Abstracts
In order to establish cut-off limits and to distinguish isolated premature thelarche (IPT) from precocious puberty (PP), we evaluated data from 79 girls with premature thelarche, comparing basal and stimulated LH and FSH serum concentrations with those from 91 healthy girls. A GnRH stimulation test was performed in 10 normal girls and in 42 with premature thelarche. Comparison among groups was performed by Kruskal-Wallis and Dunn’s tests. LH values were significantly greater in girls with IPT than in control groups. Basal gonadotropin concentrations were higher in patients with PP than in controls, but not different from patients with IPT. Peak LH levels after GnRH stimulation distinguished those two groups, with a cut-off value of 4.0 IU/L, but still with minimal overlap. In conclusion, a girl with premature thelarche and LH peak value above 4.5 IU/L has, indeed, PP, but values between 3.5 and 4.5 IU/L point to careful follow-up.
Premature thelarche; GnRH test; Laboratorial assessment; Immunochemiluminescent assay
Com o objetivo de estabelecer o valor de corte e distinguir telarca precoce isolada (TPI) de puberdade precoce (PP), avaliamos 79 meninas com telarca precoce, comparando as dosagens basais e pós-estímulo de LH e FSH com grupo-controle. O teste de estímulo com GnRH foi realizado em 10 meninas normais e em 42 com telarca precoce. Os testes de Kruskal-Wallis and Dunn foram usados na comparação dos grupos. Os níveis de LH foram significativamente mais elevados no grupo com TPI, quando comparados com controles. As gonadotrofinas basais foram mais elevadas naquelas com PP que nos controles, mas não diferiram do grupo com TPI. O pico de LH após GnRH distinguiu estes dois grupos, com valor de corte de 4,0 UI/L, apesar de pequena sobreposição. Concluímos que uma menina com telarca precoce e LH pós-estímulo acima de 4,5 UI/L apresenta PP, mas valores entre 3,5 e 4,5 UI/L requerem seguimento cuidadoso.
Telarca precoce; Teste de estímulo do GnRH; Avaliação laboratorial; Ensaio imunoquimioluminescente
ARTIGO ORIGINAL
Premature thelarche: clinical and laboratorial assessment by immunochemiluminescent assay
Telarca precoce: avaliação clínica e laboratorial pelo método imunoquimioluminescente
Maria F. Borges; Kátia D. Pacheco; Andréia A. Oliveira; Cláudia V. C. Rita; Karla D. Pacheco; Elisabete A. M. Resende; Beatriz H. J. Lara; Beatriz P. Ferreira
Disciplina de Endocrinologia [Endocrinology Department], Faculdade de Medicina da Universidade Federal do Triângulo Mineiro, Uberaba, MG, Brasil
Address for correspondence Address for correspondence: Maria de Fátima Borges Hospital Escola da Faculdade de Medicina da Universidade Federal do Triângulo Mineiro Departamento de Clínica Médica Rua Getúlio Guaritá, S/N, Bairro Abadia 38025-440 Uberaba, MG, Brazil E-mail: borgmf@uol.com.br
ABSTRACT
In order to establish cut-off limits and to distinguish isolated premature thelarche (IPT) from precocious puberty (PP), we evaluated data from 79 girls with premature thelarche, comparing basal and stimulated LH and FSH serum concentrations with those from 91 healthy girls. A GnRH stimulation test was performed in 10 normal girls and in 42 with premature thelarche. Comparison among groups was performed by Kruskal-Wallis and Dunns tests. LH values were significantly greater in girls with IPT than in control groups. Basal gonadotropin concentrations were higher in patients with PP than in controls, but not different from patients with IPT. Peak LH levels after GnRH stimulation distinguished those two groups, with a cut-off value of 4.0 IU/L, but still with minimal overlap. In conclusion, a girl with premature thelarche and LH peak value above 4.5 IU/L has, indeed, PP, but values between 3.5 and 4.5 IU/L point to careful follow-up.
