Abstracts
We examine the possibility of detecting the chemical bond overlap plasmon (CBOP) of the hydrogen molecule by electron inelastic scattering. The CBOP has been predicted to efficiently absorb and scatter electromagnetic radiation above the molecular ionization threshold in the cases of alkali halides. For the hydrogen molecule the quadrupole nature of the CBOP energy-loss cross section leads to cross section values with impacting electron energy dependence and an angular behavior which are totally distinguishable from the usual ionization, inter-band transitions and dissociation processes. Previously established relationships between the CBOP and the polarizability of the overlap region suggest this an a promising theoretical tool for quantifying covalency in the chemical bond.
energy loss; bond overlap plasmon, H2; electron scattering; covalency
Examinamos a possibilidade de detectar o plásmon da região de recobrimento da ligação química (CBOP) da molécula de hidrogênio através do espalhamento inelástico de elétrons. Foi previsto que o CBOP absorve e espalha eficientemente a radiação eletromagnética acima do limiar de ionização molecular para o caso dos halogenetos de metais alcalinos. Para a molécula de hidrogênio, a natureza quadrupolar da seção de choque do CBOP fornece valores de seção de choque para perda de energia que dependem da energia do feixe de elétrons incidente e um comportamento angular que é totalmente distinto da ionização usual, das transições inter-bandas e dos processos dissociativos. Algumas relações obtidas anteriormente entre o CBOP e a polarizabilidade da região de recobrimento sugerem ser essa grandeza uma ferramenta teórica promissora na quantificação da covalência em ligações químicas
ARTICLE
Electron energy-loss cross sections for the chemical bond overlap plasmon Of the hydrogen molecule
Oscar L. Malta; Renaldo T. Moura Jr.* * e-mail: renaldotmjr@gmail.com ; Ricardo L. Longo
Departamento de Química Fundamental - CCEN, Universidade Federal de Pernambuco, Cidade Universitária, 50740-540 Recife-PE, Brazil
ABSTRACT
We examine the possibility of detecting the chemical bond overlap plasmon (CBOP) of the hydrogen molecule by electron inelastic scattering. The CBOP has been predicted to efficiently absorb and scatter electromagnetic radiation above the molecular ionization threshold in the cases of alkali halides. For the hydrogen molecule the quadrupole nature of the CBOP energy-loss cross section leads to cross section values with impacting electron energy dependence and an angular behavior which are totally distinguishable from the usual ionization, inter-band transitions and dissociation processes. Previously established relationships between the CBOP and the polarizability of the overlap region suggest this an a promising theoretical tool for quantifying covalency in the chemical bond.
Keywords: energy loss, bond overlap plasmon, H2, electron scattering, covalency
RESUMO
Examinamos a possibilidade de detectar o plásmon da região de recobrimento da ligação química (CBOP) da molécula de hidrogênio através do espalhamento inelástico de elétrons. Foi previsto que o CBOP absorve e espalha eficientemente a radiação eletromagnética acima do limiar de ionização molecular para o caso dos halogenetos de metais alcalinos. Para a molécula de hidrogênio, a natureza quadrupolar da seção de choque do CBOP fornece valores de seção de choque para perda de energia que dependem da energia do feixe de elétrons incidente e um comportamento angular que é totalmente distinto da ionização usual, das transições inter-bandas e dos processos dissociativos. Algumas relações obtidas anteriormente entre o CBOP e a polarizabilidade da região de recobrimento sugerem ser essa grandeza uma ferramenta teórica promissora na quantificação da covalência em ligações químicas.
Introduction
In a relatively recent attempt to obtain a deeper insight on covalency in lanthanide compounds, the concepts of chemical bond overlap polarizability (OP) and ionic specific valence (ISV) have been introduced.1 This has allowed the definition of a covalency scale and a description of ligand fields in coordination compounds in terms of a potential that may be treated non phenomenologically when charge factors, appearing in the simple overlap model,2 are identified with the ligating atoms ISV's. As far as each pair lanthanide ion-ligating atom is considered as a diatomic-like molecule, these charge factors are a simple function of the chemical bond force constant, internuclear distance and LUMO-HOMO energy difference.
