Abstracts
This study identifies the adverse events related to the use of central venous catheters (CVC) in newborns admitted to a neonatal care unit. This is a quantitative, descriptive and retrospective study. The population consisted of 167 newborns admitted in the neonatal unit of the Hospital de Clínicas at Porto Alegre, RS, Brazil which used CVCs inserted through percutaneous puncture (PICC) and surgical insertion, totaling 241 catheters. There was a higher prevalence of mechanical adverse events in the PICC line insertions, with a preponderance of catheter occlusions (19.44%) and ruptures (8.8%). The surgically inserted CVCs had a higher prevalence of catheter-related infectious adverse events with the most common being clinical sepsis (16%). This study suggests that the correct insertion technique should be used and a specialized team should monitor the CVCs to ensure safety and prevent adverse events.
Infant; Newborn; Catheterization; Central Venous; Adverse Effects
O objetivo deste estudo foi identificar os eventos adversos relacionados ao uso de cateteres venosos centrais (CVC), em recém-nascidos internados em unidade neonatal. Trata-se de pesquisa quantitativa, descritiva, retrospectiva. A população foi constituída por 167 neonatos internados na unidade neonatal do Hospital de Clínicas de Porto Alegre que utilizaram CVCs, inseridos por punção percutânea (PICC) e inserção cirúrgica, totalizando 241 cateteres. Nos PICCs houve maior prevalência de eventos adversos mecânicos, predominando a oclusão (19,44%) e a ruptura do cateter (8,8%). Os CVCs por inserção cirúrgica apresentaram maior prevalência dos eventos adversos infecciosos relacionados ao cateter, sendo o mais frequente a sepse clínica (16%). O estudo sugere que, para maior segurança do uso de CVCs, é importante que seja utilizada a técnica correta de inserção do cateter e realizado o acompanhamento dos CVCs por equipe especializada e atenta para a prevenção de eventos adversos.
Recém-Nascido; Cateterismo Venoso Central; Efeitos Adversos
El objetivo de este estudio fue identificar los eventos adversos relacionados con el uso de catéteres venosos centrales (CVC), en recién nacidos internados en una unidad neonatal. Se trata de investigación cuantitativa, descriptiva, retrospectiva. La población fue constituida por 167 neonatos internados en la unidad neonatal del Hospital de Clínicas de Porto Alegre que utilizaron CVCs, inseridos por punción percutánea (PICC) e inserción quirúrgica, totalizando 241 catéteres. En los PICCs hubo mayor incidencia de eventos adversos mecánicos, predominando la oclusión (19,44%) y la ruptura del catéter (8,8%). Los CVCs por inserción quirúrgica presentaron la mayor incidencia de los eventos adversos infecciosos relacionados al catéter, siendo el más frecuente la sepsis clínica (16%). El estudio sugiere que, para mayor seguridad del uso de CVCs, es importante que sea utilizada la técnica correcta de inserción del catéter y realizado el acompañamiento de los CVCs por un equipo especializado y atento a la prevención de eventos adversos.
Recién Nacido; Cateterismo Venoso Central; Efectos Adversos
ORIGINAL ARTICLE
Adverse Events Related to the Use of Central Venous Catheters in Hospitalized Newborns
Alessandra Tomazi FranceschiI; Maria Luzia Chollopetz da CunhaII
IRN, Resident nurse in Intensive Care, Grupo Hospitalar Conceição, RS, Brazil. E-mail: alefranceschi@gmail.com
IIAdjunct Professor, Escola de Enfermagem, Universidade Federal do Rio Grande do Sul, RS, Brazil. E-mail: luzia@adufrgs.ufrgs.br
Corresponding Author Corresponding Author: Maria Luzia Chollopetz da Cunha Universidade Federal do Rio Grande do Sul Rua São Manoel, 963 Rio Branco CEP: 90620-110 Porto Alegre, RS, Brasil E-mail: luzia@adufrgs.ufrgs.br
ABSTRACT
This study identifies the adverse events related to the use of central venous catheters (CVC) in newborns admitted to a neonatal care unit. This is a quantitative, descriptive and retrospective study. The population consisted of 167 newborns admitted in the neonatal unit of the Hospital de Clínicas at Porto Alegre, RS, Brazil which used CVCs inserted through percutaneous puncture (PICC) and surgical insertion, totaling 241 catheters. There was a higher prevalence of mechanical adverse events in the PICC line insertions, with a preponderance of catheter occlusions (19.44%) and ruptures (8.8%). The surgically inserted CVCs had a higher prevalence of catheter-related infectious adverse events with the most common being clinical sepsis (16%). This study suggests that the correct insertion technique should be used and a specialized team should monitor the CVCs to ensure safety and prevent adverse events.