Keywords: Premature thelarche; GnRH test; Laboratorial assessment; Immunochemiluminescent assay
RESUMO
Com o objetivo de estabelecer o valor de corte e distinguir telarca precoce isolada (TPI) de puberdade precoce (PP), avaliamos 79 meninas com telarca precoce, comparando as dosagens basais e pós-estímulo de LH e FSH com grupo-controle. O teste de estímulo com GnRH foi realizado em 10 meninas normais e em 42 com telarca precoce. Os testes de Kruskal-Wallis and Dunn foram usados na comparação dos grupos. Os níveis de LH foram significativamente mais elevados no grupo com TPI, quando comparados com controles. As gonadotrofinas basais foram mais elevadas naquelas com PP que nos controles, mas não diferiram do grupo com TPI. O pico de LH após GnRH distinguiu estes dois grupos, com valor de corte de 4,0 UI/L, apesar de pequena sobreposição. Concluímos que uma menina com telarca precoce e LH pós-estímulo acima de 4,5 UI/L apresenta PP, mas valores entre 3,5 e 4,5 UI/L requerem seguimento cuidadoso.
Descritores: Telarca precoce; Teste de estímulo do GnRH; Avaliação laboratorial; Ensaio imunoquimioluminescente
INTRODUCTION
PREMATURE THELARCHE (PT) is a clinical condition characterized by isolated breast development in girls before 8 years of age. In the absence of other clinical signs of sexual maturation, it has been considered as a variant of normal development, and it is more prevalent during the first two years of life when the hypothalamic-pituitary-gonadal (HPG) axis has not been suppressed yet (1-3).
The pathophysiological mechanisms that cause PT are unknown and have been hypothesized to result from 1) increased breast sensitivity to estrogen (4,5); 2) increased estradiol (E2) levels (6); 3) transient estrogen secretion by follicular ovarian cysts (4); 4) increased estrogen production from adrenal precursors (7); 5) increased dietary estrogen (8); 6) transient partial activation of the HPG axis with predominant FSH secretion (5,9-12); 7) increased serum SHBG which could modify the ratio of bioavailable testosterone to estrogen, producing a relative increment in free E2 (13).
Thus, it is possible that more than one of these proposed mechanisms is involved in the pathophysiology of isolated premature thelarche (IPT). New methodologies applied to commercial LH and FSH assays improved the differential diagnosis between IPT and gonadotropin-dependent precocious puberty (GDPP) or even between variants of precocious sexual maturation in girls that have some of the clinical features of PT and yet some of the features of GDPP (12,14-16).
The aim of this study is to establish cut-off limits and to distinguish IPT from precocious puberty (PP). We evaluated 79 girls comparing basal and stimulated LH and FSH serum concentrations and E2 levels with a normal control group seen at the Endocrine Pediatric Unit between 1996 and 2005.
SUBJECTS AN METHODS
Subjects
The research protocol was approved by the Ethics Committee of the Faculdade de Medicina da Universidade Federal do Triângulo Mineiro, Uberaba, (Brazil). Written consent to participate in this study was obtained from the subjects in the healthy control group and their parents.
We compared, retrospectively, data from 79 records of girls presenting PT with 91 healthy pre-pubertal girls.
Patients were distributed into two groups (Figure 1):
a) Isolated premature thelarche (IPT) 58 girls with breast enlargement and absence of other signs of pubertal development were divided into two subgroups according to the age at onset of thelarche: 40 girls ages 0.9-2.9 yr (IPT1) and 18 girls between 3 and 8 years of age (IPT2).
b) Precocious puberty (PP) 21 girls with PT were selected and divided into two subgroups according to the time of activation of HPG axis: 9 girls ages 1.5-7.7 yr initially presented HPG axis inactivated (phase I-PP1,), but during the follow-up they developed pubarche, acceleration of growth velocity, and a second GnRH test disclosed an active HPG axis and was considered as phase II-PP1 group. The remaining 12 girls, ages 6.7-7.9 yr, were suspected to have precocious puberty by clinical signs, and GnRH stimulation test indicated an active HPG axis (PP2).
