Abstracts
Objective: To obtain a simple model for the elicitation of the heat shock response in rats. Design: Laboratory study. Setting: University research laboratories. Sample: Seventy-nine adult male albino rats (weight range 200 g to 570 g). Procedures: Exposure to heat stress by heating animals in a warm bath for 5 min after their rectal temperatures reached 107.60 F (420 C). Liver and lung samples were collected for heat-shock protein 70 (HSP70) detection (Western analysis). Results: Western analysis was positive for HSP70 in the liver and in the lungs of heated animals. There was a temporal correlation between heating and HSP70 detection: it was strongest 1 day after heating and reduced afterwards. No heated animals died. Conclusion: These data show that heating rats in a warm (45o C) bath, according to parameters set in this model, elicits efficiently the heat shock response.
Heat-shock response; Proteins; Gene expression; Rats
OBJETIVO: Obter um modelo simples para tentar esclarecer a resposta ao choque térmico em ratos. LOCAL: Laboratório de pesquisa da Universidade. MÉTODO: Amostra: 79 ratos albinos, adultos, entre 200g a 570g. Procedimentos: Exposição ao calor, em banho quente, por 5 minutos, após a temperatura retal chegar a 42 graus centigrados. Biópsias de fígado e pulmão foram obtidas para detectar a proteina 70 (HSP 70), pelo "Western blot". RESULTADOS: As análises foram positivas nos animais aquecidos, com uma correlação entre aquecimento e constatação da HSP 70. Foi mais elevada no primeiro dia e não houve óbitos nos animais aquecidos. CONCLUSÃO: Os ratos aquecidos a 45 graus centígrados respondem eficientemente ao choque térmico.
Resposta a choque térmico; Proteinas; Expressão gênica; Ratos
A SIMPLE EXPERIMENTAL MODEL OF HEAT SHOCK RESPONSE IN RATS11 Article from the Postgraduation Course on Surgery, UFMG (Federal University of Minas Gerais), Belo Horizonte, Brazil, and INCIS (Health Science Institute, UNINCOR(Vale do Rio Verde University), Três Corações, MG, Brazil.
Tufi Neder Meyer21 Article from the Postgraduation Course on Surgery, UFMG (Federal University of Minas Gerais), Belo Horizonte, Brazil, and INCIS (Health Science Institute, UNINCOR(Vale do Rio Verde University), Três Corações, MG, Brazil.
Alcino Lázaro da Silva31 Article from the Postgraduation Course on Surgery, UFMG (Federal University of Minas Gerais), Belo Horizonte, Brazil, and INCIS (Health Science Institute, UNINCOR(Vale do Rio Verde University), Três Corações, MG, Brazil.
MEYER, T.N. & LÁZARO da SILVA, A. A simple experimental model of heat shock response in rats. Acta Cir. Bras., 13(4):217-21, 1998.
SUMMARY: Objective: To obtain a simple model for the elicitation of the heat shock response in rats. Design: Laboratory study. Setting: University research laboratories. Sample: Seventy-nine adult male albino rats (weight range 200 g to 570 g). Procedures: Exposure to heat stress by heating animals in a warm bath for 5 min after their rectal temperatures reached 107.60 F (420 C). Liver and lung samples were collected for heat-shock protein 70 (HSP70) detection (Western analysis). Results: Western analysis was positive for HSP70 in the liver and in the lungs of heated animals. There was a temporal correlation between heating and HSP70 detection: it was strongest 1 day after heating and reduced afterwards. No heated animals died. Conclusion: These data show that heating rats in a warm (45o C) bath, according to parameters set in this model, elicits efficiently the heat shock response.
SUBJECT HEADINGS: Heat-shock response. Proteins. Gene expression. Rats.
INTRODUCTION
Cells, as well as organisms, have several ways to defend themselves against injuries and stress. Acute-phase response (APR), hypothalamic-pituitary-adrenal axis responses, sympathetic autonomous nervous systems responses, oxidative stress response and integrated responses to surgical trauma are some examples of defense mechanisms 21,35.
