ABSTRACT
Allophylus edulis and Cupania vernalis (Sapindaceae) are Brazilian native trees used as medicinal plants for the treatment of respiratory, digestive, circulatory, and skin diseases. Ubiquitously distributed in the Brazilian territory, these species are indicated for mixed plantations aimed at the recovery of degraded ecosystems. In this study, the total phenolic content (TPC) and total flavonoid content (TFC), and the antioxidant activity of extracts and fractions obtained from A. edulis and C. vernalis leaves were assessed. The TPC and TFC was determined spectrophotometrically. Antioxidant activity was evaluated through radical scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH). The extracts were obtained by two methods: maceration (method 1) and Soxhlet (method 2). Solvents of increasing polarity (hexane, dichloromethane, ethyl acetate, and n-butanol) were used to obtained the fractions. The results showed that the ethyl acetate fraction from A. edulis, obtained from the maceration method, had the highest TPC (442.0 ± 18.2 mg GAE g-1) and TFC (58.1 ± 0.4 mg RUE g-1), and antioxidant activity (EC50 = 43.6 ± 2.6 µg mL-1). By C. vernalis, superior results were obtained with the n-butanol fraction (TPC = 126.1 ± 5.8 mg GAE g-1, TFC = 37.7 ± 0.6 mg RUE g-1). The highest antioxidant potential was found in the crude hydroalcoholic extract (EC50 = 816.1 ± 50.9 µg mL-1) and butanol fraction (1,156.4 ± 3.8 µg mL-1). The results of this study show that the fractions obtained by maceration and liquid-liquid partition with more polar solvents (ethyl acetate and n-butanol) are the richest in TPC and TFC, and presented the greater antioxidant activity. Comparing the two plants, A. edulis showed the best results, with a high content of TPC, TFC, and antioxidant potential, and therefore may be used to treat diseases related to oxidative stress.
Keywords:
Free radical scavengers; Medicinal plants; Phytochemistry
RESUMO
Allophylus edulis e Cupania vernalis (Sapindaceae) são árvores nativas do Brasil utilizadas como medicinais para o tratamento de doenças do sistema respiratório, digestivo, circulatório e doenças da pele. Com ampla distribuição no território brasileiro, estas espécies são indicadas para plantios mistos destinados à recuperação de ecossistemas degradados. Neste estudo foram investigados o conteúdo de fenólicos totais (TPC), o conteúdo de flavonoides totais (TFC) e a atividade antioxidante de extratos e frações obtidas das folhas de A. edulis e C. vernalis. Foi utilizada metodologia espectrofotométrica para determinar o TPC e o TFC. A atividade antioxidante foi analisada através do método do radical livre 2,2-difenil-1-picrilhidrazila (DPPH). Os extratos foram obtidos por dois métodos: maceração (método 1) e Soxhlet (método 2). Solventes de polaridade crescente (hexano, diclorometano, acetato de etila e n-butanol) foram utilizados para a obtenção das frações. Os resultados mostraram que a fração acetato de etila da espécie A. edulis, obtida por maceração, apresenta o maior TPC (442,0 ± 18,2 mg GAE g-1) e TFC (58,1 ± 0,4 mg RUE g-1) e a maior atividade antioxidante (EC50 = 43,6 ± 2,6 µg mL-1). Em relação à espécie C. vernalis, os melhores resultados foram obtidos com a fração n-butanol (TPC = 126,1 ± 5,8 mg GAE g-1, TFC = 37,7 ± 0,6 mg RUE g-1). Nesta planta, o maior potencial antioxidante foi encontrado no extrato bruto hidroalcoólico (EC50 = 816,1 ± 50,9 µg mL-1) e na fração n-butanol (1.156,4 ± 3,8 µg mL-1). Os resultados deste estudo mostram que as frações obtidas por maceração e partição líquido-líquido com solventes mais polares (acetato de etila e n-butanol) são as mais ricas em TPC e TFC, apresentando também a maior atividade antioxidante. Comparando as duas plantas, A. edulis mostrou os melhores resultados, com um elevado TPC, TFC e potencial antioxidante, sugerindo sua utilização no tratamento de doenças relacionadas ao estresse oxidativo.
Palavras-Chave:
Sequestradores de radicais livres; Plantas medicinais; Fitoquímica
1.INTRODUCTION
Among several Brazilian native plants of medicinal importance, Allophylus edulis and Cupania vernalis (Sapindaceae) have shown potential use in the treatment of respiratory, digestive, circulatory, and skin diseases (Rovedder et al., 2016Rovedder APM, Piazza EM, Thomas PA, Felker RM, Hummel RB, Farias JA. Potential medicinal use of forest species of the Deciduous Seasonal Forest from Atlantic Forest Biome, South Brazil. Brazilian Archives of Biology and Technology. 2016;59:1-11. doi:10.1590/1678-4324-2016150329.
https://doi.org/10.1590/1678-4324-201615...
). Commonly known as “chal-chal, vacum, fruto-de-pombo”, among others, A. edulis is a tree that can reach up to 20 m in height, with a fenestrate trunk of 15 to 30 cm in diameter, and spiral leaves without stipules composed of three leaflets with serrate margins. Flowering occurs from September to November and fruiting from December to March. The flowers are whitish and arranged in short axillary racemes. The species is widely known for its intense flowering and especially for its red fruits with sweet pulp. It occurs from north to south of Brazil - Amazon region, and states of Ceará, Bahia, Mato Grosso, Minas Gerais, Rio de Janeiro and Rio Grande do Sul (Backes and Irgang, 2004Backes P, Irgang B. Mata Atlântica: as árvores e a paisagem. Porto Alegre: Paisagem do Sul, 2004.; Lorenzi, 2016Lorenzi H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. vol. 1. 7th ed. Nova Odessa: Instituto Plantarum; 2016.). It is the species with the largest geographical distribution in Brazil and the main representative of the genus comprising tropical seasonal forest trees, and it also occurs in Argentina, Bolivia, Paraguay, and Uruguay (Coelho, 2014Coelho RLG. Estudos sistemáticos das espécies neotropicais de Allophylus L. (Sapindaceae)[tese]. Campinas:Unicamp; 2014.). Cupania vernalis is known as “Camboatã” or “Camboatá-vermelho”, this is frequently seen in almost every forest formation and found in the states of Minas Gerais, Mato Grosso, and São Paulo, extending as far as Rio Grande do Sul. It may reach up to 22 m in height, has alternate spiral leaves and yellowish flowers. This tree flourishes from March to May, and its fruit ripens from late September to November. Both A. edulis and C. vernalis are indicated for mixed plantations intended for the recovery of degraded ecosystems, and their fruits are much appreciated and eaten by birds and other animals, which are responsible for seed dispersal (Lima Jr. et al., 2006; Lima Junior et al., 2005; Lorenzi, 2016).