The control group included children without abnormalities of pubertal development and consisted of three subgroups: 1) C1: 25 girls under 3 years of age (0.11-2.3yr) that had basal LH, FSH and E2 values determined to be compared with IPT1; 2) C2: 56 girls older than 3 years (3.1-10.6 yr) that had also LH, FSH and E2 basal values determined to be compared with the same variables from the other groups; 3) C3: 10 girls ages 4.5-9.9yr that were submitted to GnRH test and had stimulated values of LH and FSH determined.
Study Protocol
Despite being a retrospective study, children were cared for according to an assumed routine protocol. After a thorough history and physical examination, height and weight Z-score were calculated and plotted on charts appropriate for Brazilian children (17). The degree of breast development was assessed according to the stages given by Marshall & Tanner (18). Bone age was determined according to the method of Greulich & Pyle (19). Concentrations of LH, FSH, E2, TSH and bHCG were determined and children older than 3 years routinely underwent a GnRH stimulation test. Ovarian and uterine ultrasonography to rule out pelvic conditions was performed in all children. After clinical and laboratorial confirmation of PT, children were reexamined every three-four months and GnRH test was repeated if signs of sexual maturity were found. Computerized tomography scan or magnetic resonance imaging of the brain and pituitary gland was performed in all girls who had progression to GDPP.
Gonadotropins
Serum concentrations of LH, FSH, TSH and bHCG were determined by commercial, solid phase two-site immunochemiluminometric assay based on direct sandwich technique (20-22). E2 was determined by competitive immunochemiluminescent enzyme assay. The assays were routinely performed by a semi-automated Immulite System, and commercial kits obtained from DPC (Diagnostic Products Corporation, LA, USA) were used. The control group assays were standardized with the WHO Second International Standard human pituitary LH 80/552 and WHO Second International Standard FSH 78/549. The working range of the assays was established by the precision profile, which is a plot of intra-assay variation vs. concentration in the sample. The working range was defined as the interval in which the intra-assay variation was < 8% and varied from 0.1-200 IU/L for LH, from 0.1-170 IU/L for FSH and ranged from 20-2000 pg/mL to E2. In such case, the minimal detectable concentration (MDC) was set at 0.1 IU/L for LH, 0.1 IU/L for FSH and 20 pg/mL for E2. The interassay variation was lower than 10%.
A GnRH stimulation test was performed in 10 girls from the control group C3 and in 42 girls with PT. In each test, 100µg of GnRH (Relisorm®, Serono, México) were administered iv at time zero, and blood samples were drawn 15 min before, and 0, 15, 30, 45 and 60 min after GnRH infusion for serum LH and FSH measurements. The cut-off for peak LH value indicating active HPG axis had been that determined by Brito e cols. (14), until we set up our own control values when a peak LH value of 4.0 IU/L was assumed as pubertal response to GnRH, as well as basal LH value of 0.2 IU/L (23).
Statistical analysis
All data were expressed as median and range. The comparison among the several groups of normal subjects and PT was performed using nonparametric tests (Kruskal-Wallis, followed by multiple comparison procedures according to Dunn's method), as our variables did not have a normal distribution. Differences were considered significant if p<0.05. For the purposes of statistical analysis, values falling below the MDC for each hormone were taken as the MDC.
RESULTS
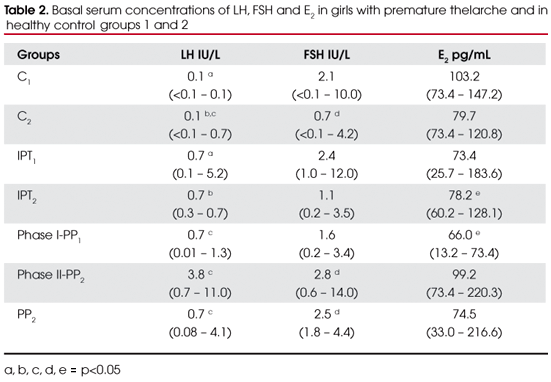
Clinical data from girls with PT and normal controls are represented in table 1. Significant differences were not found (p>0.05) in age at diagnosis, height z-score, weight z-score, BMI and bone age between IPT1 and C1; IPT2, PP1, PP2 and C2; and between the groups IPT2, PP1 and PP2. The values of basal gonadotropins and E2 of all groups are represented in table 2. Significant variables were designated with alphabetic letters.