Descriptors: Infant, Newborn; Catheterization, Central Venous / Adverse Effects.
Introduction
CVC is a common practice in Neonatal Intensive Care Units (NICU) and provides safe vascular access to newborns, though it is not an innocuous procedure and oftentimes is associated with adverse events(1).
CVCs might be tunneled, non-tunneled, Peripherally Inserted Central Catheters (PICC) or totally implantable(2). The catheters most used in neonatology are non-tunneled and PICCs. Specialized nurses can practice PICC line insertion at bedside through the percutaneous puncture of peripheral veins. The insertion of catheters through the percutaneous puncture of the large veins of the neck and thorax and the insertion of catheters through phlebotomy can only be performed by surgeons.
An adverse event is currently defined as a non-intentional lesion that results in temporary or permanent incapacity and/or extended time of hospitalization or death as a consequence of delivered care(3).
Adverse events related to the use of CVC are split into infectious adverse events, mechanical adverse events and thrombosis. According to the literature, mechanical adverse events occur in 5 to 19% of patients with a CVC, infectious adverse events in 5 to 26% and thrombosis in 2 to 26%(4).
Even with the possibility of the occurrence of adverse events, the use of CVCs should not be discarded because the survival of many newborns depends on their use. The decision to insert a CVC includes the consideration of risks and benefits.
Procedures to identify adverse events by the healthcare facility are the first step in constructing a care system designed to prevent errors. The American Academy of Pediatrics recommends identifying errors and studying their patterns of occurrence so as to diminish the chances of adverse events occurring(5).
One study suggests that indicators of results such as adverse events are essential tools to measure quality because they indicate aspects of care that can be improved and make care delivery safer for patients(6).
Realizing that the use of CVCs is essential for the survival of most of the newborns hospitalized in NICUs and that the occurrence of adverse events in this population can lead to major and irreversible consequences due to the newborns' fragility, this study identified the adverse events related to the use of CVCs in newborns hospitalized in NICUs.
Methods
This quantitative, descriptive and retrospective study was carried out through the search of the medical records of patients hospitalized in the NICU of the Hospital das Clinicas at Porto Alegre, RS, Brazil between January and December 2007. The population was composed of newborns admitted into the NICU of the Hospital das Clinicas at Porto Alegre between January 1st and December 31st 2007, who received a CVC. The participants were selected as an intentional convenience sample(7). All newborns who received a CVC through percutaneous puncture or surgical insertion in 2007 were included in the study. The exclusion criteria were: venous catheters inserted by incision in the navel vein, CVCs inserted in hospitalization units other than the neonatal unit and/or other hospital facility, and central venous catheters not removed by the team from the neonatal unit. This situation occurs when a newborn is transferred with the catheter from the neonatal unit/Hospital das Clinicas of Porto Alegre to another hospitalization unit or facility.
Participants were searched out through the numbers of medical records found in the forms of patients receiving CVCs. These forms are filled out by nurses. A sample of 167 newborns, totaling 241 catheters, was used. Data were collected from patients' medical records. The researcher herself collected information using an instrument. This study was supported by the Research Incentive Fund of the Hospital de Clínicas de Porto Alegre (FIPE/HCPA).
The following definitions were adopted regarding the type of CVC used:
- Peripherally Inserted Central Catheter (PICC), catheter inserted through the percutaneous puncture of a peripheral vessel.