Among the pharmacological activities already described for A. edulis, the repellent activity against insects stands out (Castillo et al., 2009Castillo L, González-Coloma A, González A, Díaz M, Santos E, Alonso-Paz E, et al. Screening of Uruguayan plants for deterrent activity against insects. Industrial Crops and Products. 2009;29(1):235-240. doi:10.1016/j.indcrop.2008.05.004.
https://doi.org/10.1016/j.indcrop.2008.0...
; Díaz et al., 2014Díaz M, Castillo L, Díaz CE, Álvarez RG, González-Coloma A, Rossini C. Differential deterrent activity of natural products isolated from Allophylus edulis (Sapindaceae). Advances in Biological Chemistry. 2014; 04:168-179. doi:10.4236/abc.2014.42021.
https://doi.org/10.4236/abc.2014.42021...
). The fruits revealed anticholinesterase and antioxidant activity (Umeo et al., 2011Umeo SH, Ito TM, Yokota ME, Romagnolo MB, Laverde Junior A. Avaliação das propriedades antioxidantes, anticolinesterásicas e citotóxicas dos frutos de Allophylus edulis (A.St.-Hil., Cambess. & A. Juss.) Radlk. (Sapindaceae). Arq Ciênc Saúde Unipar. 2011; 15(2):167-171.), as crude leaf extracts showed antioxidant activity and antimicrobial effect (Tirloni et al., 2015Tirloni CAS, Macorini LFB, Santos UP dos, Rocha P dos S da, Barros SV, Mello AMMF de, et al. Evaluation of the antioxidant activity, antimicrobial effect and acute toxicity from leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess.) Hieron. ex Niederl. African Journal of Pharmacy and Pharmacology. 2015; 9(11):353-362. doi:10.5897/AJPP2015.4270.
https://doi.org/10.5897/AJPP2015.4270...
). The essential oil showed anti-inflammatory, antioxidant, and antimycobacterial activity (Trevizan et al., 2016Trevizan LNF, Nascimento KF do, Santos JA, Kassuya CAL, Cardoso CAL, Vieira M do C, et al. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. Journal of Ethnopharmacology. 2016; 192:510-5. doi:10.1016/j.jep.2016.08.053.
https://doi.org/10.1016/j.jep.2016.08.05...
). The infusion is used, inter alia, to treat diabetes (Díaz et al., 2008). Nine glycosyl flavones with antihepatotoxic activity have been isolated from the leaves of A. edulis and identified (Hoffmann-Bohm et al., 1992Hoffmann-Bohm K, Lotter H, Seligmann O, Walter H. Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis. Planta Medica. 1992; 58(6):544-548. doi:10.1055/s-2006-961546.
https://doi.org/10.1055/s-2006-961546...
). L-quebrachitol has also been isolated from the leaves and shown hypoglycemic activity in rats (Díaz et al., 2008). By C. vernalis, the leaf extracts inhibited nitric oxide production in macrophages, an important inflammatory mediator (Napolitano et al., 2005Napolitano DR, Mineo JR, De Souza MA, De Paula JE, Espindola LS, Espindola FS. Down-modulation of nitric oxide production in murine macrophages treated with crude plant extracts from the Brazilian Cerrado. Journal of Ethnopharmacology. 2005; 99:37-41. doi:10.1016/j.jep.2005.01.059.
https://doi.org/10.1016/j.jep.2005.01.05...
), and showed antiplasmodial activity (Mesquita et al., 2007Mesquita ML, Grellier P, Mambu L, Paula JE, Espindola LS. In vitro antiplasmodial activity of Brazilian Cerrado plants used as traditional remedies. Journal of Ethnopharmacology. 2007; 110:165-170. doi:10.1016/j.jep.2006.09.015.
https://doi.org/10.1016/j.jep.2006.09.01...
). Regarding the chemical composition, a new glucosyl diterpene named vernanolide, with a geranyl-geraniol skeleton, has been isolated. Furthermore, mixtures of sitosterol, stigmasterol, 3-β-D-glucosyl sitosterol, 3-β-D-glucosyl stigmasterol, and coumarin scopoletin have been obtained (Cavalcanti et al., 2001Cavalcanti SBT, Teles HL, Silva DHS, Furlan M, Young MCM, Bolzani VS. New tetra-acetylated oligosaccharide diterpene from Cupania vernalis. Journal of the Brazilian Chemical Society. 2001;12(3):413-6. doi:10.1590/S0103-50532001000300014.
https://doi.org/10.1590/S0103-5053200100...
).
In the past years, free radicals and other oxidants have been held largely responsible for aging and associated degenerative diseases. In living beings, free radical formation is controlled by antioxidant compounds, which may be endogenous in origin or derived from the diet (Suleman et al., 2019). Among naturally occurring antioxidants, phenolic compounds have received much attention. The content of polyphenols in plant extracts has been used as an important antioxidant capacity parameter (Rojas and Buitrago, 2019Rojas J, Buitrago A. Antioxidant activity of phenolic compounds biosynthesized by plants and its relationship with prevention of neurodegenerative diseases. In: Campos MRS, editor. Bioactive Compounds. Sawston: Woodhead Publishing; 2019, p. 3-31. doi:10.1016/B978-0-12-814774-0.00001-3.
https://doi.org/10.1016/B978-0-12-814774...
