (Mirim Lagoon system, southern Brazil): generating subsidies for conservation and management. Biota Neotrop . 2015; 15(4):e0006. https://doi.org/10.1590/1676-0611-BN-2015-0006
» https://doi.org/10.1590/1676-0611-BN-2015-0006 - Costa WJEM, Bockmann FA. Un nouveau genre néotropical de la famille des Trichomycteridae (Siluriformes: Loricarioidei). Rev Fr Aquariol. 1993; 20(2): 43-46.
- Costa WJEM. Description de huit nouvelles espèces du genre Trichomycterus (Siluriformes: Trichomycteridae), du Brésil oriental. Rev Fr Aquariol . 1992; 18(4):101-10.
- Datovo A, Bockmann FA. Dorsolateral head muscles of the catfish families Nematogenyidae and Trichomycteridae (Siluriformes: Loricarioidei): comparative anatomy and phylogenetic analysis. Neotrop Ichthyol . 2010; 8(2):193-246. https://doi.org/10.1590/S1679-62252010000200001
- Devincenzi GJ, Teague GW. Ictiofauna del Río Uruguay Medio. Anales del Museo de Historia Natural de Montevideo. 1942; 5(4)1-100.
- DoNascimiento C. Sistemática y relaciones filogenéticas de la subfamília de bagres parasíticos Stegophilinae (Siluriformes: Trichomycteridae). [PhD Thesis]. Caracas: Universidad Central de Venezuela; 2012.
- DoNascimiento C. Morphological evidence for the monophyly of the subfamily of parasitic catfishes Stegophilinae (Siluriformes, Trichomycteridae) and phylogenetic diagnoses of its genera. Copeia. 2015; 103(4):933-60. https://doi.org/10.1643/CI-14-132
» https://doi.org/10.1643/CI-14-132 - Eigenmann CH. Catalogue of the fresh-water fishes of tropical and south temperate America. In: Scott WB, editor. Reports of the Princeton University expeditions to Patagonia, 1896-1899. Stuttgart: Princeton University; 1910. p.375-511.
- Eigenmann CH. Descriptions of sixteen new species of Pygidiidae. Proc Am Philos Soc. 1917; 56:690-703.
- Eigenmann CH. The Pygidiidae, a family of South American catfishes. Mem Carnegie Museum. 1918; 7(5):259-398.
- Eschmeyer WN. Catalog of fishes. California Academy of Sciences, San Francisco; 1998.
- Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007; 1418:1-628. http://dx.doi.org/10.11646/zootaxa.1418.1.1
» http://dx.doi.org/10.11646/zootaxa.1418.1.1 - Ferrer J. Filogenia e revisão taxonômica do gênero Scleronema (Siluriformes: Trichomycteridae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2016.
- Ferrer J, Malabarba LR. A new Trichomycterus lacking pelvic fins and pelvic girdle with a very restricted range in southern Brazil (Siluriformes: Trichomycteridae). Zootaxa, 2011: 2912:59-67. http://dx.doi.org/10.11646/zootaxa.2912.1.5
» http://dx.doi.org/10.11646/zootaxa.2912.1.5 - Ferrer J, Donin LM, Malabarba LR. A new species of Ituglanis Costa & Bockmann, 1993 (Siluriformes: Trichomycteridae) endemic to the Tramandaí-Mampituba ecoregion, southern Brazil. Zootaxa. 2015; 4020(2):375-89. http://dx.doi.org/10.11646/zootaxa.4020.2.8
» http://dx.doi.org/10.11646/zootaxa.4020.2.8 - Fowler HW. Os peixes de água doce do Brasil. São Paulo: Arq Zool Estado São Paulo. 1954; (vol 2).
- Fricke R, Eschmeyer WN, Fong JD. Species by family/subfamily [Internet]. San Francisco: California Academy of Science; 2020 [updated 2020 Mar 02; cited 2020 Mar 09]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp - González-Bergonzoni I, Loureiro M, Oviedo S. A new species of Gymnogeophagus from the río Negro and río Tacuarí basins, Uruguay (Teleostei: Perciformes). Neotrop Ichthyol . 2009; 7(1):19-24. https://doi.org/10.1590/S1679-62252009000100003
» https://doi.org/10.1590/S1679-62252009000100003 - Gosline WA. Catálogo dos nematognatos de água-doce da América do sul e central. Bol Mus Nac Rio de Janeiro Zool. 1945; 33:1-138.
- Haseman JD, Eigenmann CH. A brief report upon the expedition of the Carnegie Museum to Central South America, together with a list of localities at which Mr. Haseman collected. Ann Carnegie Mus. 1911; 7:287-314.