- Central Venous Catheter through surgical insertion (CVCSI), catheter inserted through surgical incision or the puncture of a central vein (subclavian, jugular or femoral) by a surgeon.
Statistical Analysis
Data were analyzed through descriptive statistics. The categorical variables were described by absolute frequency and relative percentage; the symmetrical quantitative variables were described by the median and standard error and the asymmetric variables by the median and inter-quantile range.
Ethical aspects
Ethical standards were complied with through the use of consent forms authorizing the use of data. The consent form establishes that the project's researchers are required to maintain the privacy of patients whose data were collected from medical records and databases of the HCPA. It also states that data are solely and exclusively used for the development of this research project.
The Project was approved by the Ethics Research Committee at the Federal University of Rio Grande do Sul (COMPESQ/EEUFRGS) and at the Hospital de Clínicas de Porto Alegre (GPPG/HCPA).
Results
Data from all newborns admitted into the NICU at HCPA in 2007 and who received central venous catheters surgically inserted or by percutaneous puncture were used.
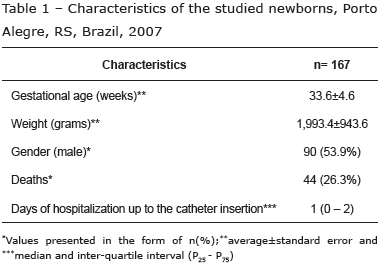
The sample was composed of 167 newborns: 35 newborns received two catheters, five received three catheters, seven received four catheters and two newborns received five catheters, totaling 241 inserted catheters. Regarding the characteristics of the newborns, the average gestational age was 33.6 (±4,6) weeks and most of the infants were male (53.9%) (Table 1).
The CVCs were analyzed according to the type of insertion, PICC or CVCSI: 216 PICCs and 25 CVCSIs were inserted. In relation to the CVCSI used: 21 double lumen catheters were inserted by phlebotomy, three double lumen catheters were inserted by percutaneous puncture and one monolumen catheter was inserted by percutaneous puncture. Of the 241 inserted catheters (PICC and CVCSI), 118 presented adverse events: 103 PICCs and 15 CVCSI.
Infectious adverse events are presented in this study in three categories: sepsis with positive blood culture, clinical sepsis and suspected infection. We considered sepsis with positive blood cultures those with laboratory confirmation and those patients who were treated with antibiotics. Clinical sepsis were those that presented clinical signs of sepsis but had no laboratory confirmation and newborns were treated with antibiotics. Suspected infection was considered in cases with no laboratory confirmation in which patients were not treated with antibiotics.
The most prevalent adverse event in newborns who received PICC was catheter occlusion, present in 19.44% (n=42) of the PICCs. Newborns who received CVCSI did not present any cases of catheter occlusion. The most prevalent adverse event in these patients was clinical sepsis, present in 16% (n=4) of the catheters (Table 2).
Of the 44 studied newborns who died, 19 had a PICC and none of them had CVCSI. The deaths of these newborns were investigated and none were associated with the presence of a catheter.
Discussion
The catheter's intraluminal occlusion may occur due to blood clot or by the formation of fibrin that results from the presence of blood in the catheter after inappropriate washing of the catheter or retrograde flow; occlusion might have other origins not related to thrombosis, such as precipitate minerals from infused solutions or incompatible drugs(2). Among the studied catheters, the rate of occlusion of PICCs was 19.44% (n=42), a rate similar to that found in the literature. One study carried out with 135 catheters, in patients in a neonatology unit, reveals a total of 22.9% (n=31) of catheters developed occlusions, similar to these findings(8).
In order to prevent intraluminal occlusion, the use of phenytoin and diazepam in a PICC is not recommended because crystals form inside the catheter during their infusion(2). Other non-recommended actions are: the infusion of blood products, due to the risk of hemolysis and obstruction(9); collecting blood through the catheter because there is a risk of the catheter's walls collapsing during reflux due to its small caliber. (An exception is PICCs with a Groshong valve.) Some authors report the use of urokinase 5000iu/ml or tissue plasminogen activator to unblock catheters occluded by blood clots(10-11). However, the use of these solutions in neonatology has to be evaluated because none of these studies was carried out with this newborns.