). Spectrophotometric methods for the analysis of phytochemical content in plant species are often used because of their simplicity and reproducibility. The Folin-Ciocalteu assay is one of the most widely used for the determination of total phenolic content (TPC). Spectrophotometric analyses of total flavonoid content (TFC) are based on aluminum chloride complexation. For evaluation of in vitro antioxidant activity of plant extracts, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical assay is a great alternative. DPPH is an unstable organic nitrogen radical with a purple color. In this method, antioxidants reduce DPPH radicals by donating hydrogen, converting them to DPPH-H. The color changes from purple to yellow, with a decrease in absorbance that allows the determination of antioxidant capacity (Huyut et al., 2017Huyut Z, Beydemir F, Gülçin E. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochemistry Research International. 2017;1-10. doi:10.1155/2017/7616791
https://doi.org/10.1155/2017/7616791...
).
In this work, total phenolic and flavonoid contents and antioxidant activity of Allophylus edulis and Cupania vernalis leaves were investigated. By using two extraction methods and solvents with different polarities, the purpose of this study was also determined the best method and solvent for extraction of phenols, flavonoids and antioxidant activity in the two plants.
2.MATERIAL AND METHODS
2.1 Reagents and equipment
All solvents and reagents used were analytical grade. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical was obtained from Sigma-Aldrich and the Folin-Ciocalteu reagent was obtained from Merck. Gallic acid and rutin (Sigma-Aldrich) were used as standards. A lyophilizer (LS 3000, Terroni), visible spectrophotometer (Kasuaki, II-227), and rotary evaporator (Büchi R-200) were utilized.
2.2 Plant material
Allophylus edulis leaves were collected in July 2017, in Casca, state of Rio Grande do Sul, Brazil (28º33’20.9”S, 51º57’44.2”W), and Cupania vernalis leaves were collected in May 2016, in Rio da Várzea, municipality of Passo Fundo, state of Rio Grande do Sul, Brazil (28º13’24.4”S, 52º29’37.4”W). The plants were identified and deposited in the herbarium of the Universidade de Passo Fundo (voucher numbers RSPF 14385 and RSPF 14166, respectively). The plant material was air-dried at 35 °C for about 48 h and crushed into a fine powder.
2.3 Extraction
The extracts were obtained by two different methods: maceration with subsequent liquid-liquid partition at room temperature (method 1) and exhaustive extraction with a Soxhlet extractor, using a heat source (method 2).
Method 1: The crushed leaves were macerated (ratio of plant material to solvent of 1:10) for eight days with methanol:water (1:1) to obtain the crude hydroalcoholic extract. In a rotary evaporator, the extract was concentrated until methanol elimination and successive liquid-liquid partitions were carried out with solvents of increasing polarity: hexane, dichloromethane, ethyl acetate, and n-butanol. The obtained fractions were concentrated and lyophilized. A portion of the crude hydroalcoholic extract was also dried and lyophilized. The samples obtained were thus coded: ECH (crude hydroalcoholic extract); FH1 (hexane fraction); FEA1 (ethyl acetate fraction); and FB1 (n-butanol fraction).
Method 2: The dried and ground plant material from both plants was packed in a Soxhlet extractor and subjected to exhaustive extraction with hexane, dichloromethane, ethyl acetate, and n-butanol, followed by concentration on a rotary evaporator. Each extraction lasted approximately 10 h. The following samples were then obtained: FH2 (hexane fraction); FD2 (dichloromethane fraction); FEA2 (ethyl acetate fraction); and FB2 (n-butanol fraction).
2.4 Phytochemical screening
General characterization reactions of flavonoids and tannins (polyphenolic compounds) were performed in the samples obtained from the two extraction methods. The presence of tannins was investigated by reaction with 1 % (w/v) ferric chloride solution in ethanol and with 10 % (w/v) aqueous lead acetate solution. The bluish-green color and the reddish-brown precipitate indicate positivity in the assays, respectively. For the detection of flavonoids, the oxalo-boric reaction was used, which is performed with 3 % (w/v) boric acid in ethanol and 10 % (w/v) oxalic acid in ethanol. The observation of green-yellow fluorescence at 365 nm is a positive reaction for flavonoids (Harborne, 1980Harborne JB. Phytochemical Methods. Dordrecht: Springer Netherlands; 1980. https://doi.org/10.1007/978-94-009-5921-7.
https://doi.org/10.1007/978-94-009-5921-...
).
2.5 Total phenolic and flavonoid contents
The TPC of crude extract and fractions was determined by the Folin-Ciocalteu assay (Sousa et al., 2007). The lyophilized samples were diluted in methanol at an accurately weighed final concentration of about 200 µg mL-1. An aliquot of 0.5 mL was transferred to a 10 mL volumetric flask, adding 0.5 mL of Folin-Ciocalteu reagent, 3 to 4 mL of distilled water, and 2 mL of 14 % aqueous sodium carbonate solution, completing the volume with distilled water. After incubation for 2 h at room temperature, absorbance was measured at 750 nm against a blank, prepared with all reagents, except the sample. The total phenolic content was expressed in milligram of gallic acid equivalents (GAE) per gram of dry extract and obtained from a calibration curve of standard gallic acid (5 to 150 µg mL-1). The assay was performed in triplicate.
A spectrophotometric assay based on aluminum complex formation (Schmidt and González-Ortega, 1993Schmidt PC, González-Ortega G. Passionsblumenkraut Bestimmung des Gesamtflavonoidgehaltes von Passiflorae herba. Deutsche Apotheker Zeitung. 1993;133:4457-4466.) was used for TFC analysis. The lyophilized samples were diluted in 70 % methanol at concentrations around 350 µg mL-1 (FBH, FEA1, and FB1), 500 µg mL-1 (FH1), and 1,500 µg mL-1 (FD1). Aliquots of 1 mL of samples were transferred to a 10-mL volumetric flask and mixed with 1 mL of 5 % aluminum trichloride (AlCl3) in methanol and completed with 70 % methanol. A blank without AlCl3 was prepared for all samples. After 10 min, absorbance was read at 425 nm. The TFC in the samples was determined based on the calibration curve of rutin, diluted in methanol 70 % (from 20 to 150 µg mL-1). The results were expressed in milligram of rutin equivalents (RUE) per gram of dry extracts. The assay was performed in triplicate.