The OP and ISV concepts have also been explored in a more general context outside the scope of ligand field theory. They have proven to be useful in the case of diatomic molecules, allowing to establish a new covalency scale in excellent agreement with Pauling's scale3,4 and analytically quantifiable in terms of the OP. Relevant questions could be raised on possible relationships between macroscopic properties of materials and these concepts. Thus, for example, a good correlation has been found between the non-linear index of refraction (n2) and the OP.4 More recently, a proposal in which the overlap region is regarded as a localized plasmon-like charge distribution (chemical bond overlap plasmonCBOP), characterized by the OP, has raised the question on the possibility of absorption and inelastic scattering of radiation by the overlap region, above the first ionization threshold.5 Predicted oscillator strengths and scattering cross sections for diatomic molecules are considerably high and can be measured in the UV and near soft-X-rays spectral regions. An interesting aspect is that, formally, oscillation modes corresponding to s and p bonds can be distinguished by these processes, on the grounds of their quite distinct electronic clouds as, for instance, in the carbon monoxide molecule.
In the present work we calculate, within a Born-type approximation, the electron energy-loss cross section for the CBOP of the H2 molecule as a function of the incident electron energy and scattering angle. The aim is to examine how this inelastic scattering cross section compares with the cross section values for processes involving bound-to-bound (inter-band) transitions, ionization and dissociation. An interesting feature of the present model and calculations is that no molecular continuum wave functions are involved.
Results and Discussion
Electron energy-loss cross sections for the CBOP
Chemical bond overlap plasmon
The CBOP theory has been developed in reference 1 as a consequence of the concepts of overlap polarizability and ionic specific valence introduced in reference 1. In this subsection we briefly present the main points of the theory and their application to the case of the H2 molecule.
The overlap polarizability, aOP, is given by1
where
with Sξ,ζ being the overlap integral between orbitals and
and  involved in the formation of a given chemical bond. Distinguishing between σ and πbonds is a matter of taking the appropriate sum over valence shell orbitals in equation 2 for each case. R is the internuclear distance, ΔE is the energy difference between the LUMO and HOMO associated with the chemical bond, and e is the elementary charge.
involved in the formation of a given chemical bond. Distinguishing between σ and πbonds is a matter of taking the appropriate sum over valence shell orbitals in equation 2 for each case. R is the internuclear distance, ΔE is the energy difference between the LUMO and HOMO associated with the chemical bond, and e is the elementary charge.
The overlap charge, q, is postulated to satisfy the following relations:
and
where k is the force constant of the chemical bond and the ionic specific valence, v, is given by
The theory also postulates that the CBOP excitation frequency, ω(), is given by the harmonic oscillator behavior according to
where
and me being the electron mass. Thus, the CBOP may act as an open scattering channel.
Electron energy-loss cross sections
The inelastic scattering process by the CBOP is schematized in Figure 1. An incident electron on the z axis, with wave vector  , hits a target molecule, considered as a spherical system located at the origin of the reference frame, and is scattered with a wave vector
, hits a target molecule, considered as a spherical system located at the origin of the reference frame, and is scattered with a wave vector  '. From the addition theorem for spherical harmonics the interaction Hamiltonian between an incident electron and the CBOP is given by
'. From the addition theorem for spherical harmonics the interaction Hamiltonian between an incident electron and the CBOP is given by
where the position vector  ' stands for the overlap region and
' stands for the overlap region and  stands for the impacting electron. It is assumed that r' < r.
stands for the impacting electron. It is assumed that r' < r.
The scattering cross section, in a unitary interaction volume, per scattering solid angle is given by6
where the total initial and final wave functions are expressed as
and
where  and
and  indicate the initial and final state of the CBOP. Conservation of energy requires that
indicate the initial and final state of the CBOP. Conservation of energy requires that
ħω0 being the CBOP energy.