Unlike the PICCs, CVCSIs did not present any cases of occlusion. This might be related to the larger caliber of the CVCSI (Fr 4) in relation to the PICCs (Fr 1.2 or 1.19). In addition to the small caliber used in neonatology, the PICC also travels a greater distance into the venous network, which can leave the patient susceptible to mechanical obstruction due to folds in the catheter.
Data in the literature reports a 4 to 5% frequency of rupture, which occurred in 19 (8.8%) of the studied newborns receiving PICCs. No rupture was reported in CVCSIs(8,12). Ruptures in PICCs are associated with poorly handling the catheter and infusing with too much intraluminal pressure(2). In this study, all ruptures occurred at the catheter's point of insertion; catheter rupture may become an adverse event if it occurs in the bloodstream. To avoid catheter rupture, it is recommended not to use force to infuse any solution or to use syringes smaller than 10ml to infuse fluids; syringes smaller than this caliber have an infusion pressure higher than that supported by PICCs(9,12).
Contamination of central venous catheters may occur through direct invasion of microorganisms extant on the skin or at the catheter's point of insertion due to inappropriate handling of parenteral solutions and the catheter's connections or endogenous contamination(13). The cases of catheter-related sepsis fall in the incidence of late onset sepsis. Late onset sepsis has a nosocomial origin and occurs within 48 hours of the newborn's life(14). The criteria used in this study to determine catheter-related infection is similar to another study found in the literature(15). The infectious events were split into three categories: sepsis with positive blood cultures, clinical sepsis and suspected infection.
Twenty PICCs were removed due to infectious causes: five with positive blood cultures, nine due to clinical sepsis and six due to suspected infection. Of these 20 catheters, four (1.9%) presented a positive culture of the catheter's tip. In relation to CVCSI, eight were removed due to infectious causes (three with a positive blood culture, four due to clinical sepsis and one due to suspected infection). Of these eight catheters, seven (28%) presented a positive culture of the catheter's tip.
One study comparing the use of CVCSI and PICC in adults obtained findings similar to those found in this study. The study found a total of six (21%) PICCs removed due to infection, of which only half were confirmed to be catheter-related infections. In the cases of CVCSIs, on the other hand, all the 21 catheters removed due to catheter-related suspected infection were later confirmed cases of infection(10).
Another study presents the experience of a healthcare facility that inserted 135 PICCs into newborns over a given period. Only three (2.2%) catheters out of 135 PICCs were presented as sources of infection(8).
One study reports that the incident rate of PICC-related sepsis is between 2 and 21%. This study suggests that the lower incidence of infection in PICCs, when compared to other CVCs, might be related to the low concentration of bacteria in peripheral areas (50 to 100 colonies of bacteria per cm2 of skin) when compared to the thorax (1,000 to 10,000 colonies of bacteria per cm2 of skin)(12). PICCs are rarely inserted in the thoracic region. Sometimes the axillary vein is used to insert catheters, though this is the last choice for the insertion of PICCs. In this study, only 11 PICCs (5%) were inserted in this vein and only one was removed due to sepsis.
The literature shows that there are microorganisms more prevalent in catheter-related primary sepsis. The gram-positive cocci are responsible for 65% of infections, while the most prevalent are the Staphylococcus epidermidis (31%) and the Staphylococcus aureus (14%). The gram-negative bacilli account for 30% of infections and the most prevalent are the Pseudomonas sp (7%) and the Escherichia coli (6%). Infection by Candida SP is responsible for the remaining 5% of catheter-related infections. However, the most frequent microorganism isolated in cultures in this study was the Staphylococcus sp coagulase negative(13). Here, four positive cultures of catheter tips in PICCs presented colonization by the Staphylococcus sp coagulase negative. In the CVCSI, of the seven positive cultures of catheter tips, six were colonized by Staphylococcus sp coagulase negative and one by multiple microbiota.