2.6 Free-radical scavenging activity
Antioxidant activity was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical assay, following the methodology described by Sousa et al. (2007), with modifications. A stock solution of DPPH was initially prepared in methanol at the concentration of 40 µg mL-1 (0.1 mM). Dilutions of 35, 30, 25, 20, 15, 10, 5, and 1 µg mL-1 were obtained from the solution, and absorbances were read at 516 nm, using methanol as blank. The calibration curve of DPPH was built using the absorbance results. The assay was carried out in triplicate.
Methanol solutions containing different concentrations of lyophilized samples of A. edulis and C. vernalis were tested using DPPH. Aliquots of 0.3 mL of the samples and 2.7 mL of the DPPH stock solution (0.1 mM) were used. The solutions obtained were protected from the light, and absorbances were read at 516 nm, 30 min after addition of DPPH. The mixture of methanol (2.7 mL) and of the highly concentrated methanol solution of each sample (0.3 mL) was used as blank. Methanol (0.3 mL) and DPPH 0.1 mM (2.7 mL) were mixed and used as control. The assays were carried out in triplicate.
The equation obtained from the DPPH calibration curve and the absorbance data read within 30 min were used to determine the concentration of DPPH after reaction with the samples, denoted by [DPPH]30. By also using the initial concentration of DPPH, denoted as [DPPH]o, the percentage of remaining DPPH was estimated by the following equation:
Based on an exponential curve graph between the percentage of remaining DPPH and the concentration of the sample, the effective concentration (in µg mL-1), which is necessary for reduction of the initial concentration of DPPH by 50 % (EC50), within 30 min, was obtained.
2.7 Statistical analysis
The results for FD1, FEA1, and FB1 (method 1) were compared with those obtained for FD2, FEA2, and FB2 (method 2) by Student’s t test. Student’s t test was also used to compare the two plants in samples obtained by the same extraction procedure and solvent regarding TPC, TFC and antioxidant activity. The results for samples obtained from the same plant were compared by one-way analysis of variance (ANOVA), followed by Tukey’s test. Pearson’s correlation coefficient was used to check the correlation among: TPC and TFC; TPC and EC50; TFC and EC50. The significance level was set at 5 %. Data were obtained in triplicate and the results were expressed as mean (n = 3) ± standard deviation (SD).
3.RESULTS
3.1 Phytochemical screening
General tests for the identification of secondary metabolites revealed presence of flavonoids and tannins in the crude extract and more polar fractions of both plants, obtained from extractions performed with ethyl acetate and n-butanol (ECH, FEA1, FEA2, FB1, and FB2 fractions). There was more intense positive reaction to flavonoids in FB1 fraction of C. vernalis. This was the most polar fraction obtained, with extraction at room temperature. The nonpolar fractions, hexane (FH1, FH2) and dichloromethane (FD1, FD2), did not yield positive results in these tests (Table 1).
Results of phytochemical screening for identification of flavonoids and tannins in crude extract and fractions of A. edulis and C. vernalis leaves.
Tabela 1
Resultados da prospecção fitoquímica para identificação de flavonoides e taninos no extrato bruto e frações das folhas de A. edulis e C. vernalis.
3.2 Total phenolic and flavonoid contents
TPC in A. edulis samples obtained by maceration and later liquid-liquid partition (method 1) were compared with those of samples obtained by Soxhlet extraction (method 2). Method 1 proved to be more efficient, with significant differences among samples (Table 2).
Comparison of extraction methods 1 (maceration) and 2 (Soxhlet), regarding total phenolic compounds, expressed as mg of gallic acid equivalents (mg GAE) per gram of dry extract, in A. edulis samples.
Tabela 2
Comparação entre os métodos de extração 1 (maceração) e 2 (Soxhlet) em relação ao conteúdo de fenólicos totais, expressos em mg equivalentes de ácido gálico (mg GAE) por g de extrato seco, em A edulis.
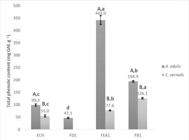
Based on these results, we decided not to proceed with the tests in samples obtained by Soxhlet extraction (method 2) , but only in those obtained by maceration (method 1). From A. edulis samples obtained by method 1, the ethyl acetate fraction (FEA1) had the highest TPC (442.0 ± 18.2 mg GAE g-1), followed by the FB1 fraction (194.9 ± 1.3), the crude extract ECH (99.1 ± 4.4), and the FD1 fraction (47.1 ± 1.2). The hexane fraction (FH1) revealed undetectable phenolic content. Cupania vernalis had higher TPC in FB1 (126.1 ± 5.8 mg GAE g-1), followed by FEA1 (77.6 ± 2.6), and ECH (55.0 ± 2.3). Phenolic content was also undetectable in FH1 and FD1 for this plant. All samples of both plants showed significant differences. TPC was also compared between the two plants in samples obtained with the same solvent. Allophylus edulis revealed a significantly higher TPC in all tested samples (Figure 1).
Total phenolic content, expressed as mg of gallic acid equivalents (mg GAE) per gram of dry extract, in A. edulis and C. vernalis samples obtained from the maceration method followed by liquid-liquid partition (method 1). *Lowercase letters compare the samples in the same plant. Means followed by the same lowercase letter do not diff er signifi cantly by Tukey’s test. Uppercase letters compare the fractions obtained from both plants using the same extraction method and solvent. Means followed by the same uppercase letter do not diff er signifi cantly from each other in the Student’s t test. The signifi cance level was set at 5%. Each value represents the mean of three experiments. The lines in the bars refer to standard deviation. ECH: crude hydroalcoholic extract; FD1: dichloromethane fraction (method 1); FEA1: ethyl acetate fraction (method 1); FB1: n-butanol fraction (method 1).
The analysis of flavonoids revealed that, in A. edulis samples, FEA1 had a higher content (58.1 ± 0.4 mg RUE g-1), followed by FB1 (45.0 ± 0.1), ECH (42.8 ± 0.0), FH1 (36.1 ± 0.4), and FD1 (13.8 ± 0.2). All samples were significantly different in this assay. For C. vernalis, FB1 had a higher TFC (37.7 ± 0.6 mg RUE g-1), thus confirming the result of the phytochemical screening of this group of substances, in which this sample showed a more intense positive reaction. The FH1 fraction (18.4 ± 0.1) and the FD1 fraction (11.5 ± 0.2) showed inferior results. Except for ECH (28.3 ± 0.7) and FEA1 (28.6 ± 0.2), which did not differ significantly, all the other C. vernalis samples revealed differences in the test results. TFC was also compared between the species and A. edulis showed significant higher results than C. vernalis in all tested samples (Figure 2).
