Abstracts
With the aim of creating an inventory of the metazoan gill parasites of Salminus hilarii in the Taquari River, state of São Paulo, Brazil, five species of monogeneans (Anacanthorus contortus, A. bicuspidatus, Annulotrematoides parisellei, Jainus iocensis and Tereancistrum arcuatus) are reported the first time for this host. A total of 28 fish were sampled quarterly between April 2011 and January 2012, with 10 hosts in a lentic ecosystem and 18 in a lotic ecosystem. Quantitative ecological descriptors (prevalence, intensity of infestation and abundance) were calculated for the purpose to comparing the two ecosystems sampled (lentic and lotic ecosystems). However, no quantitative difference between the lentic and lotic ecosystems was observed. The present study has made available a checklist for species of the genus Anacanthorus and their hosts and geographical distribution in the Neotropical region up to the present time.
Ectoparasite; Monogenea; freshwater fish; Taquari River
Com o objetivo de inventariar os metazoários parasitos de brânquias de Salminus hilarii do rio Taquari, estado de São Paulo, Brasil, cinco espécies de monogenéticos (Anacanthorus contortus, A. bicuspidatus, Annulotrematoides parisellei, Jainus iocensis, e Tereancistrum arcuatus) foram registradas pela primeira vez para o presente hospedeiro. Um total de 28 peixes foi amostrado trimestralmente entre abril/2011 e janeiro/2012, com 10 hospedeiros no ecossistema lêntico e 18 no ecossistema lótico. Os descritores ecológicos quantitativos (prevalência, intensidade de infestação e abundância) foram calculados com o objetivo de comparar os dois ecossistemas amostrados (ecossistema lêntico versus ecossistema lótico). No entanto, nenhuma diferença quantitativa desses foi verificada entre os ecossistemas lêntico e lótico. O presente estudo disponibiliza uma lista de espécies do gênero Anacanthorus, os hospedeiro e distribuição geográfica na região Neotropical até o presente momento.
Ectoparasito; Monogenea; peixe de água doce; rio Taquari
Introduction
Monogeneans included in Dactylogyridae are primarily parasites of the
gills of marine and freshwater fish. The class Monogenea is the most diversified
group and contains the largest number of species parasitizing Neotropical fish
(BOEGER; VIANNA, 2006). Specific monogeneans are common helminths parasitizing bony
fish worldwide (AKOLL et al., 2012Akoll P, Fioravanti ML, Konecny R, Schiemer F. Infection dynamics of
Cichlidogyrus tilapiae and C. sclerosus (Monogenea, Ancyrocephalinae) in Nile
tilapia (Oreochromis niloticus L.) from Uganda. J Helminthol 2012; 86(3):
302-310. PMid:21791155.
http://dx.doi.org/10.1017/S0022149X11000411
http://dx.doi.org/10.1017/S0022149X11000...
), which is
a factor justifying the importance of taxonomic knowledge of species of this class.
Eiras et al. (2011)Eiras JC, Takemoto RM, Pavanelli GC, Adriano EA. About the
biodiversity of parasites of freshwater fish from Brazil. Bull Eur Assoc Fish
Pathol 2011; 31(4): 161-168. highlighted the
increasing numbers of studies on monogeneans. However, the high number of monogenean
species does not necessarily imply that this group has more species than other
groups. This may simply have occurred because monogeneans have been more intensively
studied than other groups.
Salminus hilarii Valenciennes, 1850, is a freshwater fish popularly
known as "tabarana" in Brazil, and is considered to be a migratory species over
large distances (BARBIERI et al., 2004Barbieri G, Salles AF, Cestarolli MA, Teixeira-Filho AR. Estratégias
reprodutivas do dourado, Salminus maxillosus, e do curimbatá, Prochilodus
lineatus no rio Mogi Guaçu, Estado de São Paulo, com ênfase nos
parâmetros matemáticos da dinâmica populacional. Acta Sci Biol Sci
2004; 26(2): 169-174.
http://dx.doi.org/10.4025/actascibiolsci.v26i2.1631
http://dx.doi.org/10.4025/actascibiolsci...
). The
geographical distribution of this species covers the main Brazilian river basins
(Paraná, São Francisco, Tocantins, Amazon and Orinoco) (AGOSTINHO et al., 2007Agostinho AA, Gomes LC, Pelicice FM. Ecologia e Manejo de Recursos
Pesqueiros em Reservatórios do Brasil. Maringá: Eduem; 2007.; GRAçA;
PAVANELLI, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio
Paraná e áreas adjacentes. Maringá: Eduem; 2007.).
The present study underscores and broadens the importance of taxonomic
knowledge of species of this parasite group. This is the first study to record
occurrences of monogeneans at species level, parasitizing the gills of S.
hilarii. Kohn et al. (1985)Kohn A, Fernandes BMM, Macedo B, Abramson B. Helminths parasites of
Freshwater fishes from Pirassununga, SP, Brazil. Mem Inst Oswaldo Cruz 1985;
80(3): 327-336.
http://dx.doi.org/10.1590/S0074-02761985000300009
http://dx.doi.org/10.1590/S0074-02761985...
identified one nematode, two digenean, and two monogenean parasites of S.
hilarii, but the last ones were only identified at superfamily level
(Dactylogyridae).