The LH values were significantly increased (p<0.05) in group IPT1 in relation to C1 (0.7 vs. 0.1 IU/L) and the same was found in groups IPT2, phase I-PP1 when compared to control group C2. Basal LH values in groups phase II-PP1 and PP2 were significantly higher than in C2 (p<0.05). FSH serum concentrations were significantly increased in groups phase II-PP1 and PP2 compared with control group C2 (2.8 and 2.5 vs. 0.7 IU/L respectively), but differences were not found between IPT1 and C1 (2.4 vs. 2.1 IU/L) and IPT2 vs. C2 (1.1 vs. 0.7 IU/L) or between the groups of patients.
Considering E2serum concentrations, there were no significant differences between the groups, except for a decreased value in phase1-PP1 compared with IPT2 (p<0.05) [18.0 pg/ml (3.6-20.0) vs. 21.3 pg/ml (16.4-34.9)].
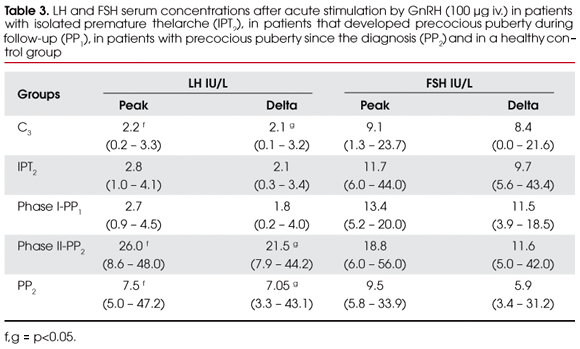
GnRH-stimulated gonadotropin levels of PT groups, as well as of the control group C3, are represented in table 3. No significant differences were found in peak (or delta) LH levels between IPT2 and C3 (2.8 vs. 2.2 IU/L) or phase I-PP1 and C3 (2.7 vs. 2.2 IU/L) (p<0.05), but peak (or delta) LH levels of phase II-PP1 and PP2 were significantly increased compared with control group C3 (26.0 and 7.5 vs. 2.2 IU/L) (p<0.05).
Ovarian and uterine volumes were within the expected values for age.
For girls under 3 years (IPT1 group) a long-term follow-up was performed in 20 (50%); 14 girls were treated with medroxiprogesterone acetate, with regression of the breast enlargement; 4 girls had spontaneous and complete regression of the thelarche. In the IPT2 group, no treatment was proposed to reduce the breast enlargement and there were 3 cases of spontaneous regression. Girls whose diagnosis was PP, performed CT or MRI, but no hypothalamic or pituitary disorder was found, and the PP was considered idiopathic. These girls were treated with leuprolide acetate, 3.75 mg/month.
DISCUSSION
The present study, as well as several previous reports (1-5), indicates that variants of PT occur on a spectrum towards complete precocious puberty. In the differential diagnosis between these conditions, new gonadotropin assays with better sensitivity and specificity are particularly useful in guiding who needs treatment and the precise moment to begin therapy.
According to the literature, the majority of our subjects (73.4%), in fact, had IPT, therefore idiopathic or normal variant and in 40 of them (50.6%), thelarche was identified in the first 3 years of life (IPT1 group), whereas the remainder 18 girls were 6.6 yr (4.117.5 yr). However, 21 girls (26.6%) progressed to idiopathic GDPP from three months to four years after thelarche was identified, and in 12 of them (PP2 group), serum concentrations of LH stimulated by GnRH indicated, from the beginning, that PT was due to PP, before pubarche became apparent, and driven treatment promptly to GnRH analogue. Instead, in 9 girls, only follow-up and new GnRH test disclosed the PP diagnosis.