Flavonoid content, expressed as mg of rutin equivalents (mg RUT) per gram of dry extract, in A. edulis and C. vernalis samples obtained from the maceration method followed by liquid-liquid partition (method 1). *Lowercase letters compare the samples in the same plant. Means followed by the same lowercase letter do not diff er signifi cantly by Tukey’s test. Uppercase letters compare the samples obtained from both plants using the same extraction method and solvent. Means followed by the same uppercase letter do not diff er signifi cantly from each other in the Student’s t test. The signifi cance level was set at 5%. Each value represents the mean of three experiments. The lines in the bars refer to standard deviation. ECH: crude hydroalcoholic extract; FH1: hexane fraction (method 1); FD1: dichloromethane fraction (method 1); FEA1: ethyl acetate fraction (method 1); FB1: n-butanol fraction (method 1).
3.3 Antioxidant activity
The effective concentration (µg mL-1) necessary to reduce the initial concentration of DPPH free radical by 50 % (EC50) was assessed. Allophylus edulis, when compared to C. vernalis, had already revealed higher TPC and TFC, and it also demonstrated higher antioxidant activity, with significant differences for all tested fractions. This result is corroborated by lower EC50 values in A. edulis samples when compared to C. vernalis. In A. edulis, FEA1 yielded superior results (43.6 ± 2.6 µg mL-1), confirming its potential as antioxidant. Following, FB1 (98.9 ± 3.4) and ECH (134.4 ± 1.2) did not differ statistically. Lastly, FH1 (646.3 ± 5.7) and FD1 (698.7 ± 14.7) showed inferior results. EC50 values were quite high for C. vernalis. ECH and FB1 also did not show any statistically significant differences and had the highest antioxidant potential, with an EC50 of 816.1 ± 50.9 and 1,156.4 ± 3.8 µg mL-1, respectively. Following, the results of FEA1 (1,232.3 ± 142.1) and FD1 (1,382.4 ± 285.7) did not show differences between them. There was significant difference in FH1, with an EC50 of 2,325.8 ± 7.7 µg mL-1 (Figure 3).
Eff ective concentrations (μg mL-1) of A. edulis and C. vernalis samples needed for the initial concentration of DPPH to be reduced by 50% (EC50) within 30 minutes. *Lowercase letters compare the samples in the same plant. Means followed by the same lowercase letter do not diff er signifi cantly by Tukey’s test. Uppercase letters compare the samples obtained from both plants using the same extraction method and solvent. Means followed by the same uppercase letter do not diff er signifi cantly from each other in the Student’s t test. The signifi cance level was set at 5%. Each value represents the mean of three experiments. The lines in the bars refer to standard deviation. ECH: crude hydroalcoholic extract; FH1: hexane fraction (method 1); FD1: dichloromethane fraction (method 1); FEA1: ethyl acetate fraction (method 1); FB1: n-butanol fraction (method 1).
3.4 Correlation between assays
A positive Pearson’s correlation was observed between TPC and TFC, while negative correlations were found between TPC and EC50, TFC and EC50. A statistically significant strong positive correlation was observed between TPC and TFC for C. vernalis (Person’s value = 0.985; p = 0.01). The Pearson’s correlation for this plant was of -0.585 (p = 0.300) between TPC and EC50, and of -0.500 (p = 0.391) between TFC and EC50. In A. edulis samples, Person’s value was of 0.761 for TPC and TFC (p = 0.135), -0.755 for TPC and EC50 (p = 0.140), and -0.855 for TFC and EC50 (p = 0.065).
4.DISCUSSION
This work demonstrated that A. edulis and C. vernalis contain phenolic compounds in their leaves and that their extracts have antioxidant properties. Phenolic compounds extracted from plants have been intensively investigated due to their benefits for human health (Tungmunnithum et al., 2018Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5:93. doi.org/10.3390/medicines5030093.
https://doi.org/10.3390/medicines5030093...
). Several studies have showed that these compounds possess antioxidant activity (Aryal et al., 2019Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants. 2019;8:96. doi.org/10.3390/plants8040096.
https://doi.org/10.3390/plants8040096...
; Dong et al., 2019Dong X, Hu Y, Li Y, Zhou Z. The maturity degree, phenolic compounds and antioxidant activity of Eureka lemon [Citrus limon (L.) Burm. f.]: A negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Scientia Horticulturae. 2019;243:281-289. doi.org/10.1016/j.scienta.2018.08.036.
https://doi.org/10.1016/j.scienta.2018.0...
; Kakouri et al., 2019Kakouri E, Kanakis C, Trigas P, Tarantilis PA. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: study of their total phenolic content and antioxidant activity. Anal Bioanal Chem. 2019;411:3135-50. doi.org/10.1007/s00216-019-01781-7.
https://doi.org/10.1007/s00216-019-01781...
; Sarikurkcu et al., 2020Sarikurkcu C, Andrade JC, Ozer MS, Silva JMF de L, Ceylan O, de Sousa EO, et al. LC-MS/MS profiles and interrelationships between the enzyme inhibition activity, total phenolic content and antioxidant potential of Micromeria nervosa extracts. Food Chemistry. 2020;328:126930. doi.org/10.1016/j.foodchem.2020.126930.
https://doi.org/10.1016/j.foodchem.2020....
). Therefore, it is important to find suitable methods of extraction of these compounds. In the present study, it was observed that the extraction of phenolics by the Soxhlet method, which involves exposing the sample to intense heat, does not provide effective results. The samples obtained by maceration and liquid-liquid partition with ethyl acetate (in A. edulis) and n-butanol (in C. vernalis) are the richest in TPC and TFC, presenting greater antioxidant activity. Some studies evaluated the influence of extraction conditions and solvents on the phenolic content and antioxidant activity in plant extracts. Narimane et al. (2017Narimane S, Demircan E, Salah A, Özçelik B, Salah R. Correlation between antioxidant activity and phenolic acids profile and content of Algerian propolis: Influence of solvent. Pak J Pharm Sci. 2017:8.) reported the impact of using various solvents in the extraction of potentially active compounds from Algerian propolis. The authors conclude that the use of ethyl acetate and n-butanol result in samples with the strongest antioxidant activity and the highest amount of TPC and TFC. Ismail et al. (2019Ismail BB, Pu Y, Guo M, Ma X, Liu D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chemistry. 2019;277:279-88. doi.org/10.1016/j.foodchem.2018.10.056.
https://doi.org/10.1016/j.foodchem.2018....