Heat shock response (HSR), or stress response, is the oldest and most conserved form of reaction to stress. It is nearly universal in all living organisms 13. Heating cells 29 or organisms generate the expression of a class of proteins, known as heat shock proteins (HSP). Other stresses, like water immersion 26, exposure to sodium arsenite 27, dinitrophenol 29 and other metabolic poisons 40 may also elicit HSR.
HSP act as molecular chaperones 5: they escort other proteins during the assembling, translocation and folding process. According to their molecular masses (in kD kilodaltons), HSP may be divided into families: HSP27, HSP47, HSP60, HSP70, HSP90 and HSP110 13. HSP70 is the most conserved family its structure, in man, is 72 % homologous to its counterpart in Drosophilae 12.
Cells and organisms previously submitted to stresses that induce HSR become protected against a second exposure to the same stress, as well as against other types of injury, provided that this happens during the period in which HSP are significantly expressed. The HSR has important protective effects. In rabbits, HSR decreases myocardial ischemia/reperfusion injury 4. Rat cardiomyocytes in culture are protected against further injury when preconditioned by heat shock 25. In rats, induction of HSP70 protects the retina from light-provoked injury 1. HSP may have cytoprotective effects on gastric mucosa 9, as well as on experimental acute ileitis 33. Ischemic intestinal injury is reduced by previous HSP70 induction 34. HSP70 protects rat liver against thioacetamide-induced injury 7. HSR reduces ischemic injury in rat skeletal muscle 8. Experimental skin flaps previously submitted to heat shock show increased surviving length 19 and less necrosis 30. Also, free myocutaneous flaps show increased skin survival when previously conditioned by heat shock 38.
HSR reduces mortality in experimental models of septic shock and adult respiratory distress syndrome 36,37, and also in models of endotoxemia 17,18. HSR may modulate inflammatory responses. HSP inhibit production of TNF-a and IL-1 in lipopolysaccharide-activated human monocytes and rat macrophages 10. Heat shock protects in vivo against several injuries associated with increased production of cytokines and/or reactive oxygen metabolites15. HSR increases production of superoxide-dismutase in rat lung 11, as well as of catalase in rats 16. Overexpression of HSP70 may protect cells from necrosis induced by TNF-a and TNF-b 14. RIBEIRO and co-workers 28 have shown that, in an experimental sepsis model, HSR-associated mortality reduction is linked to decreasing plasma levels of TNF-a . KLOSTERHALFEN and associates 17 have shown that HSR reduces liberation of pro-inflammatory cytokines and decreases apoptosis rates. In experimental endotoxic shock, HSR decreases IL-b levels 3. HS-treated endothelial cells become more resistant to neutrophil-induced necrosis 39. HSR seems to exclude acute-phase response2.
We have previously shown22 that HSR reduces mortality in severe experimental burns.
Many pathological states related to surgery may be, in the future, amenable to modulation using the HSR. Therefore, studying this fundamental response can be important for surgeons. In this work, the viability of a simple, reproducible experimental model of HSR was sought for.
METHOD
The animal experiments carried out in the present study were performed in accordance to Institutional Review Board (UNINCOR) norms and to those contained in the Guide for the care and use of laboratory animals (NIH National Institutes of Health, 1996).
A total of 79 adult male albino rats ( 17 Wistar and 62 Fischer F-344) (UNINCOR, Três Corações, MG, Brasil) weighing 200 g-560 g were used. All animals were placed in individual cages in the same experimental room 7 days before the procedures, with free access to standard laboratory chow and tap water. Natural light and darkness cycles were allowed. Room temperature was maintained at 25o C. Follow-up observations were performed at 12-hour intervals. Anesthesia was administered with intraperitoneal ketamine hydrochloride (Ketalar, Parke-Davis, Guarulhos, SP, Brazil), 90 mg/kg.
Heating protocol. The anesthetized animal was secured to a supporting device and placed in warm water at 450C. Its rectal temperature was monitored with a digital thermomether (Digithermo, Sigma T1916), or with a glass clinical thermometer, until it reached 42o C. The animal was kept in the bath for five additional minutes. Time intervals elapsed between the beginning of the bath and the attainment of rectal temperatures of 40o C, 41o C and 42o C were measured. The rectal temperature at the end of the bath, as well as total bath time, were measured.