To avoid contaminating central venous catheters, several measures should be implemented in their insertion and maintenance. Central catheter insertion, whether it is a PICC or a CVCSI, should be aseptic and include measures of barrier precaution such as wearing a cap, mask, sterile gown, sterile gloves and drapes. It is recommended to wash hands with chlorhexidine detergent or alcohol gel before and after contact with the catheter during CVC maintenance. The dressing has to be changed every seven days or when it is wet or for other reasons taken off, change taps, equipment and extensions every 72 hours and the equipment for parenteral nutrition should be changed every 24 hours, always swabbing the connections and taps of the catheter with 10% concentration of alcohol before handling them(9).
The literature describes the experience of a hospital where the rates of PICC-related infection significantly decreased after the implementation of a "PICC maintenance team". This team, composed of a neonatologist (fellow), who follows-up preterm newborns, and two nurses, is responsible for proactive care through the daily inspection of catheters and has the autonomous authority to remove or retract it. This protocol diminished the rates of PICC-related infections from 25 to 7.1% in preterm newborns in the facility(15).
Venous thrombosis occurs due to the continuous contact of the catheter with the endocardium, in the case of CVCSI, or endothelium, in the case of PICCs, which causes irritation, inflammation and the formation of thrombi. The prolonged presence of a CVC can cause blood clots in up to 70% of cases. Several factors might influence the incidence of thrombosis associated with CVCs, including composition, size, duration and number of entrances(16-17). One study revealed that the incidence of venous thrombosis in PICCs varies between 4 and 38%. The factors that lead to the formation of a clot are trauma to the endothelial wall, interruption of therapy for a long period of time, reflux of blood through the catheter, and slow infusion. The incidence of thrombosis also increases as the diameter of catheters increases(12).
The occurrence of venous thrombosis was observed only in CVCSIs in this study. The occurrence of all these events was related to the prolonged duration of the CVC: one event occurred on the 14th day of use, another on the 22nd day and the third event occurred on the 23rd day of the catheter's use.
Infiltration is the accumulation of non-irritating substances infused into the tissue surrounding the vein due to the displacement of the catheter from the vein's intima to the subcutaneous tissue. Infiltration of vesicant solution is called extravasation(2). Studies present a frequency of infiltration equivalent to 3.7% (n=5) in PICCs and 3.8% (n=14) in CVCICs(8,18). This frequency is less than that presented in this study, which found a frequency of infiltration of 5.09% (n=11) for PICCs and 12% (n=3) for CVCs.
Several events caused by the poor positioning of the catheter tip are described in the literature such as: pneumothorax, hydrothorax, hemothorax, hydro mediastinum, arteriovenous fistula, perforation and cardiac tamponade, among others(9,17). No adverse events occurred in this study due to poor catheter positioning. Such a fact is related to the intravenous therapy occurring after analysis of the first X-ray in which the need to retract the catheter or remove it due to inappropriate positioning is evaluated. The ideal positioning of the PICC is in the vena cava in the distal third of the thorax level and the CVCSI has to be placed between the vena cava and the right atrium(17).
A limitation was identified in this study. Because this is a retrospective study, the findings were based on medical records and were limited by access to information such as radiographic images and reports of the catheters' X-rays.
Final considerations
Adverse events in central catheters were frequent in neonatal populations, both for PICCs as in CVCSIs. The most prevalent adverse event in PICCs was catheter occlusion, while clinical sepsis prevailed in CVCSIs.
PICCs presented a higher frequency of mechanical adverse events, especially catheter occlusion and rupture. However, its use presented very low rates of catheter-related infections; these rates are similar or less than those reported in the literature. Therefore, we assert that PICC is a safe means for parenteral administration in the neonatal population due to the low risk of infection found in this study and in the literature. The use of CVCSI resulted in a lower rate of mechanical adverse events: occlusions or ruptures were not found for this catheter in this study. However, the rates of infectious adverse events related to this catheter are the most prevalent.
This study's findings reveal the need for further studies to evaluate the factors associated with the occurrence of sepsis in CVCSIs. In relation to PICCs, further research is suggested in order to investigate the factors associated with catheter occlusion. Studies identifying the factors that predispose PICCs to such adverse event can contribute to a safer use of PICCs.