There were no significant differences in clinical and axiological characteristics between the groups and none of them proved to be useful in predicting a PP progression, although individually, increased growth velocity, as well as bone age, were relevant indicators pointing to review of the diagnosis and to a new GnRH stimulation test (phase I to II-PP1).
Premature thelarche progressing to idiopathic GDPP was reported by Pasquino e cols. (9) and Verrotti e cols. (24). Stanhope & Brooke (16) named the situation as "thelarche variant" of precocious sexual maturation and Garibaldi e cols. (12) refer to the same as "exaggerated thelarche" . Regarding this subject, we identified two patterns: 1) a subgroup with suppressed HPG axis when challenged by iv GnRH that became liberated during follow-up (PP1); 2) a subgroup with stimulated LH by GnRH clearly indicating activated HPG axis and, in this case, PP was easily recognized, even though bone age advancement was not relevant or height Z-score was not very positive.
Based on Herman-Giddens e cols. (25), who evaluated 17,077 American girls between 3 and 12 years of age and recommended lowering the normal age of puberty to 7 years in white girls and to 6 in black ones, someone could argue that, in our study, girls between 7 and 8 years that were diagnosed as PP in fact had normal puberty. So far, we have no information about similar guidelines applicable to Brazilian children. Even for American children these new limits have been disputed by Midyett e cols. (3). They stated "signs of puberty in 6-8 years old girls should not be considered normal or benign. Implementation of new guidelines for the evaluation of puberty will result in failure to identify conditions that respond to early intervention".
Despite this study is retrospective in nature, by employing a highly sensitive and specific ICMA assay,, we had the opportunity to compare hormonal data from PT children with normative data previously published (23). Up to 1995, we employed AutoDelphia fluorometric assays and determined our own cut-off limits of gonadotropins used to distinguish normal pre-pubertal children from pubertal or GDPP children (14). However, towards 1996, when ICMA assays became available in our laboratory, it was evident that LH peaks of 6.9 IU/L and 9.6 IU/L, for girls and boys respectively, determined by IFMA, do not fit for ICMA assay. Thus we analyzed another normal pre-pubertal and pubertal population in different stages of puberty (I-V).
Cut-off values of the LH peak indicating maturity of HPG axis were 3.3 IU/L for girls and 4.1 IU/L for boys, and basal LH levels above 0.2 IU/L were consistent with puberty. However, significant overlap in basal (53.8%) and stimulated (17.6%) LH values were found indicating that laboratorial measurements must always be analyzed according to clinical context (23).
Considering basal LH values, the comparison between normal population and girls with PT resulted in an unexpected finding. Despite overlap, basal LH levels in IPT1, IPT2 and phaseI-PP1 groups were significantly increased, expressing a greater secretion of LH in children with PT than in normal ones. The same was not observed concerning FSH, and differences between these three groups and healthy control groups were not found.
As expected, basal LH and FSH serum concentrations were significantly increased in girls that had PT as the first sign of GDPP (PP2) or after PP development in follow-up (phase II-PP1).
Stanhope e cols. (10), Pescovitz e cols. (11), Stanhope & Brooke (16) and Garibaldi e cols. (12), by studying gonadotropin profile of girls with IPT, observed a secretion pattern characterized by predominance of pulsatile release of FSH over LH, suggesting that this FSH secretion could induce the development of single or small numbers of follicular cysts early in the ovary, resulting in E2 production and consequently breast enlargement. The first three authors cited above employed radioimmunoassay and their findings were not compared with a healthy control group. Secretion of FSH and LH during pre-pubertal period has that reported pattern and it is considered physiological, so, this finding cannot be accounted as a mechanism to explain PT.