Animals submitted to this protocol were sacrificed 1, 3, 5 and 7 days after the procedures with a lethal dosis of ketamine. Liver and lung samples were collected, washed in saline and frozen in liquid nitrogen.
HSP detection (Western analysis) briefly: liver and lung were thawed, finely minced, weighed (0.22 g-0.23 g liver, 0.33 g-0.34 g lung) and homogenized in cold phosphate buffered saline containing protease inhibitors. Homogenate was centrifuged at 18,000 rpm, 4o-10o C for 45 min. Protein concentration in the supernatant was measured by the biuret method (Analisa, Belo Horizonte, MG, Brazil). Samples were suspended in Läemmlis sample buffer and submitted to heat denaturation. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in a BioRad 10-well Mini-PROTEAN system (BioRad, Hercules, CA, USA), using 0.75 mm gel and 200-220 mg of total protein per lane. Positive control was a purified human recombinant HSP70 (SPP-755, StressGen, Victoria, BC, Canada). After gel electrophoresis, proteins were transferred to a nitrocellulose membrane (Sigma N2764). Transfer was checked with Ponceau-S (Sigma P7170) and membranes were thoroughly washed in PBS-Tween. After nonspecific membrane block for 2 hours at room temperature, proteins were labeled with anti-HSP70 monoclonal antibody (SPA-810, StressGen) at 1:1000 concentration for 1 hour at room temperature. After secondary labeling with antimouse IgG conjugated with alkaline phosphatase at 1:1000 concentration (1 hour, room temperature), protein was visualized using color development with Nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) (Sigma FAST BCIP/NBT, B 5655) in alkaline phosphatase buffer. Blots were scanned for densitometric analysis. Bar graphs were constructed with data from these analysis.
RESULTS
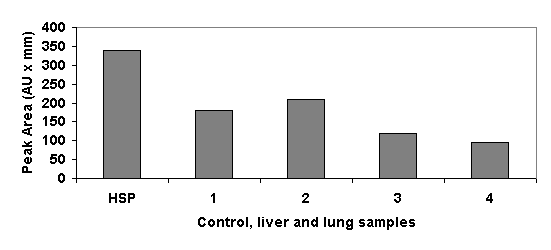
Heated animals attained an internal temperature of 40o C at a mean time of 6 min, 41o C at 8.5 min, and 42o C at 12 min. Mean total time for the bath was 17 min. Mean final internal temperature was 43o C (Fig.1). Mortality was null until 25 days in all animals. Western analysis detected HSP70 production in lungs and liver of animals killed for this purpose. A representative blot is shown in Figure 2, in which a bar graph constructed with data from the densitometric analysis can also be seen.
Figure 1. Rats were placed in warm(450 C) water bath, in which they were kept for 5 additional minutes after their rectal temperatures reached 420 C. Rectal temperatures reached 400 C in 6 minutes (mean), 410 C in 8.5 minutes (mean) and 420 C in 12 minutes (mean). Mean total bath time was 17 minutes. Mean final internal temperature was 430 C.
Western blot (top) and densitometric analysis (bottom) of the expression of HSP70 in the liver and lung of rats killed 1 day and 3 days after heating. HSP: heat-shock protein 70 (positive control). 1: Liver, one day after heating; 2: Lung, one day after heating; 3: Liver, 3 days after heating; 4: Lung, 3 days after heating. Optical density is expressed in absorbance units (AU).
DISCUSSION
In this work, a heating protocol using a warm water bath induced HSR in an efficient way. This is a simple and inexpensive method for HSR induction. In this work, no animals died due to anesthesia alone or combined with HS.
HSR can be induced through other means: heating cell cultures24,29,39; contact of cultured cells with herbimycin-A23, sodium arsenite39, indomethacin20 or gold sodium thiomalate and auranofin32; heating whole animals (rats) in heating boards6, heating chambers31,33 or adapted neonatal incubators3,28,36,37; treating rats with sodium arsenite28 or zinc aspartate23; prolonged immersion of animals in water at room temperature26.