Since the dipole moment of the excited CBOP vanishes for A2-type molecules, the relevant term in the expansion in equation 8 corresponds to t = 2 (the quadrupole term). The matrix element in equation 9 reduces to
where  =
= 
 ' . Using the partial waves expansion
' . Using the partial waves expansion
where jl(Qr) is a spherical Bessel's function, equation 13 becomes
The orthonormality relation for the spherical harmonics has been used in the above equation. An interesting feature is that the radial integral involving the spherical Bessel's function in this equation is independent of Q, having a value equal to 1/3. Evaluation of the matrix element given by equation 15 is not an easy task due to the difficulty in evaluating the p-spherical component of the quadrupole transition moment of the CBOP. This difficulty can be considerably facilitated if this p-dependent quadrupole transition moment is replaced by an average quadrupole moment of the CBOP, M(2). This approximation is better justified on the basis of the rotational degrees of freedom of the target molecule. Thus, equation 15 may be approximated as
The quadrupole contribution to the differential cross section in equation 9 is then given by
where E is the kinetic energy of the incident electron.
The angular dependence of the r.h.s. of equation 17 is better analyzed if the following relation is used:7
where ( ) is a 3-j symbol. The cross section is totally symmetric with respect to the azimuthal angle
Q. Therefore, the r.h.s. of equation 17 may be replaced by an average over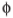 Q and, from equation 18, it is not difficult to see that the summation over the product of spherical harmonics reduces to 5/4π, giving
Q and, from equation 18, it is not difficult to see that the summation over the product of spherical harmonics reduces to 5/4π, giving
The total cross section in a scattering solid angle specified by the position and characteristics of the detection instrument is then given by
The distance from the target molecule to the detection instrument and its aperture define the angles Δ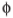 =
= 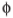 2
2 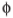 1 , θ1 and θ2. This cross section has a maximum for a scattering angle, θs (between θ1 and θ2), equal to π/2.
1 , θ1 and θ2. This cross section has a maximum for a scattering angle, θs (between θ1 and θ2), equal to π/2.
Numerical estimates
In order to discuss the important features of the cross section given by equation 20 an estimate of the CBOP average quadrupole moment is needed. The quadrupole moment of the H2 molecule has been estimated to be 0.22 Å2.8 Thus, it is reasonable to assume (in cm2) that
The overlap integral between the two 1s orbitals is expressed as,9
for R in a0 and with ζ being the orbital exponent in a01. Using R = 0.74 Å = 1.40 a0 and ζ = 1.0 a01 we find r = 0.753. Considering the force constant, k = 5.1 × 105 dyne cm1,10 and v = 1, the CBOP excitation energy, from equations 6 and 7, is found to be 18 eV, which is ca. 2.5 eV above the ionization threshold of the H2 molecule. This result should be considered as a lower bound value, since if the orbital exponent is optimized,11 at the equilibrium distance, 1.42 a0, it is equal to 1.17 a01, so that r = 0.680 and the CBOP excitation energy becomes 19 eV. In addition, the valence bond treatment can be improved by including the ionic contribution (ψI) to the Heitler-London covalent function (ψC). Indeed, if the covalent and ionic functions are non-orthogonal, the optimized wavefunction at the energy minimum (optimum ζ) is9
If the ionic contribution is orthogonalized to the covalent function, this wavefunction becomes
In any case, there is an ionic contribution to the wavefunction when localized (optimum) atomic orbitals are used. Thus, the ionic specific valence, v, should be smaller than 1.0, which leads to an increase of the CBOP excitation energy. For instance, for non-orthogonal functions the ionic contribution is nearly 5% so that v ca. 0.95 which yields a CBOP excitation energy ca. 19.5 eV. Ascribing ΔE in equation 5 as the first excitation energy of H2, namely ΔE ca. 12.7 eV yields a value of 0.82 for the ionic specific valence, according to equation 5, and thus a value of ca. 21 eV for the CBOP excitation energy. As a result, a good estimate of the CBOP excitation energy would be 20 ± 1 eV. We performed TDHF/aug-ccpVQZ calculations12 for H2 at 0.736 Å and found some highly excited states at 18.9, 20.2 and 24.0 eV. All these states have a Rydberg character, thus having a very distinct electronic density from the CBPO charge density. It is thus expected that, in despite of the CBOP excitation energy being in the energy range of highly excited discrete states they should have very distinct angular dependence in the differential cross section electron energy loss spectra.