) analyzed baobab (Adansonia digitata) fruit pulp, and concluded that, in this case, 80 % acetone is the best solvent. Sarikurkcu et al. (2020) worked with ethyl acetate, methanolic, and aqueous extracts from Micromeria nervosa aerial parts, and concluded that the different extracts possess different mechanisms responsible for the antioxidant activity. Array et al. (2018Array EJ, Tonfack Djikeng F, Kingne Kingne F, Kingne EE, Womeni HM. Effect of different extraction solvents on the phenolic content and antioxidant activity of turmeric (Curcuma longa) from South-West Region, Cameroon. Food Res. 2018;3:86-90. http://doi.org/10.26656/fr.2017.3(1).227.
http://doi.org/10.26656/fr.2017.3(1).227...
) worked with Curcuma longa and showed that the alcoholic extracts, obtained at room temperature, exhibited the highest phenolic content and antioxidant activities. Even with variations in the employed methods of extraction, the studies indicate the efficacy of extraction of phenolic compounds at room temperature and with more polar solvents. The results of the phytochemical screening in the present work also revealed the presence of phenolic compounds (flavonoids and tannins) in the fractions obtained with more polar solvents (Table 1).
Phenolic compounds have been described in A. edulis extracts, with isolation of glycosyl flavones and flavonols, among other substances (Hoffmann-Bohm et al., 1992Hoffmann-Bohm K, Lotter H, Seligmann O, Walter H. Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis. Planta Medica. 1992; 58(6):544-548. doi:10.1055/s-2006-961546.
https://doi.org/10.1055/s-2006-961546...
) (Arisawa et al., 1989Arisawa M, Morinaga Y, Nishi Y, Ueno H, Suzuki S, Hayashy T, et al. Chemical and pharmaceutical studies on medicinal plants in Paraguay. Constituents of angiotensin converting enzyme inhibitory fraction from “Cocu”, Allophylus edulis RADLK. The Japanese Society of Pharmacognosy. 1989;43:78-80.). TPC was measured in ethanol crude extract (176 mg GAE g-1) and aqueous extract (90 mg GAE g-1) obtained from the leaves of A. edulis (Tirloni et al., 2015Tirloni CAS, Macorini LFB, Santos UP dos, Rocha P dos S da, Barros SV, Mello AMMF de, et al. Evaluation of the antioxidant activity, antimicrobial effect and acute toxicity from leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess.) Hieron. ex Niederl. African Journal of Pharmacy and Pharmacology. 2015; 9(11):353-362. doi:10.5897/AJPP2015.4270.
https://doi.org/10.5897/AJPP2015.4270...
). In the present study, the results showed a concentration of 99.1 ± 4.4 mg GAE g-1 in ECH (hydroalcoholic crude extract). This extract was used for partitioning with solvents in increasing order of polarity, and fractions with a higher phenolic content were obtained (FEA1 = 442.0 ± 18.2; FB1 = 194.9 ± 1.3 mg GAE g-1) (Figure 1).
By using quercetin as standard, Tirloni et al. (2015Tirloni CAS, Macorini LFB, Santos UP dos, Rocha P dos S da, Barros SV, Mello AMMF de, et al. Evaluation of the antioxidant activity, antimicrobial effect and acute toxicity from leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess.) Hieron. ex Niederl. African Journal of Pharmacy and Pharmacology. 2015; 9(11):353-362. doi:10.5897/AJPP2015.4270.
https://doi.org/10.5897/AJPP2015.4270...
) obtained 20 mg g-1 of flavonoids for the ethanol crude extract, and 10 mg g-1 for the aqueous extract of A. edulis leaves. The flavonoid content obtained in the present study was much higher: 42.8 ± 0.0 mg RUE g-1 in the crude extract (ECH) and 58.1 ± 0.4 mg RUE g-1 in the ethyl acetate fraction (FEA1) (Figure 2). Despite small variations in the technique used, it could be observed, once again, that the choice of the appropriate solvent yielded better results.
Allophylus edulis has also been investigated using the DPPH method. Umeo et al. (2011Umeo SH, Ito TM, Yokota ME, Romagnolo MB, Laverde Junior A. Avaliação das propriedades antioxidantes, anticolinesterásicas e citotóxicas dos frutos de Allophylus edulis (A.St.-Hil., Cambess. & A. Juss.) Radlk. (Sapindaceae). Arq Ciênc Saúde Unipar. 2011; 15(2):167-171.) found an EC50 of 46.7 µg mL-1 in alcoholic extracts of A. edulis fruits. Trevizan et al. (2016Trevizan LNF, Nascimento KF do, Santos JA, Kassuya CAL, Cardoso CAL, Vieira M do C, et al. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. Journal of Ethnopharmacology. 2016; 192:510-5. doi:10.1016/j.jep.2016.08.053.
https://doi.org/10.1016/j.jep.2016.08.05...
) obtained an EC50 of 82.9 µg mL-1 for essential oil of A. edulis, and 74.7 µg mL-1 for viridiflorol, the major constituent of this oil. Tirloni et al. (2015Tirloni CAS, Macorini LFB, Santos UP dos, Rocha P dos S da, Barros SV, Mello AMMF de, et al. Evaluation of the antioxidant activity, antimicrobial effect and acute toxicity from leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess.) Hieron. ex Niederl. African Journal of Pharmacy and Pharmacology. 2015; 9(11):353-362. doi:10.5897/AJPP2015.4270.
https://doi.org/10.5897/AJPP2015.4270...
) found an EC50 of 17.7 and 45.8 µg mL-1 in ethanolic and aqueous crude extracts, respectively, from A. edulis leaves. Our findings for FEA1, with an EC50 of 43.6 ± 2.6 µg mL-1, were similar to those described for this species (Figure 3).