- Henn AW. List of types of fishes in the collection of the Carnegie Museum on September 1, 1928. Ann Carnegie Mus . 1928; 19(4):51-99.
- Ibarra M, Stewart DJ. Catalogue of type specimens of Recent fishes in Field Museum of Natural History. Fieldiana Zool (New Series). 1987; 35:1-112.
- Ihering H von. Die Süßwasser-Fische von Rio Grande do Sul. In: Koseritz’ Deutscher Volkskalender für Brasilien. Rio Grande do Sul; 1893. p.89-119.
- Ihering H von. Os peixes de água doce do Rio Grande do Sul. In: Annuario do estado do Rio Grande do Sul. Porto Alegre; 1898. p.161-90.
- Instituto Brasileiro de Geografia e Estatística (IBGE). Mapa de biomas do Brasil [Internet]. Rio de Janeiro; 2004. Available from: http://www.ibge.gov.br/home/presidencia/noticias/21052004biomashtml.shtm
» http://www.ibge.gov.br/home/presidencia/noticias/21052004biomashtml.shtm - Instituto Brasileiro de Geografia e Estatística (IBGE). Biomas do Brasil 1:250 000 [Internet]. Rio de Janeiro; 2019. Available from: https://www.ibge.gov.br/geociencias/informacoes-ambientais/15842-biomas.html?=&t=acesso-ao-produto
» https://www.ibge.gov.br/geociencias/informacoes-ambientais/15842-biomas.html?=&t=acesso-ao-produto - Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). SISBIO-Estatísticas [Internet]. Brasília; 2016. Available from: http://www.icmbio.gov.br/sisbio/estatisticas.html
» http://www.icmbio.gov.br/sisbio/estatisticas.html - International Commission on Zoological Nomenclature (ICZN). International code of zoological nomenclature. 4th ed. London: International trust for zoological nomenclature Natural History Museum [Internet]. London; 1999. Available from: https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/
» https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/ - International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Gland; 2019. DOI: 10.1002/joc.3480. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
» https://doi.org/10.1002/joc.3480» http://www.iucnredlist.org/documents/RedListGuidelines.pdf - Katz AM, Barbosa MA, Mattos JLO, Costa WJEM. Multigene analysis of the catfish genus Trichomycterus and description of a new South American trichomycterine genus (Siluriformes, Trichomycteridae). Zoosyst Evol. 2018; 94(2):557-66. https://doi.10.3897/zse.94.29872
» https://doi.10.3897/zse.94.29872 - Liotta J. Distribución geográfica de los peces de aguas continentales de la República Argentina. Buenos Aires: ProBiota, FCNyM, UNLP; 2005. (Serie Documentos; 3)
- Litz TO, Koerber S. Check list of the freshwater fishes of Uruguay (CLOFF-UY). ICP. 2014; 28:1-40.
- Lopes-Neto JS. Lendas do Sul. Pelotas: Echenique & C.; 1913.
- López HL, Miquelarena AM, Menni RC. Lista comentada de los peces continentales de la Argentina. Buenos Aires: ProBiota, FCNyM, UNLP ; 2003. (Serie Técnica y Didáctica; 5)
- López HL, Miquelarena AM, Ponte Gómez J. Biodiversidad y distribución de la ictiofauna mesopotámica. Miscelánea. 2005; 14:311-54.
- Loureiro M, Duarte A, Zarucki M. A new species of Austrolebias Costa (Cyprinodontiformes: Rivulidae) from northeastern Uruguay, with comments on distribution patterns. Neotrop Ichthyol . 2011; 9(2):335-42. https://doi.org/10.1590/S1679-62252011000200010
» https://doi.org/10.1590/S1679-62252011000200010 - Loureiro M, Zarucki M, González I, Vidal N, Fabiano G. Peces Continentales. In: Soutullo A, Clavijo C, Martínez-Lanfranco JA, editors. Especies prioritarias para la conservación en Uruguay. Vertebrados, moluscos continentales y plantas vasculares. Montevideo: SNAP/DINAMA/MVOTMA y DICYT/MEC; 2013. p.90-112
- Loureiro M, Serra WS, Scarabino F. Colecciones ictiológicas del Uruguay: pasado y presente. In: Del Moral-Flores LFD, Villalobos AJR, Pérez JAM, Acosta AFG, López JF, editors. Colecciones ictiológicas de Latinoamérica. Ciudad de México: Facultad de Estudios Superiores Iztacala, UNAM Sociedad Ictiológica Mexicana; 2016. p.400-14.
- Lundberg JG, Baskin JN. The caudal skeleton of the catfishes, order Siluriformes. Am Mus Novit. 1969; 2398:1-49.