The cross section in equation 20 presents interesting and distinguishable features in comparison with electron energy-loss cross sections for usual processes like inter-band transitions, ionization and dissociation. The first aspect to be noted is that the differential cross section (dσ/dΩs) in equation 19 assumes values between 0.023 and 0.071, in units of πa20, for incident electron energies between 20 eV and 1000 eV, respectively. These values are basically of the same order as the ones estimated, both experimentally and theoretically, for the usual processes mentioned above.13,14 They are only a factor of three smaller then the values for ionization at these two energies.14 The first distinguishable aspect to be noted is the energy dependence presented by equation 19. In contrast to the cases of inter-band, ionization and dissociation processes, the CBOP differential cross section presents no maximum; it increases with increasing incident energy and goes asymptotically to a value around 0.07 (in units of πa20), as shown in Figure 2. This behavior is a consequence of the quadrupole nature of the electron scattering process by the CBOP in A2-type molecules.
The second aspect is the angular dependence presented by the total cross section given by equation 20. The sinusoidal angular dependence is predicted to be independent of the incident energy, also in contrast to the inter-band, ionization and dissociation cases. In these cases, both the differential and total cross sections have a maximum between 15 eV and 100 eV, and decrease as the incident energy increases, with angular dependence peaked between 30° and 40°,13 while in the CBOP case it is peaked at 90°. The angular dependence of the total cross section is depicted in Figure 3 for an incident energy equals to 1000 eV, aperture angular resolutions Δ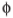 = 5° (fixed) and Δq = 5° θwith q varying from 0° to 180° and plotted as a function of the final angle θ2.
= 5° (fixed) and Δq = 5° θwith q varying from 0° to 180° and plotted as a function of the final angle θ2.
Conclusions
Once the CBOP is directly related to the polarizability of the overlap region, which in turn is directly associated to the degree of covalency, its assessment by means of spectroscopic techniques may constitute a tool for quantifying the sharing of electrons in a chemical bond. In addition to the previously analyzed oscillator strengths and Raman cross sections by the CBOP in heteropolar diatomic molecules, the results obtained in the present work, though for the particular case of the hydrogen molecule, indicate that electron energy-loss spectra may also provide useful information on the CBOP.
The hydrogen molecule, as a case study, has been particularly illustrative due to the quadrupole nature of the CBOP electron energy-loss cross section, which presents an impacting electron energy dependence and angular behavior distinguishable from the scattering cross sections for inter-band transitions, ionization and dissociation.
Acknowledgements
The authors are grateful for the financial support from the CNPq (Brazilian Agency) and from RENAMI and IMMC (Brazilian Scientific Programs).
References
1. Malta, O. L.; Batista, H. J.; Carlos, L. D.; Chem. Phys. 2002, 282, 21.
2. Malta, O. L.; Chem. Phys. Lett. 1982, 87, 27; 1982, 88, 353.
3. Albuquerque, R. Q.; Malta, O. L.; In Nato Science Series II: Math. Phys. Chem., vol. 126, Krupa, J. C.; Kulagin N. A. eds.; Kluwer Academic Publishers: Dordrecht, 2003, p. 141.