The Taquari River, located in the state of São Paulo, is a tributary of the Paranapanema River, which is influenced by the Jurumirim reservoir. From studies on gill parasites of S. hilarii in this context, occurrences of five monogenean species were reported and their quantitative parameters were compared between lentic and lotic ecosystems.
Materials and Methods
Study area
The Jurumirim dam (23° 12′ 17″ S and 49° 13′ 19″ W) is the first of a cascade of dams on the Paranapanema River. The reservoir behind this dam is operated such that it regulates others further downstream. The dam was built in the late 1950s and operations began in 1962 (HENRY; NOGUEIRA, 1999Henry R, Nogueira MG. A Represa de Jurumirim (São Paulo): Primeira síntese sobre o conhecimento limnológico e uma proposta preliminar de manejo ambiental. In: Henry R. Ecologia de reservatórios: estrutura, função e aspectos sociais. Botucatu: FUNDIBIO, FAPESP; 1999. p. 651-686). The Taquari River (23° 15′ 12″ S and 49° 12′ 34″ W), located in the state of São Paulo, is the second largest tributary of the Jurumirim reservoir (HENRY et al., 1999Henry R, Santos AAN, Camargo YR. Transporte de sólidos suspensos, N e P total pelos Rios Paranapanema e Taquari e uma avaliação de sua exportação na represa de Jurumirim. In: Henry R. Ecologia de reservatórios: estrutura, função e aspectos sociais. Botucatu: FUNDIBIO, FAPESP; 1999. p. 687-710.) (Figure 1).
Jurumirim reservoir on the upper Paranapanema River, Brazil. Sampling areas: *lentic and ** lotic ecosystems in the Taquari River (arrow). Source: satellite image from Google Earth Digital Globe
Fish collection and laboratory procedure
Twenty-eight specimens of S. hilarii from the Taquari River were collected between April 2011 and January 2012 in order to study monogenean parasites. The fish were collected using nylon monofilament gillnets with mesh sizes of 3 to 14 cm and with standardized effort. Nets were deployed at 5:00 pm and gathered in at 7:00 am the following day (total exposure time: 14 h). Limnological parameters such as temperature (°C), pH and dissolved oxygen (mg O2.L-1) were measured with aid of a multi-parameter analyzer. Individuals were measured in terms of standard length (Ls , to the nearest 0.1 mm) and weight (to the nearest 0.1 g). The gills were frozen and subsequently removed and placed in vials containing 5% formalin solution. The parasites collected were preserved in alcohol 70%. Some parasite specimens were mounted unstained in Hoyer's medium in order to study the sclerotized structures, while others were stained with Gomori's trichrome to observe internal organs (EIRAS et al., 2006Eiras JC, Takemoto RM, Pavanelli GC. Métodos de Estudo e Técnicas Laboratoriais em Parasitologia de Peixes. Maringá: Eduem; 2006.).
Hierarchical levels of study and statistical analysis
In accordance with Bush et al.
(1997)Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets
ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4):
575-583. PMid:9267395. http://dx.doi.org/10.2307/3284227
http://dx.doi.org/10.2307/3284227...
, the following community descriptors were calculated at the
infracommunity level: prevalence (%), mean intensity of infestation and mean
abundance for each parasite species. After checking the assumptions of normality
(Lilliefors test), Pearson's linear correlation (r) was
applied to examine the relationships between parasite abundance and the
limnological parameters. The Mann-Whitney U test was performed
to measure the effects of the lentic and lotic ecosystems on the abundance of
each parasite species. Yates' corrected X
2 together with Spearman's rank correlation (rs) were also
performed on pairs of co-occurring parasite species to investigate possible
interspecific associations (i.e. co-operative or antagonistic relationships)
(LUDWIG; REYNOLDS, 1988Ludwig JA, Reynolds JF. Statistical Ecology: a Primer on Methods and
Computing. New York: Wiley-Interscience Publications; 1988.). The
Z-test for proportions was performance to check differences
in prevalence between infrapopulations of the lentic and lotic ecosystems.
The Berger-Parker index was applied to appraise the numerical
dominance trends among parasite species (MAGURRAN, 1988Magurran AE. Ecological diversity and its measurement. New Jersey:
Princeton University Press; 1988.
http://dx.doi.org/10.1007/978-94-015-7358-0
http://dx.doi.org/10.1007/978-94-015-735...
). The variance-to-mean ratio of parasite abundance
(dispersion index) and the discrepancy index, computed using the Quantitative
Parasitology 3.0 software (RóZSA et al.,
2000Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of
hosts. J Parasitol 2000; 86(2): 228-232. PMid:10780537.), were used to detect distribution patterns of the
infrapopulations (POULIN, 1993Poulin R. The disparity between observed and uniform distributions -
A new look at parasite aggregation. Int J Parasitol 1993; 23(7): 937-944.
http://dx.doi.org/10.1016/0020-7519(93)90060-C
http://dx.doi.org/10.1016/0020-7519(93)9...
). The
Shannon index (H') was calculated to compared the biological diversity of
communities in the lentic and lotic ecosystems (MAGURRAN, 1988Magurran AE. Ecological diversity and its measurement. New Jersey:
Princeton University Press; 1988.
http://dx.doi.org/10.1007/978-94-015-7358-0
http://dx.doi.org/10.1007/978-94-015-735...