The use of CVCs is essential to the survival of many newborns. We expect the results of this study to encourage the analysis of the patterns of occurrence of adverse events. We also assert the importance of a specialized and attentive team for the follow-up of CVCs in preventing such events.
Received: Jan. 21st 2009
Accepted: Nov. 16th 2009
- 1. Menon G. Neonatal long lines. Arch Dis Child Fetal Neonatal. 2003 July; 88:F260-2.
- 2. Phillips LD. Cateteres de acesso venoso central. In: Phillips LD. Manual de Terapia Intravenosa. Porto Alegre (RS): Artmed; 2001. p. 334-72.
- 3. Mendes W, Travassos C, Martins M, Noronha J. Revisão dos estudos de avaliação da ocorrência de eventos adversos em hospitais. Rev Bras Epidemiol 2005 dezembro; 8(4):393-406.
- 4. Mcgee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003 March; 348:1123-33.
- 5. Lannon CM, Coven BJ, France FL, Hickson GB, Miles PV, Swanson JT, et al. Principles of patient safety in pediatrics. Pediatrics 2001 June; 107(6):1473-4.
- 6. Nascimento CCP, Toffoletto MC, Gonçalves LA, Freitas WG, Padilha KG. Indicadores de resultados da assistência: análise dos eventos adversos durante a internação hospitalar. Rev Latino-am Enfermagem 2008 julho-agosto; 16(4):746-51.
- 7. Hulley SB, Newman TB, Cummings SR. Escolhendo os sujeitos do estudo: especificação, amostragem e recrutamento. In: Hulley SB. Delineando a pesquisa clínica: uma abordagem epidemiológica.Porto Alegre (RS): Artmed; 2003. p. 374.
- 8. Lourenço AS, Kakehashi TY. Avaliação da implantação do cateter venoso central de inserção periférica em neonatologia. Acta Paul Enferm 2003 abril; 16(2):26-32.
- 9. Secretaria do Estado do Rio de Janeiro. Rotina para cateter venoso central de inserção periférica em neonatos. Rio de Janeiro (RJ): Secretaria do Estado do Rio de Janeiro; 2002.
- 10. Griffiths VR, Philpot P. Peripherally inserted central catheters (PICCs): do they have a role in the care of the critically ill patient? Intensive Crit Care Nurs. 2002 February; 18:37-47.
- 11. Fetzer SJ, Manning GPD. Safety and efficacy of the POP technique for restoring patency to occluded PIC catheters. Applied Nurs Res. 2004 November; 17(4):297-300.
- 12. Jesus VC, Secoli SR. Complicações acerca do cateter venoso central de inserção periférica (PICC). Ciência, Cuidado e Saúde. 2007 abril; 6(2):252-60.
- 13. Basile-Filho A, Castro PTO, Pereira Júnior GA, Marson F, Mattar L Júnior, Costa JC. Sepse primária relacionada ao cateter venoso central. Medicina (Ribeirão Preto) 1998 julho; 31:363-8.
- 14. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD neonatal research network. Pediatrics. 2002 August; 110(2):285-91.
- 15. Golombek SG, Rohan AJ, Parvez B, Salice AL, LaGamma EF. Proactive management of percutaneously inserted central catheters results in decreased incidence of infection in the ELBW population. J Perinatol. 2002 April; 22(3):209-13.
- 16. Doria S, Noguchi DT, Paccez JP. Trombose venosa profunda na faixa etária pediátrica. RBTI - Rev Bras Terapia Intensiva 2001 abril; 13(1):15-20.
- 17. Tannuris U. Acessos vasculares. In: Piva JP. Terapia intensiva pediátrica. São Paulo (SP): Atheneu; 2006. p. 1563-87.
- 18. Karapinar B, Cura A. Complications of central venous catheterization in critically ill children. Pediatr Int 2007 October; 49(5):593-9.
Corresponding Author:
Publication Dates
-
Publication in this collection
16 June 2010 -
Date of issue
Apr 2010
History
-
Received
21 Jan 2009 -
Accepted
16 Nov 2009