- Malabarba LR. Histórico sistemático e lista comentada das espécies de peixes de água doce do sistema da Laguna dos Patos, Rio Grande do Sul, Brasil. Comun Mus Ciênc Tecnol PUCRS. 1989; 2(8):107-79. (Série Zoologia)
- Menni RC. Peces y ambientes en la Argentina continental. Monogr Mus Argentino Cienc Nat. 2004; 5:1-316.
- Morris PJ, Yager HM, Sabaj Pérez MH. ACSImagebase: A digital archive of catfish images compiled by participants in the All Catfish Species Inventory [Internet]. 2006 Available from: http://acsi.acnatsci.org/base
» http://acsi.acnatsci.org/base - Myers GS. Two extraordinary new blind nematognath fishes from the Rio Negro, representing a new subfamily of Pygidiidae, with a rearrangement of the genera of the family, and illustrations of some previously described genera and species from Venezuela and Brazil. Proc Calif Acad Sci. 1944; 23(40):591-602.
- Myers GS, Weitzman SH. Two remarkable new trichomycterid catfishes from the Amazon basin in Brazil and Colombia. J Zool Lond. 1966; 149(3):277-87.
- Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres communes. Montevideo: Dinara; 2002.
- Nion H, Ríos C, Meneses P. Peces del Uruguay. Lista systemática y nombres comunes; segunda edición corregida y ampliada. Montevideo: Dinara ; 2016.
- Ochoa LE, Roxo FF, DoNascimiento C, Sabaj MH, Datovo A, Alfaro M, Oliveira C. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae. Mol phylogenet evol. 2017a; 115:71-81. http://dx.doi.org/10.1016/j.ympev.2017.07.007
» http://dx.doi.org/10.1016/j.ympev.2017.07.007 - Ochoa LE, Silva GSC, Costa e Silva GJ, Oliveira C, Datovo A. New species of Trichomycterus (Siluriformes: Trichomycteridae) lacking pelvic fins from Paranapanema basin, southeastern Brazil. Zootaxa. 2017b; 4319(3):550-60. http://dx.doi.org/10.11646/zootaxa.4319.3.7
» http://dx.doi.org/10.11646/zootaxa.4319.3.7 - Ochoa LE, Datovo A, DoNascimiento C, Roxo FF, Sabaj MH, Chang J et al . Phylogenomic analysis of trichomycterid catfishes (Teleostei: Siluriformes) inferred from ultraconserved elements. Sci Rep. 2020; 10:2697. http://doi.org/ 10.1038/s41598-020-59519-w
» http://doi.org/ 10.1038/s41598-020-59519-w - Olazarri J, Mones A, Ximénez A, Philippi ME. Lista de los ejemplares-tipo depositados en el Museo Nacional de Historia Natural de Montevideo, Uruguay. Comun Zool Mus Hist Nat Montev. 1970; 10(131):1-12.
- Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GV, Underwood E et al Terrestrial ecoregions of the world: a new map of life on earth. A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience. 2001; 51(11):933-38. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
- Overbeck GE, Vélez-Martin E, Scarano FR, Lewinsohn TM, Fonseca CR, Meyer ST et al . Conservation in Brazil needs to include non-forest ecosystems. Divers Distrib. 2015; 21(12):1455-60. https://doi.org/10.1111/ddi.12380
» https://doi.org/10.1111/ddi.12380 - Pallarés OR, Berretta EJ, Maraschin GE. The south american campos ecosystem. In: Suttie J, Reynolds SG, Batello C, editors. Grasslands of the world. Rome: Food and agriculture organization of the United Nations; 2005. p.171-219.
- Paullier S, Bessonart J, Brum E, Loureiro M. Lista de especies de peces de la cuenca del río Queguay, río Uruguay Bajo. Bol Soc Zool Uruguay. 2019; 28(2):66-78.
- de Pinna MCC. A new sarcoglanidine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am Mus Novit . 1989; 2950:1-25.
- de Pinna MCC. A new subfamily of Trichomycteridae (Teleostei, Siluriformes), lower loricarioid relationships and a discussion on the impact of additional taxa for phylogenetic analysis. Zool J Linn Soc. 1992; 106(3):175-229. https://doi.org/10.1111/j.1096-3642.1992.tb01247.x
» https://doi.org/10.1111/j.1096-3642.1992.tb01247.x - de Pinna MCC. Phylogenetic relationships of Neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.279-330.
- de Pinna MCC. Trichomycteridae. In: Queiroz LJ, Torrente-Vilara G, Ohara WM, Pires THS, Zuanon J, Doria CRC, editors. Peixes do Rio Madeira. São Paulo: Dialeto Latin American Documentary; 2013. p.142-79.