). The hierarchical levels and terminology used in
this study followed Bush et al. (1997)Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets
ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4):
575-583. PMid:9267395. http://dx.doi.org/10.2307/3284227
http://dx.doi.org/10.2307/3284227...
and Poulin (2004)Poulin R. Macroecological patterns of species richness in parasite
assemblages. Basic Appl Ecol 2004; 5(5): 423-434.
http://dx.doi.org/10.1016/j.baae.2004.08.003
http://dx.doi.org/10.1016/j.baae.2004.08...
and the significance
level used was p < 0.05.
The monogenean species were identified as described by Cohen et al. (2012)Cohen SC, Kohn A, Boeger WA. Neotropical Monogenoidea. 57. Nine new species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis (Characidae, Characiformes) from the Paraná River, State of Paraná, Brazil. Zootaxa 2012; 3049: 57-68. and Boeger and Vianna (2006). Voucher specimens were deposited in the Helminthological Collection of the Institute of Biosciences (CHIBB), UNESP, Botucatu, São Paulo, Brazil.
Results
A total of 28 fish were captured and all were parasitized by one or more monogenean species (overall prevalence = 100%). About 35% of the fish examined were parasitized by three parasite species (Figure 2). Calculations on the absolute frequencies of the total parasites collected highlighted Anacanthorus contortus as the most important numerically (53.2%), followed by Annulotrematoides parisellei (20.9%), Tereancistrum arcuatus (15.1%), Jainus iocensis (7.4%) and A. bicuspidatus (3.4%). Salminus hilarii is a new host record for all the monogeneans identified.
Richness and intensity of infection in communities of monogeneans in the gills of Salminus hilarii in the Taquari River, upper Paranapanema River, Brazil.
The component community was composed of five monogenean species totaling 417 specimens. Among these, 183 were collected in a lentic ecosystem and 234 in a lotic ecosystem, with means of 3.36 ± 7.53 and 2.6 ± 4.21, respectively. Comparing the prevalence and abundance of the component communities of the lotic and lentic ecosystems, no significant difference was observed. Fish from both ecosystems had the same magnitude of species richness and no differences in diversity (H'lentic = 1.19 and H'lotic = 1.27; degrees of freedom = 27; t test = 0.988; p > 0.05).
Comparing the component communities, A. contortus showed high values for quantitative descriptors in both the lentic and the lotic ecosystems, except for the prevalence values. Among these, A. parisellei in the lentic ecosystem stood out with prevalence of 80%. The parasite A. bicuspidatus presented the lowest prevalence and J. iocensis and T. arcuatus presented similar prevalences (Table 1).
Prevalence (%), total abundance (TA), mean intensity ± standard error (MI ± SE) and mean abundance (MA) of monogenean species in Salminus hilarii in the Taquari River, upper Paranapanema River, Brazil.
Significant covariations of abundance were observed between pairs: A. contortus versus A. bicuspidatus, A. contortus versus T. arcuatus and A. parisellei versus T. arcuatus. Significant associations of prevalence were observed between pairs: A. contortus versus A. bicuspidatus and A. parisellei versus A. bicuspidatus (Table 2).
Paired associations for monogenean species in Salminus hilarii in the Taquari River, upper Paranapanema River, Brazil. Spearman rank correlation (rs). Significant values *p ≤ 0.05.
Anacanthorus contortus had the highest dominance frequency at both sites (0.59 and 0.48 in lentic and lotic ecosystems, respectively). All the parasites had a typically aggregated distribution pattern, and A. bicuspidatus was the monogenean species that showed the highest discrepancy index values (Table 3).
Values of variance-to-mean ratio for parasite abundance (ID) and discrepancy index (D) among monogenean parasites of Salminus hilarii in the Taquari River, upper Paranapanema River, Brazil.
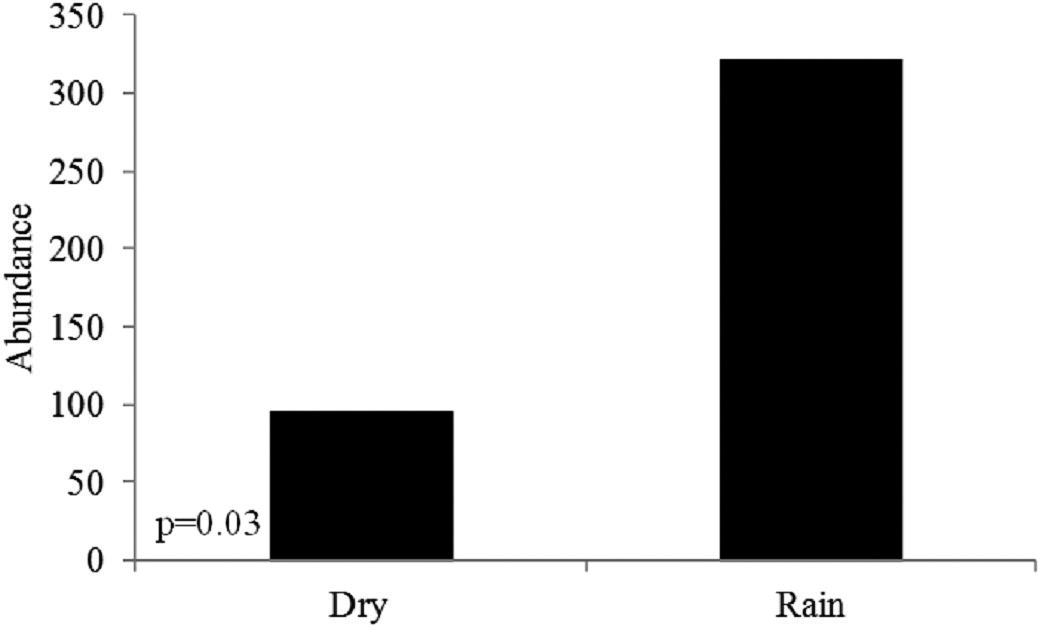
In relation to the limnological variables (Table 4), the Pearson linear correlation (r) only revealed a statistical difference in the abundance of monogeneans in relation to pH: abundance versus temperature (R2 = 0.81; p = 0.09); abundance versus oxygen (R2 = 0.15; p = 0.61); and abundance versus pH (R2 = 0.89; p = 0.05). Considering the months of May to July to be the dry period and October to January to be the rainy period, greater abundance of monogeneans was observed during the rainy period (Figure 3).