Stimulated serum concentrations of LH and FSH by GnRH test in IPT1, IPT2 and phase I-PP1 groups were not different from control group C3 showing the classical pre-pubertal pattern i.e., suppressed LH and liberated FSH. Therefore, in the IPT, LH suppression is a working mechanism, but increased basal levels suggest that the set point of this inhibition is more elevated. Such finding could explain breast enlargement in cases of raised E2; however, E2 MDC of our control group was 29 pg/mL, so ICMA assay as well as the other methods are not sensitive enough to study E2 levels in pre-pubertal concentrations (23). Klein e cols. (6) using an ultrasensitive recombinant cell bioassay detected higher E2 levels in 20 girls with PT than in control girls, a finding consistent with the hypothesis that the mechanism of PT involves increased E2 levels rather than increased sensitivity of breast tissue to normal E2. In addition, increased LH basal levels could account for ovary micro-cysts reported in PT (26,27).
As expected, LH and FSH basal concentrations were significantly higher in patients that progress to PP (phase II-PP1 and PP2) than in control groups, keeping above basal cut-off limit of 0.2 IU/L in 71% of them (15/21), but they were not different from patients of IPT1, IPT2 and phase I-PP1 groups. On the other hand, peak LH levels after GnRH stimulation allowed the distinction between PT and PP. These findings still point to the usefulness of performing GnRH test as a diagnostic resource.
Iughetti e cols. (28) compared basal and stimulated LH and FSH obtained by RIA, IFMA, and ICMA and concluded that the LH peak is the gold standard to differentiate PT and PP. Neely e cols. (22), using ICMA, considered that LH peak level greater than 5.0 IU/L and 8.0 IU/L in girls and boys, respectively, is indicative of maturity of the HPG axis. Maybe such limits result in a lesser overlap, but our data suggest that these limits in the ICMA assays are still smaller. In the present study, we had one patient with IPT and LH peak level of 4.1 IU/L (D=3.4) and up to this moment there was no progression to PP, but in a group where at first the girls seemed to have IPT that was shown to be PP (phase I-PP1) over follow-up, we had a patient with an initial LH peak level of 4.5 IU/L (D=4.0). Based on these findings, we believe that cut-off value for LH peak must be around 4.5 and 5.0 IU/L, but values between 3.5 and 4.5 IU/L point to a careful clinical correlation and follow-up because it is a borderline response to GnRH stimulation test.
In brief, this study reports basal and GnRH stimulated levels of LH, FSH and E2 measured by ICMA in girls with PT analyzed against matched control groups. Our results demonstrated that basal values of LH are increased in girls with IPT and the usefulness of GnRH stimulation test in differential diagnosis of IPT and PP. LH peak values greater than 4.5 IU/L are indicative of maturity of HPG axis and values between 3.5 and 4.5 IU/L should be analyzed with caution.
Recebido em 29/08/2007
Aceito em 02/10/2007
- 1. Rosenfield RL. Normal and almost normal precocious variations in pubertal development, premature pubarche and premature thelarche revisited. Horm Res. 1994; 41:7-13.
- 2. Della Mana T, Setian N, Damiani D, Kuperman H, Dichtchekenian V. Premature thelarche: identification of clinical and laboratory data for the diagnosis of precocious puberty. Rev Hosp Clín Fac Med S Paulo. 2002; 57:49-54.
- 3. Midyett LK, Moore WV, Jacobson JD. Are pubertal changes in girls before age 8 benign? Pediatrics. 2003; 111:47-51.
- 4. Sizonenko PC. Preadolescent and adolescent endocrinology: physiology and physiopathology. Hormonal changes during abnormal pubertal development. Am J Dis Child. 1978; 132:797-805.
- 5. Ilicki A, Lewin RP, Kauli R, Kaufman H, Schachter A, Laron Z. Premature thelarche: natural history and sex hormone secretion in 68 girls. Acta Paediatr Scand. 1984; 73:75662.
- 6. Klein KO, Mericq V, Brown- Dowson JM, Larmore KA, Cabezas P, Cortinez A. Estrogen levels in girls with premature thelarche compared with normal prepubertal girls as determined by an ultrasensitive recombinant cell bioassay. J Pediatr. 1999; 134:190-2.
- 7. Dumic M, Tajic M, Mardesic D, Kalafatic Z. Premature thelarche: a possible adrenal disorder. Arch Dis Child. 1982; 57:200-3.