In A. edulis, TPC, TFC, and antioxidant activity were significantly higher in all tested samples when compared to C. vernalis. In the samples obtained from C. vernalis, FB1 showed a significantly higher content of total phenols and flavonoids, followed by ECH (Figure 1 and 2). The highest antioxidant activity in C. vernalis was detected in ECH and FB1, and the lowest one in FH1. The statistical analysis did not show significant difference between ECH and FB1. Also, there was no significant difference between FD1, FEA1, and FB1, but only between FH1 and the other samples. By looking at the result for FH1, the influence of polarity of the solvent used for the extraction of compounds of interest is quite clear. Based on the fact that the mechanism of DPPH reduction is correlated with the presence of hydroxyl groups in the antioxidant molecule, it may be inferred that higher antioxidant capacity is encountered in polar extracts owing to the presence of substances with an available hydroxyl group (Mensor et al., 2001Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research. 2001;15:127-130. doi:10.1002/ptr.687
https://doi.org/10.1002/ptr.687...
). A nonpolar solvent was used to obtain FH1, which exhibited lower antioxidant capacity (Figure 3).
The correlation between TPC and TFC, TPC and EC50, and TFC and EC50 was assessed. A significant Person’s correlation was obtained between TPC and TFC for C. vernalis. Despite the lack of statistical significance, probably due to the small number of repetitions in each sample, the Pearson’s value in all A. edulis samples is considerable. The results indicate the relationship between phenolic content and antioxidant potential of these plant extracts.
5.CONCLUSION
The results of this study show that extraction solvents of different polarities vary significantly in their extraction capacity and selectivity for phenolic and flavonoid contents, as well as in antioxidant activity. The use of maceration with posterior liquid-liquid partition with solvents of increasing polarity (a method of extraction without heat use) is essential for obtaining samples with higher content of these phytochemicals and, consequently, greater antioxidant capacity. The ethyl acetate and n-butanol fractions are the richest in TPC and TFC, and presented the greater antioxidant activity. The comparison between the results obtained with the two plant species showed the antioxidant potential of A. edulis, given its high content of total phenolics and flavonoids. Therefore, these results suggest that A. edulis may be used to treat diseases related to oxidative stress.
7. ACKNOWLEDGMENTS
The authors are grateful to Universidade de Passo Fundo for the granting of scientific initiation scholarships, and to Renato Michel for statistical assistance.
8. REFERENCES
- Arisawa M, Morinaga Y, Nishi Y, Ueno H, Suzuki S, Hayashy T, et al. Chemical and pharmaceutical studies on medicinal plants in Paraguay. Constituents of angiotensin converting enzyme inhibitory fraction from “Cocu”, Allophylus edulis RADLK. The Japanese Society of Pharmacognosy. 1989;43:78-80.
- Array EJ, Tonfack Djikeng F, Kingne Kingne F, Kingne EE, Womeni HM. Effect of different extraction solvents on the phenolic content and antioxidant activity of turmeric (Curcuma longa) from South-West Region, Cameroon. Food Res. 2018;3:86-90. http://doi.org/10.26656/fr.2017.3(1).227
» http://doi.org/10.26656/fr.2017.3(1).227 - Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants. 2019;8:96. doi.org/10.3390/plants8040096.
» https://doi.org/10.3390/plants8040096 - Backes P, Irgang B. Mata Atlântica: as árvores e a paisagem. Porto Alegre: Paisagem do Sul, 2004.
- Castillo L, González-Coloma A, González A, Díaz M, Santos E, Alonso-Paz E, et al. Screening of Uruguayan plants for deterrent activity against insects. Industrial Crops and Products. 2009;29(1):235-240. doi:10.1016/j.indcrop.2008.05.004.
» https://doi.org/10.1016/j.indcrop.2008.05.004 - Cavalcanti SBT, Teles HL, Silva DHS, Furlan M, Young MCM, Bolzani VS. New tetra-acetylated oligosaccharide diterpene from Cupania vernalis Journal of the Brazilian Chemical Society. 2001;12(3):413-6. doi:10.1590/S0103-50532001000300014.
» https://doi.org/10.1590/S0103-50532001000300014 - Coelho RLG. Estudos sistemáticos das espécies neotropicais de Allophylus L. (Sapindaceae)[tese]. Campinas:Unicamp; 2014.
- Díaz M, Castillo L, Díaz CE, Álvarez RG, González-Coloma A, Rossini C. Differential deterrent activity of natural products isolated from Allophylus edulis (Sapindaceae). Advances in Biological Chemistry. 2014; 04:168-179. doi:10.4236/abc.2014.42021.
» https://doi.org/10.4236/abc.2014.42021 - Díaz M, González A, Castro-Gamboa I, Gonzalez D, Rossini C. First record of l-quebrachitol in Allophylus edulis (Sapindaceae). Carbohydrate Research. 2008; 343:2699-700. doi:10.1016/j.carres.2008.07.014.
» https://doi.org/10.1016/j.carres.2008.07.014 - Dong X, Hu Y, Li Y, Zhou Z. The maturity degree, phenolic compounds and antioxidant activity of Eureka lemon [Citrus limon (L.) Burm. f.]: A negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Scientia Horticulturae. 2019;243:281-289. doi.org/10.1016/j.scienta.2018.08.036.
» https://doi.org/10.1016/j.scienta.2018.08.036 - Harborne JB. Phytochemical Methods. Dordrecht: Springer Netherlands; 1980. https://doi.org/10.1007/978-94-009-5921-7
» https://doi.org/10.1007/978-94-009-5921-7 - Hoffmann-Bohm K, Lotter H, Seligmann O, Walter H. Antihepatotoxic C-glycosylflavones from the leaves of Allophyllus edulis var. edulis and gracilis. Planta Medica. 1992; 58(6):544-548. doi:10.1055/s-2006-961546.