Comparison of the abundance of monogeneans parasitizing the gills of S. hilarii in the Taquari River, upper Paranapanema River, Brazil, between the dry period (April to July) and the rainy period (October to January). Significantly different values in Mann-Whitney U test (p < 0.05).
Mean values and standard deviations of monthly limnological parameters in the Taquari River, between April 2011 and January 2012.
Discussion
The present study reports occurrences of five monogenean species parasitizing S. hilarii in the Taquari River, Brazil. Individual species of Anacanthorus show varying ability to infest closely related host species. Furthermore, subgroups within Anacanthorus, based on the general morphology of the copulatory complex, appear to express high host specificity to familial groups within the Characidae. Because of these traits, species of Anacanthorus may provide valuable models for studying biogeography (KRITSKY et al., 1992Kritsky DC, Boeger WA, Van Every LR. Neotropical Monogenoidea. 17. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) from Characoid Fishes of the Central amazon. J Helminthol Soc Wash 1992; 59(1): 25-51.). These characteristics may explain the higher abundance and mean intensity of infestations relating to A. contortus.
The genus Anacanthorus has large species diversity. To
date, 70 species have been described, mostly from the Amazon region (Table 5). According to Van Every and Kritsky (1992)Kritsky DC, Boeger WA, Van Every LR. Neotropical Monogenoidea. 17.
Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) from
Characoid Fishes of the Central amazon. J Helminthol Soc Wash 1992; 59(1):
25-51., species diversity among
Amazonian fish and their parasites may have resulted from lacustrine resource
partitioning, similar to that proposed to explain cichlid diversity in some African
lakes (LOWE-MCCONNELL, 1987Lowe-McConnell RH. Ecological Studies in Tropical Fish Communities.
Cambridge: Cambridge University Press; 1987.
http://dx.doi.org/10.1017/CBO9780511721892
http://dx.doi.org/10.1017/CBO97805117218...
) and Pliocene
diversity in lake Idaho (SMITH, 1975Smith GR. Fishes of the Pliocene Glenns Ferry Formation, Southwest
Idaho. Claude W Hibbard Memorial 1975; 5(14): 1-68.).
Checklist of Anacanthorus species and their hosts and geographical distribution in the Neotropical region.
So far, monogenean species of Anacanthorus have been
recorded parasitizing twenty species of fish of the order Characiformes in the
Neotropical region: Brycon amazonicus, Brycon
melanopterus, Brycon orthotaenia, Catoprion
mento, Colossoma bidens, Colossoma
macropomum, Myleus rubripinnis, Mylossoma
duriventris, Oreochromis mossambicus (=
Tilapia mossambica), Piaractus mesopotamicus,
Pristobrycon eigenmanni (= Serrasalmus
eigenmanni), Pristobrycon striolatus,
Pristobrycon sp., Pygocentrus nattereri,
Roeboides myersii, Salminus brasiliensis,
Serrasalmus elongatus, Serrasalmus rhombeus,
Serrasalmus spilopleura, Serrasalmus sp.,
Triportheus albus, Triportheus angulatus,
Triportheus elongatus, Triportheus sp. and
Salminus affinis (KRITSKY et
al., 1979Kritsky DC, Thatcher VE, Kayton RJ. Neotropical Monogenoidea 2. The
Anacanthorinae Price, 1967, with the proposal of four new species of
Anacanthorus Mizelle & Price, 1965, from Amazonian fishes. Acta Amaz 1979;
9(2): 355-361.; KRITSKY et al., 1992Kritsky DC, Boeger WA, Van Every LR. Neotropical Monogenoidea. 17.
Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) from
Characoid Fishes of the Central amazon. J Helminthol Soc Wash 1992; 59(1):
25-51.;
KOHN; COHEN, 1998Kohn A, Cohen SC. South American Monogenea - list of species, hosts
and geographical distribution. Int J Parasitol 1998; 28(10): 1517-1554.
http://dx.doi.org/10.1016/S0020-7519(98)00083-6
http://dx.doi.org/10.1016/S0020-7519(98)...