- 8. Saenz de Rodriguez CA, Bongiovanni AM, Conde de Barrego L. An epidemic of precocious development in Puerto Rican children. J Pediatr. 1985; 107:393-6.
- 9. Pasquino AM, Piccolo F, Scalamandre A , Malvaso M, Ortolani R, Boscherini B. Hypothalamic - pituitary - gonadotropic function in girls with premature thelarche. Arch Dis Child. 1980; 55:941-4.
- 10. Stanhope R, Abdulwahid NA, Adams J, Brook CGD. Studies of gonadotropin pulsatility and pelvic ultrasound examinations distinguish between isolated premature thelarche and central precocious puberty. Eur J Pediatr. 1986; 145:190-4.
- 11. Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler Jr. GB. Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone releasing hormone. J Clin Endocrinol Metabol. 1988; 67:474-9.
- 12. Garibaldi LR, Aceto T Jr, Weber C. The pattern of gonadotropin and estradiol secretions in exaggerated thelarche. Acta Endocrinol. 1993; 126:345-50.
- 13. Belgorosky A, Chaler E, Rivarola MA. High serum sex hormone-binding globulin (SHBG) in premature thelarche. Clin Endocrinol. 1992; 37:203-6.
- 14. Brito VN, Batista MC, Borges MF, et al. Diagnostic value of fluorimetric assays in the evaluation of precocious puberty. J Clin Endocrinol Metabol. 1999; 84:3539-44.
- 15. Pasquino AM, Tebaldi L, Cioschi L, et al. Premature thelarche: a follow up study of 40 girls. Natural history and endocrine findings. Arch Dis Child. 1999; 60:1180-92.
- 16. Stanhope RT, Brook CD. Thelarche variant: a new syndrome of precocious sexual maturation. Acta Endocrinol (Copenh). 1990; 123:481-6.
- 17. Marques RM, Marcondes E, Berquó E, Prandi R, Yunes J. Crescimento e desenvolvimento pubertário em crianças e adolescentes brasileiras. Altura e peso. São Paulo: Editora Brasileira de Ciências;1982.
- 18. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969; 44:291-303.
- 19. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford: Stanford University Press; 1959.
- 20. Broinstein I, Voyta JC, Thorpe G, Kricka LJ, Armstrong G. Chemiluminescent assay of alkaline phosphatase applied in an ultra-sensitive enzyme immunoassay of thyrotropin. Clin Chem. 1989; 35:1441-6
- 21. Padian MR, Odell WD, Carton E, Fisher DA. Development of a third generation immunoassay for follitropin and lutropin using chemiluminescence and their clinical application. Clin Chem. 1993; 39:1815-9.
- 22. Neely EK, Hintz RL, Wilson DM, et al. 1995 Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. 1993; 127:40-6.
- 23. Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. 2007; 92(4):1424-9.
- 24. Verrotti A, Ferrari M, Morgese G, Chiarelli F. Premature thelarche: a long term follow up. Gynecol Endocrinol. 1996; 10:241-7.
- 25. Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings Network. Pediatr. 1997; 99:505-12.
- 26. Nakamuri M, Okabe I, Shimoizumi H, Yanagisawa M, Tonigushi N, Itoh K. Ultrassonography of ovary, uterus and breast in premature thelarche. Acta Paediatr Jpn. 1991; 33:645-8.
- 27. Freedman SM, Kreitzer PM, Elkowitz SS, Soberman N, Leonidas JC. Ovarian microcystis with isolated premature thelarche. J Pediatr. 1993; 122:246-9.
- 28. Iughetti L, Predieri B, Ferrari M, et al. Diagnosis of central precocious puberty: endocrine assessment. J Pediatric Endocrinol Metabol. 2000; 13:709-15.
Address for correspondence:
Publication Dates
-
Publication in this collection
11 Mar 2008 -
Date of issue
Feb 2008
History
-
Accepted
02 Oct 2007 -
Received
29 Aug 2007