» https://doi.org/10.1055/s-2006-961546 - Huyut Z, Beydemir F, Gülçin E. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochemistry Research International. 2017;1-10. doi:10.1155/2017/7616791
» https://doi.org/10.1155/2017/7616791 - Ismail BB, Pu Y, Guo M, Ma X, Liu D. LC-MS/QTOF identification of phytochemicals and the effects of solvents on phenolic constituents and antioxidant activity of baobab (Adansonia digitata) fruit pulp. Food Chemistry. 2019;277:279-88. doi.org/10.1016/j.foodchem.2018.10.056.
» https://doi.org/10.1016/j.foodchem.2018.10.056 - Kakouri E, Kanakis C, Trigas P, Tarantilis PA. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: study of their total phenolic content and antioxidant activity. Anal Bioanal Chem. 2019;411:3135-50. doi.org/10.1007/s00216-019-01781-7.
» https://doi.org/10.1007/s00216-019-01781-7 - Lima Jr É de C, Alvarenga AA de, Castro EM de, Vieira CV, Barbosa JPRAD. Aspectos fisioanatômicos de plantas jovens de Cupania vernalis Camb. submetidas a diferentes níveis de sombramento. Revista Árvore. 2006;30(1):33-41. doi:10.1590/S0100-7622006000100005.
» https://doi.org/10.1590/S0100-7622006000100005 - Lima Junior É de C, Alvarenga AA de, Castro EM de, Vieira CV, Oliveira HM de. Trocas gasosas, características das folhas e crescimento de plantas jovens de Cupania vernalis Camb. submetidas a diferentes níveis de sombreamento. Ciência Rural. 2005;35(5):1092-1097. doi:10.1590/S0103-84782005000500016.
» https://doi.org/10.1590/S0103-84782005000500016 - Lorenzi H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. vol. 1. 7th ed. Nova Odessa: Instituto Plantarum; 2016.
- Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TC, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research. 2001;15:127-130. doi:10.1002/ptr.687
» https://doi.org/10.1002/ptr.687 - Mesquita ML, Grellier P, Mambu L, Paula JE, Espindola LS. In vitro antiplasmodial activity of Brazilian Cerrado plants used as traditional remedies. Journal of Ethnopharmacology. 2007; 110:165-170. doi:10.1016/j.jep.2006.09.015.
» https://doi.org/10.1016/j.jep.2006.09.015 - Napolitano DR, Mineo JR, De Souza MA, De Paula JE, Espindola LS, Espindola FS. Down-modulation of nitric oxide production in murine macrophages treated with crude plant extracts from the Brazilian Cerrado. Journal of Ethnopharmacology. 2005; 99:37-41. doi:10.1016/j.jep.2005.01.059.
» https://doi.org/10.1016/j.jep.2005.01.059 - Narimane S, Demircan E, Salah A, Özçelik B, Salah R. Correlation between antioxidant activity and phenolic acids profile and content of Algerian propolis: Influence of solvent. Pak J Pharm Sci. 2017:8.
- Rojas J, Buitrago A. Antioxidant activity of phenolic compounds biosynthesized by plants and its relationship with prevention of neurodegenerative diseases. In: Campos MRS, editor. Bioactive Compounds. Sawston: Woodhead Publishing; 2019, p. 3-31. doi:10.1016/B978-0-12-814774-0.00001-3.
» https://doi.org/10.1016/B978-0-12-814774-0.00001-3 - Rovedder APM, Piazza EM, Thomas PA, Felker RM, Hummel RB, Farias JA. Potential medicinal use of forest species of the Deciduous Seasonal Forest from Atlantic Forest Biome, South Brazil. Brazilian Archives of Biology and Technology. 2016;59:1-11. doi:10.1590/1678-4324-2016150329.
» https://doi.org/10.1590/1678-4324-2016150329 - Sarikurkcu C, Andrade JC, Ozer MS, Silva JMF de L, Ceylan O, de Sousa EO, et al. LC-MS/MS profiles and interrelationships between the enzyme inhibition activity, total phenolic content and antioxidant potential of Micromeria nervosa extracts. Food Chemistry. 2020;328:126930. doi.org/10.1016/j.foodchem.2020.126930.
» https://doi.org/10.1016/j.foodchem.2020.126930 - Schmidt PC, González-Ortega G. Passionsblumenkraut Bestimmung des Gesamtflavonoidgehaltes von Passiflorae herba. Deutsche Apotheker Zeitung. 1993;133:4457-4466.
- Sousa CM de M, Rocha e Silva H, Vieira-Jr GM, Ayres MCC, Costa CLS da, Araújo DS, et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quimica Nova. 2007;30(2):351-355.
- Suleman M, Khan A, Baqi A, Kakar MS, Samiullah, Ayub M. Antioxidants, its role in preventing free radicals and infectious diseases in human body. Pure and Applied Biology. 2019;8(1):380-388. doi:10.19045/bspab.2018.700197.
» https://doi.org/10.19045/bspab.2018.700197 - Tirloni CAS, Macorini LFB, Santos UP dos, Rocha P dos S da, Barros SV, Mello AMMF de, et al. Evaluation of the antioxidant activity, antimicrobial effect and acute toxicity from leaves of Allophylus edulis (A. St.-Hil., A. Juss. Cambess.) Hieron. ex Niederl. African Journal of Pharmacy and Pharmacology. 2015; 9(11):353-362. doi:10.5897/AJPP2015.4270.
» https://doi.org/10.5897/AJPP2015.4270 - Trevizan LNF, Nascimento KF do, Santos JA, Kassuya CAL, Cardoso CAL, Vieira M do C, et al. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. Journal of Ethnopharmacology. 2016; 192:510-5. doi:10.1016/j.jep.2016.08.053.
» https://doi.org/10.1016/j.jep.2016.08.053 - Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5:93. doi.org/10.3390/medicines5030093.
» https://doi.org/10.3390/medicines5030093 - Umeo SH, Ito TM, Yokota ME, Romagnolo MB, Laverde Junior A. Avaliação das propriedades antioxidantes, anticolinesterásicas e citotóxicas dos frutos de Allophylus edulis (A.St.-Hil., Cambess. & A. Juss.) Radlk. (Sapindaceae). Arq Ciênc Saúde Unipar. 2011; 15(2):167-171.
Publication Dates
-
Publication in this collection
02 July 2021 -
Date of issue
2021
History
-
Received
04 May 2020 -
Accepted
17 Dec 2020