; FISCHER et al., 2003Fischer C, Malta JCO, Varella AMB. A Fauna de Parasitas do Tambaqui,
Colossoma macropomum (CUVIER, 1818) (Characiformes: Characidae) do Médio Rio
Solimões, Estado do Amazonas (AM) e do Baixo Rio Amazonas, Estado do Pará
(PA), e seu Potencial como Indicadores Biológicos. Acta Amaz 2003; 33(4):
651-662.; ANDRADE;
MALTA, 2006Andrade SMS, Malta JCO. Parasite fauna monitoring of matrinxã Brycon
amazonicus (Spix & Agassiz, 1829) raised in an intensive husbandry system a
stream channel in the state of Amazonas, Brazil. Braz J Biol 2006; 66(4):
1123-1132. http://dx.doi.org/10.1590/S1519-69842006000600020
http://dx.doi.org/10.1590/S1519-69842006...
; MONTEIRO et al.,
2010Monteiro CM, Kritsky DC, Brasil-Sato MC. Neotropical Monogenoidea.
56. New species of Anacanthorus (Dactylogyridae) from the gills of matrinchã,
Brycon orthotaenia (Characiformes: Characidae), in the Rio São Francisco,
Brazil. Folia Parasitol 2010; 57(3): 164-168.; COHEN et al., 2012Cohen SC, Kohn A, Boeger WA. Neotropical Monogenoidea. 57. Nine new
species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis
(Characidae, Characiformes) from the Paraná River, State of Paraná, Brazil.
Zootaxa 2012; 3049: 57-68.).
The genus Jainus has been recorded in the following
hosts in South America: Brycon amazonicus, B.
cephalus, B. melanopterus, Chalceus
macrolepidotus, Creatochanes affinis,
Leporinus copelandii, Moenkhausia
sanctaefilomenae, Salminus brasiliensis and
Schizodon borellii (MIZELLE et
al., 1969Mizelle JD, Kritsky DC, Crane JW. Studies on monogenetic trematodes.
XXXVIII. Ancyrocephalinae from South America with the proposal of Jainus gen. n.
Am Midl Nat 1969; 80(1): 186-198.
http://dx.doi.org/10.2307/2423609
http://dx.doi.org/10.2307/2423609...
; KRITSKY et al., 1980; KOHN;
COHEN, 1998Kohn A, Cohen SC. South American Monogenea - list of species, hosts
and geographical distribution. Int J Parasitol 1998; 28(10): 1517-1554.
http://dx.doi.org/10.1016/S0020-7519(98)00083-6
http://dx.doi.org/10.1016/S0020-7519(98)...
; ANDRADE et al., 2001Andrade SMS, Malta JC, Ferraz E. Fauna parasitológica de alevinos de
Matrinchã, Brycon cephalus (Günther, 1869) coletados nos rios Negro e
Solimões, na Amazônia Central. Acta Amaz 2001; 31(2):
263-273.;
ANDRADE; MALTA, 2006Andrade SMS, Malta JCO. Parasite fauna monitoring of matrinxã Brycon
amazonicus (Spix & Agassiz, 1829) raised in an intensive husbandry system a
stream channel in the state of Amazonas, Brazil. Braz J Biol 2006; 66(4):
1123-1132. http://dx.doi.org/10.1590/S1519-69842006000600020
http://dx.doi.org/10.1590/S1519-69842006...
; TAKEMOTO et al.,
2009; KARLING et al., 2011Karling LC, Bellay S, Takemoto RM, Pavanelli GC. A new species of
Jainus (Monogenea), gill parasite of Schizodon borellii (Characiformes,
Anostomidae) from the upper Paraná river floodplain, Brazil. Acta Sci Biol Sci
2011: 33(2): 227-231.
http://dx.doi.org/10.4025/actascibiolsci.v33i2.6168
http://dx.doi.org/10.4025/actascibiolsci...
; COHEN et al., 2012Cohen SC, Kohn A, Boeger WA. Neotropical Monogenoidea. 57. Nine new
species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis
(Characidae, Characiformes) from the Paraná River, State of Paraná, Brazil.
Zootaxa 2012; 3049: 57-68.).
Tereancistrum has been recorded in Brycon
amazonicus, B. melanopterus, Leporinus
fasciatus, Prochilodus reticulatus and
Salminus brasiliensis (KRITSKY et al., 1980; KOHN; COHEN, 1998Kohn A, Cohen SC. South American Monogenea - list of species, hosts
and geographical distribution. Int J Parasitol 1998; 28(10): 1517-1554.
http://dx.doi.org/10.1016/S0020-7519(98)00083-6
http://dx.doi.org/10.1016/S0020-7519(98)...
; ANDRADE; MALTA, 2006Andrade SMS, Malta JCO. Parasite fauna monitoring of matrinxã Brycon
amazonicus (Spix & Agassiz, 1829) raised in an intensive husbandry system a
stream channel in the state of Amazonas, Brazil. Braz J Biol 2006; 66(4):
1123-1132. http://dx.doi.org/10.1590/S1519-69842006000600020
http://dx.doi.org/10.1590/S1519-69842006...
; COHEN et
al., 2012Cohen SC, Kohn A, Boeger WA. Neotropical Monogenoidea. 57. Nine new
species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis
(Characidae, Characiformes) from the Paraná River, State of Paraná, Brazil.
Zootaxa 2012; 3049: 57-68.).
During this study, we observed differences in correlations between the abundance of monogeneans and pH, which possibly influenced the presence or absence of these organisms. However, studies on the physiology of the host are needed in order to confirm the degree of influence of the pH of these organisms.