- de Pinna MCC, Wosiacki WB. Family Trichomycteridae (Pencil or parasitic catfishes). In: Reis RE, Kullander SO, Ferraris CJ Jr, organizers. Check List of the Freshwater Fishes of South America. Porto Alegre: Edipucrs ; 2003. p.270-90.
- Ramos-Fregonezi A, Malabarba LR, Fagundes NJ. Population genetic structure of Cnesterodon decemmaculatus (Poeciliidae): a freshwater look at the Pampa biome in Southern South America. Front Genet. 2017; 8:214. https://doi.org/10.3389/fgene.2017.00214
» https://doi.org/10.3389/fgene.2017.00214 - Ringuelet RA, Arámburu RH, Arámburu AA. Los peces argentinos de agua dulce. La Plata: Comisión de Investigación Científica; 1967.
- Rizzato PP, Bichuette ME. The laterosensory canal system in epigean and subterranean Ituglanis (Siluriformes: Trichomycteridae), with comments about troglomorphism and the phylogeny of the genus. J Morphol. 2016; 278(1):4-28. https://doi.org/10.1002/jmor.20616
» https://doi.org/10.1002/jmor.20616 - Serra S, Bessonart J, Melo FT, Duarte A, Malabarba LR, Loureiro M. Peces del Río Negro. Montevideo: MGAP-DINARA; 2014.
- Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9:107-19.
- Tchernavin VV. A revision of some Trichomycterinae based on material preserved in the British Museum (Natural History). Proc Zool Soc London . 1944; 114(1-2):234-75. https://doi.org/10.1590/S1679-62252008000100003
» https://doi.org/10.1590/S1679-62252008000100003 - Turcati A, Serra-Alanis WS, Malabarba LR. A new mouth brooder species of Gymnogeophagus with hypertrophied lips (Cichliformes: Cichlidae). Neotrop Ichthyol . 2018; 16(4):e180118. https://doi.org/10.1590/1982-0224-20180118
» https://doi.org/10.1590/1982-0224-20180118 - Vaz-Ferreira R, Soriano BS. Relación entre las longitudes antedorsal y anteventral y la longitud del cuerpo en dos Trichomycteridae (Pisces, Siluroidei). Arch Soc Biol Montevideo. 1959; 24:76-81.
- Vaz-Ferreira R, Soriano BS. Dos Trichomycteridae (Pisces, Siluroidei) poco conocidos. Rev Fac Humanid Cienc (Univ Repub, Montevideo). 1960; 18:315-38.
- Wosiacki WB. Estudo das relações filogenéticas de Trichomycterinae (Teleostei, Siluriformes, Trichomycteridae) com uma proposta de classificação. [PhD Thesis]. São Paulo: Universidade de São Paulo; 2002.
- Wosiacki WB, de Pinna MCC. Família Trichomycteridae. In: Buckup PA, Menezes NA, Ghazzi MAS, editors. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional; 2007. p.67-75.
- Wosiacki WB, de Pinna MCC. Trichomycterus igobi, a new catfish species from the Rio Iguaçu drainage: the largest head in Trichomycteridae (Siluriformes: Trichomycteridae). Neotrop Ichthyol . 2008; 6(1):17-23. https://doi.org/10.1590/S1679-62252008000100003
» https://doi.org/10.1590/S1679-62252008000100003 - Zarucki M, González-Bergonzoni I, Teixeira-de-Mello F, Loureiro M. Fish diversity loss in an urban stream of Uruguay throughout the last century. Panam J Aquat Sci . 2011; 6(1):71-75
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Ferrer J, Malabarba LR. Systematic revision of the Neotropical catfish genus Scleronema (Siluriformes: Trichomycteridae), with descriptions of six new species from Pampa grasslands. Neotrop Ichthyol. 2020; 18(2):e190081. https://doi.org/10.1590/1982-0224-2019-0081 -
ZOOBANK REGISTER
urn:lsid:zoobank.org:pub:B9AB7803-A395-404E-A000-50C8B3811A0F
Edited by
Data availability
Data citations
Fricke R, Eschmeyer WN, Fong JD. Species by family/subfamily [Internet]. San Francisco: California Academy of Science; 2020 [updated 2020 Mar 02; cited 2020 Mar 09]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
Morris PJ, Yager HM, Sabaj Pérez MH. ACSImagebase: A digital archive of catfish images compiled by participants in the All Catfish Species Inventory [Internet]. 2006 Available from: http://acsi.acnatsci.org/base
Publication Dates
-
Publication in this collection
12 June 2020 -
Date of issue
2020
History
-
Received
30 July 2019 -
Accepted
09 Mar 2020