4. Albuquerque, R. Q.; PhD Thesis, Universidade Federal de Pernambuco, Brazil, 2004. (http://www.bdtd.ufpe.br/tedeSimplificado//tde_busca/arquivo.php?codArquivo=2539)
5. Malta, O. L.; Chem. Phys. Lett. 2005, 406, 192.
6. Davydov, A. S.; Quantum Mechanics, 2nd ed., Pergamon Press: Oxford, 1976.
7. Edmonds, A. R.; Angular Momentum in Quantum Mechanics, 2nd ed., Princeton University Press: New Jersey, 1960.
8. Carter, C.; March, N. H.; Vincent, D.; Proc. Phys. Soc. (London) 1958, 71, 2.
9. Gallup, G. A.; Valence Bond Methods - Theory and Application, Cambridge University Press: New York, 2002.
10. McQuarrie, D. A.; Simon, J. D.; Physical Chemistry - A Molecular Approach, University Science Books: Sausalito, CA, 1997.
11. Magnasco, V.; J. Chem. Educ. 2008, 85, 1686.
12. Helgaker, T.; Jorgensen, P.; Olsen, J.; Molecular Electronic-Structure Theory, John Wiley & Sons: New York, 2000.
13. Rescigno, T. N.; McCurdy Jr., C. W.; McKoy, V.; Phys. Rev. A: At., Mol., Opt. Phys. 1976, 13, 216.
14. Straube, H. C.; Renault, P.; Lindsay, B. G.; Smith, K. A.; Stebbings, R. F.; Phys. Rev. A: At., Mol., Opt. Phys. 1995, 54, 2146.
Received: September 11, 2009
Web Release Date: March 4, 2010
- 1. Malta, O. L.; Batista, H. J.; Carlos, L. D.; Chem. Phys. 2002, 282, 21.
- 2. Malta, O. L.; Chem. Phys. Lett. 1982, 87, 27; 1982, 88, 353.
- 3. Albuquerque, R. Q.; Malta, O. L.; In Nato Science Series II: Math. Phys. Chem., vol. 126,
- Krupa, J. C.; Kulagin N. A. eds.; Kluwer Academic Publishers: Dordrecht, 2003, p. 141.
- 4. Albuquerque, R. Q.; PhD Thesis, Universidade Federal de Pernambuco, Brazil, 2004. (http://www.bdtd.ufpe.br/tedeSimplificado//tde_busca/arquivo.php?codArquivo=2539)
» link - 5. Malta, O. L.; Chem. Phys. Lett. 2005, 406, 192.
- 6. Davydov, A. S.; Quantum Mechanics, 2nd ed., Pergamon Press: Oxford, 1976.
- 7. Edmonds, A. R.; Angular Momentum in Quantum Mechanics, 2nd ed., Princeton University Press: New Jersey, 1960.
- 8. Carter, C.; March, N. H.; Vincent, D.; Proc. Phys. Soc. (London) 1958, 71, 2.
- 9. Gallup, G. A.; Valence Bond Methods - Theory and Application, Cambridge University Press: New York, 2002.
- 10. McQuarrie, D. A.; Simon, J. D.; Physical Chemistry - A Molecular Approach, University Science Books: Sausalito, CA, 1997.
- 11. Magnasco, V.; J. Chem. Educ. 2008, 85, 1686.
- 12. Helgaker, T.; Jorgensen, P.; Olsen, J.; Molecular Electronic-Structure Theory, John Wiley & Sons: New York, 2000.
- 13. Rescigno, T. N.; McCurdy Jr., C. W.; McKoy, V.; Phys. Rev. A: At., Mol., Opt. Phys. 1976, 13, 216.
- 14. Straube, H. C.; Renault, P.; Lindsay, B. G.; Smith, K. A.; Stebbings, R. F.; Phys. Rev. A: At., Mol., Opt. Phys. 1995, 54, 2146.
Publication Dates
-
Publication in this collection
30 Apr 2010 -
Date of issue
2010
History
-
Received
11 Sept 2009 -
Accepted
04 Mar 2010




