Regarding parasite dispersion, in most cases, the parasites are almost
universally aggregated between their hosts (KRASNOV;
POULIN, 2010Krasnov BR, Poulin R. Ecological properties of a parasite: species
specific stability and geographical variation. In: Morand S, Krasnov BR. The
biogeography of host-parasite interactions. New York: Oxford University Press
Inc.; 2010. p. 99-114.). That is to say, most of the hosts have few if any
parasites, while a small number of hosts are infected with many parasites (POULIN, 1993Poulin R. The disparity between observed and uniform distributions -
A new look at parasite aggregation. Int J Parasitol 1993; 23(7): 937-944.
http://dx.doi.org/10.1016/0020-7519(93)90060-C
http://dx.doi.org/10.1016/0020-7519(93)9...
). This pattern is expected in most
animals in nature, as observed regarding monogeneans parasitizing the gills of
S. hilarii in the present study, except for A.
parisellei in the lentic gradient. This type of distribution is
possibly related to variations in the characteristics of the environment or the
behavior of living beings that try to group (NERING;
ZUBEN, 2010Nering MB, Zuben CJV. Métodos Quantitativos em Parasitologia.
Jaboticabal: Funep; 2010.).
According Thomas et al. (2005)Thomas F, Bonsall MB, Dobson AP. Parasitism, biodiversity, and
conservation. In: Thomas F, Renaud F, Guégan J. Parasitism and Ecosystems. New
York: Oxford University Press; 2005. p. 124-139.
http://dx.doi.org/10.1093/acprof:oso/9780198529873.003.0009
http://dx.doi.org/10.1093/acprof:oso/978...
,
considerable progress has been made in understanding the functional value of
parasites in ecosystems. Numerous theoretical and empirical studies have shown that
parasites, in spite of their small size, are biologically and ecologically important
in ecosystems. The influence of monogeneans on fish behavior in nature is difficult
to measure, but we considered them within the life history of the host in order to
gain better understanding of both organisms within their ecological niches.
Knowledge of the geographical and systematic distribution of monogenean species is also of considerable importance, since these organisms can be used as tools to aid in understanding, conserving and preserving aquatic ecosystems.
The present study provides the first record of occurrences of the monogeneans A. contortus, A. bicuspidatus, A. parisellei, J. iocensis and T. arcuatus parasitizing the gills of the host S. hilarii in the Taquari River in the state of São Paulo, Brazil. This study contributes towards the knowledge of occurrences of these species of parasites in fish of the family Characidae and their geographical distribution, as well as listing the host species and geographical distribution of the genus Anacanthorus in South America.
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP process: 2011/22603-3) for the scholarship granted to the first author and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES process: AUX-PE-PNPD 3005/2010). They are grateful to the staff of the Fish Biology and Ecology Laboratory of the Institute of Biosciences, Botucatu, and Universidade Estadual Paulista (UNESP) for the structure provided for developing this work.
References
- Agostinho AA, Gomes LC, Pelicice FM. Ecologia e Manejo de Recursos Pesqueiros em Reservatórios do Brasil. Maringá: Eduem; 2007.
- Akoll P, Fioravanti ML, Konecny R, Schiemer F. Infection dynamics of Cichlidogyrus tilapiae and C. sclerosus (Monogenea, Ancyrocephalinae) in Nile tilapia (Oreochromis niloticus L.) from Uganda. J Helminthol 2012; 86(3): 302-310. PMid:21791155. http://dx.doi.org/10.1017/S0022149X11000411
» http://dx.doi.org/10.1017/S0022149X11000411 - Andrade SMS, Malta JCO. Parasite fauna monitoring of matrinxã Brycon amazonicus (Spix & Agassiz, 1829) raised in an intensive husbandry system a stream channel in the state of Amazonas, Brazil. Braz J Biol 2006; 66(4): 1123-1132. http://dx.doi.org/10.1590/S1519-69842006000600020
» http://dx.doi.org/10.1590/S1519-69842006000600020 - Andrade SMS, Malta JC, Ferraz E. Fauna parasitológica de alevinos de Matrinchã, Brycon cephalus (Günther, 1869) coletados nos rios Negro e Solimões, na Amazônia Central. Acta Amaz 2001; 31(2): 263-273.
- Aragort W, Morales G, Leon E, Pino LA, Guillén A, Silva M. Patologías asociadas a monogeneos branquiales en cachama bajo cultivo. Vet Trop 2002; 27(2): 75-85.
- Azevedo RK, Abdallah VD, Luque JL. Acanthocephala, Annelida, Arthropoda, Myxozoa, Nematoda and Platyhelminthes parasites of fishes from the Guandu river, Rio de Janeiro, Brazil. Check List 2010; 6(4): 659-667.
- Barbieri G, Salles AF, Cestarolli MA, Teixeira-Filho AR. Estratégias reprodutivas do dourado, Salminus maxillosus, e do curimbatá, Prochilodus lineatus no rio Mogi Guaçu, Estado de São Paulo, com ênfase nos parâmetros matemáticos da dinâmica populacional. Acta Sci Biol Sci 2004; 26(2): 169-174. http://dx.doi.org/10.4025/actascibiolsci.v26i2.1631
» http://dx.doi.org/10.4025/actascibiolsci.v26i2.1631 - Boeger WA, Kritsky DC. Neotropical Monogenea. 12. Dactylogyridae from Serrasalmus nattereri (Cypriniformes, Serrasalmidae) and aspects of their morphologic variation and distribution in the Brazilian Amazon. Proc Helminthol Soc Wash 1988; 55(2): 188-213.
- Boeger WA, Vianna RT. Monogenoidea. In: Thatcher VE. Amazon Fish Parasites. Sofia: Pensoft Publishers. 2006. p. 42-116.
- Boeger WA, Husak WS, Martins ML. Neotropical monogenoidea. 25. Anacanthorus penilabiatus n. sp. (Dactylogyridae, Anacanthorinae) from Piaractus mesopotamicus (Osteichthyes, Serrasalmidae), cultivated in the State of São Paulo, Brazil. Mem Inst Oswaldo Cruz 1995; 90(6): 699-701. http://dx.doi.org/10.1590/S0074-02761995000600008
» http://dx.doi.org/10.1590/S0074-02761995000600008 - Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 1997; 83(4): 575-583. PMid:9267395. http://dx.doi.org/10.2307/3284227
» http://dx.doi.org/10.2307/3284227 - Centeno L, Silva-Acuña A, Silva-Acuña R, Pérez JL. Fauna Ectoparasitaria Asociada a Colossoma macropomum y al Híbrido de C. macropomum x Piaractus brachypomus, Cultivados en el Estado Delta Amacuro, Venezuela. Bioagro 2004; 16(2): 121-126.
- Cohen SC, Kohn A, Boeger WA. Neotropical Monogenoidea. 57. Nine new species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis (Characidae, Characiformes) from the Paraná River, State of Paraná, Brazil. Zootaxa 2012; 3049: 57-68.
- Córdova L, Pariselle A. Monogenoidea en Serrasalmus rhombeus (Linnaeus, 1766) de la Cuenca Amazónica Boliviana. Rev Peru Biol 2007; 14(1): 11-16.
- Eiras JC, Takemoto RM, Pavanelli GC, Adriano EA. About the biodiversity of parasites of freshwater fish from Brazil. Bull Eur Assoc Fish Pathol 2011; 31(4): 161-168.
- Eiras JC, Takemoto RM, Pavanelli GC. Métodos de Estudo e Técnicas Laboratoriais em Parasitologia de Peixes. Maringá: Eduem; 2006.
- Fischer C, Malta JCO, Varella AMB. A Fauna de Parasitas do Tambaqui, Colossoma macropomum (CUVIER, 1818) (Characiformes: Characidae) do Médio Rio Solimões, Estado do Amazonas (AM) e do Baixo Rio Amazonas, Estado do Pará (PA), e seu Potencial como Indicadores Biológicos. Acta Amaz 2003; 33(4): 651-662.
- Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem; 2007.
- Henry R, Nogueira MG. A Represa de Jurumirim (São Paulo): Primeira síntese sobre o conhecimento limnológico e uma proposta preliminar de manejo ambiental. In: Henry R. Ecologia de reservatórios: estrutura, função e aspectos sociais. Botucatu: FUNDIBIO, FAPESP; 1999. p. 651-686
- Henry R, Santos AAN, Camargo YR. Transporte de sólidos suspensos, N e P total pelos Rios Paranapanema e Taquari e uma avaliação de sua exportação na represa de Jurumirim. In: Henry R. Ecologia de reservatórios: estrutura, função e aspectos sociais. Botucatu: FUNDIBIO, FAPESP; 1999. p. 687-710.
- Iannacone JA, Luque JL. New records of helminths parasitic on Peruvian Amazonian fishes (Osteichthyes). Rev Biol Trop 1993; 41(2): 303-305.
- Karling LC, Bellay S, Takemoto RM, Pavanelli GC. A new species of Jainus (Monogenea), gill parasite of Schizodon borellii (Characiformes, Anostomidae) from the upper Paraná river floodplain, Brazil. Acta Sci Biol Sci 2011: 33(2): 227-231. http://dx.doi.org/10.4025/actascibiolsci.v33i2.6168
» http://dx.doi.org/10.4025/actascibiolsci.v33i2.6168 - Kohn A, Cohen SC. South American Monogenea - list of species, hosts and geographical distribution. Int J Parasitol 1998; 28(10): 1517-1554. http://dx.doi.org/10.1016/S0020-7519(98)00083-6
» http://dx.doi.org/10.1016/S0020-7519(98)00083-6 - Kohn A, Fernandes BMM, Macedo B, Abramson B. Helminths parasites of Freshwater fishes from Pirassununga, SP, Brazil. Mem Inst Oswaldo Cruz 1985; 80(3): 327-336. http://dx.doi.org/10.1590/S0074-02761985000300009
» http://dx.doi.org/10.1590/S0074-02761985000300009 - Krasnov BR, Poulin R. Ecological properties of a parasite: species specific stability and geographical variation. In: Morand S, Krasnov BR. The biogeography of host-parasite interactions. New York: Oxford University Press Inc.; 2010. p. 99-114.
- Kritsky DC, Thatcher V. Monogenetic trematodes (Monopisthocotylea: Dactylogyridae) from freshwater fishes of Colombia, South America. J Helminthol 1974; 48(1): 59-66. http://dx.doi.org/10.1017/S0022149X00022604
» http://dx.doi.org/10.1017/S0022149X00022604 - Kritsky DC, Thatcher VE, Kayton RJ. Neotropical Monogenoidea 2. The Anacanthorinae Price, 1967, with the proposal of four new species of Anacanthorus Mizelle & Price, 1965, from Amazonian fishes. Acta Amaz 1979; 9(2): 355-361.
- Kristky DC, Thatcher VE, Kayton RJ. Neotropical Monogenoidea. 3. Five new species from South America with the proposal of Tereancistrum gen. n. and Trinibaculum gen. n. (Dactylogyridae: Ancyrocephalinae). Acta Amaz 1980; 10(2): 411-417.
- Kritsky DC, Boeger WA, Van Every LR. Neotropical Monogenoidea. 17. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) from Characoid Fishes of the Central amazon. J Helminthol Soc Wash 1992; 59(1): 25-51.
- Lowe-McConnell RH. Ecological Studies in Tropical Fish Communities. Cambridge: Cambridge University Press; 1987. http://dx.doi.org/10.1017/CBO9780511721892
» http://dx.doi.org/10.1017/CBO9780511721892 - Ludwig JA, Reynolds JF. Statistical Ecology: a Primer on Methods and Computing. New York: Wiley-Interscience Publications; 1988.
- Magurran AE. Ecological diversity and its measurement. New Jersey: Princeton University Press; 1988. http://dx.doi.org/10.1007/978-94-015-7358-0
» http://dx.doi.org/10.1007/978-94-015-7358-0 - Mizelle JD, Kritsky DC. Studies on monogenetic trematodes. XL. New species from marine and freshwater fishes. Am Midl Nat 1969; 82(2): 417-428. http://dx.doi.org/10.2307/2423787
» http://dx.doi.org/10.2307/2423787 - Mizelle JD, Price CE. Studies on Monogenetic Trematodes. XXVIII. Gill Parasites of the Piranha with Proposal of Anacanthorus gen. n. J Parasitol 1965; 51(1): 30-36. PMid:14259477. http://dx.doi.org/10.2307/3275640
» http://dx.doi.org/10.2307/3275640 - Mizelle JD, Kritsky DC, Crane JW. Studies on monogenetic trematodes. XXXVIII. Ancyrocephalinae from South America with the proposal of Jainus gen. n. Am Midl Nat 1969; 80(1): 186-198. http://dx.doi.org/10.2307/2423609
» http://dx.doi.org/10.2307/2423609 - Monteiro CM, Kritsky DC, Brasil-Sato MC. Neotropical Monogenoidea. 56. New species of Anacanthorus (Dactylogyridae) from the gills of matrinchã, Brycon orthotaenia (Characiformes: Characidae), in the Rio São Francisco, Brazil. Folia Parasitol 2010; 57(3): 164-168.
- Nering MB, Zuben CJV. Métodos Quantitativos em Parasitologia. Jaboticabal: Funep; 2010.
- Pamplona-Basilio MC, Kohn A, Feitosa VA. New Host Records and Description of the Egg of Anacanthorus penilabiatus (Monogenea, Dactylogyridae). Mem Inst Oswaldo Cruz 2001; 96(5): 667-668. PMid:11500767. http://dx.doi.org/10.1590/S0074-02762001000500014
» http://dx.doi.org/10.1590/S0074-02762001000500014 - Poulin R. The disparity between observed and uniform distributions - A new look at parasite aggregation. Int J Parasitol 1993; 23(7): 937-944. http://dx.doi.org/10.1016/0020-7519(93)90060-C
» http://dx.doi.org/10.1016/0020-7519(93)90060-C - Poulin R. Macroecological patterns of species richness in parasite assemblages. Basic Appl Ecol 2004; 5(5): 423-434. http://dx.doi.org/10.1016/j.baae.2004.08.003
» http://dx.doi.org/10.1016/j.baae.2004.08.003 - Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. J Parasitol 2000; 86(2): 228-232. PMid:10780537.
- Smith GR. Fishes of the Pliocene Glenns Ferry Formation, Southwest Idaho. Claude W Hibbard Memorial 1975; 5(14): 1-68.
- Takemoto RM, Pavanelli GC, Lizama MAP, Lacerda ACF, Yamada FH, Moreira LHA, et al. Diversity of parasites of fish from the Upper Paraná River floodplain, Brazil. Braz J Biol 2009; 69(2 Supll): 691-705.
- Thomas F, Bonsall MB, Dobson AP. Parasitism, biodiversity, and conservation. In: Thomas F, Renaud F, Guégan J. Parasitism and Ecosystems. New York: Oxford University Press; 2005. p. 124-139. http://dx.doi.org/10.1093/acprof:oso/9780198529873.003.0009
» http://dx.doi.org/10.1093/acprof:oso/9780198529873.003.0009 - Van Every LR, Kritsky DC. Neotropical Monogenoidea. 18. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) of Piranha (Characoidea, Serrasalmidae) from the Central Amazon, their Phylogeny, and aspects of Host-Parasite Coevolution. J Helminthol Soc Washington 1992; 59(1): 52-75.
Publication Dates
-
Publication in this collection
Oct-Dec 2013
History
-
Received
13 Aug 2013 -
Accepted
1 Nov 2013