Induction of HSR by a warm water bath has been done before8. Our model, therefore, is not completely original. Notwithstanding this, the adaptation of this model to conditions prevailing in our institutions, its simplification, as well as its development and detailed description, may be useful to those researchers who may be interested in studying this fundamental response. Using a warm water bath is less expensive than obtaining and adapting heating boards, heating chambers or neonatal incubators. Trying to reach simple and inexpensive research means is very important in countries like ours.
In this model, we have not studied transcriptional effects of HSR induction. This can be done in institutions where conditions allow the performance of Northern analysis. We have studied translational effects of HSR, that is, production of HSP70, which was made by Western analysis. We have used a colorimetric reaction for the final detection of HSP70 in blots, using a secondary antibody linked to alkaline phosphatase plus NBT/BCIP. Peroxidase-linked secondary antibodies may also be utilized, with DAB and/or 4-chloro-naphtol as substrates. Whenever possible, enhanced chemoluminescence (ECL) and auto-radiography should be used to reveal the blots, as this process allows better densitometry and quantitative analysis.
CONCLUSION
Wistar and Fischer F-344 adult male rats submitted to warm water baths, according to heating protocol described in this work, HSR is efficiently elicited.
ACKNOWLEDGMENTS
The technical assistance of Regina de Fátima Lopes in animal experiments is gratefully acknowledged. Animal experiments were performed in the Department of Anatomy, UNINCOR (Vale do Rio Verde University). The authors acknowledge the technical assistance of Antonio Carlos Vassalo Alves, M.Sc., in Western analysis. These experiments were performed in the Nutrition and Gnotobiology Laboratory, ICB (Institute of Biological Sciences, UFMG, Belo Horizonte); to its head, Prof. Ênio Cardillo Vieira, we express our thanks.
REFERENCES
1. BARBE, M.F.; TYTELL, M.; GOWER, D.J.; WELCH, W.J.- Hyperthermia protects against light damage in the rat retina. Science (Wash DC); 241:1817- 1820, 1988.
2. BUCHMAN, T.G & CABIN, D.E. - Molecular biology of circulatory shock III. HepG2 cells demonstrate two patterns of shock-induced gene expression which are independent, exclusive and prioritized. Surgery; 108:902-911, 1990.
3. CHU, E.K.; RIBEIRO, S.P.; SLUTSKY, A.S.- Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit. Care Med.; 25:1727-1732, 1997.
4. CURRIE, R.W.; TANGUAY, R.M.; KINGMA, J.G.- Heat shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation; 87:963-971, 1993.
5. ELLIS, R.J.- Proteins as molecular chaperones. Nature; 328:378-379, 1987.
6. EMAMI, A.; SCHWARTZ, J.H.; BORKAN, S. -Transient ischemia or heat stress induced a cytoprotectant protein in rat kidney. Am. J. Physiol.; 260:479-485, 1991.
7. FUJIMORI , S.; OTAKA, M.; OTANI, S.; JIN, M.; OKUYAMA, A.; ITOH, S.; IWABUCHI, A.; SASAHARA, H.; ITOH, H.; TASHIMA, Y.; KOMATSU, M.; MASAMUNE, O. - Induction of a 72-kDa heat shock protein and cytoprotection against thioacetamide-induced liver injury in rats. Dig. Dis. Sci.; 42:1987-1994, 1997.
8. GARRAMONE, R.R.; WINTERS, R.M.; DAS, D.K.; DECKERS, P.J.- Reduction of skeletal muscle injury through stress conditioning using the heat-shock response. Plast. Reconstr. Surg.; 93:1242-1247, 1994.
9. HAHM, K.B.; PARK, I.S.; KIM, Y.S.; KIM, J.H.; CHO, S.W.; LEE, S.I.; YOUN, J.K. - Role of rebapimide on induction of heat-shock proteins and protection against reactive oxygen metabolites-mediated cell damage in cultured gastric mucosal cells. Free Radic. Biol. Med.; 22:711-716, 1997.
10. HALL, T.J. - Role of hsp70 in cytokine production. Experientia; 50:1048-1053, 1994.
11. HASS, M.A.; MASSARO, D. - Regulation of the synthesis of superoxide dismutase in ratlings during oxidant and hyperthermic stresses. J. Biol. Chem.; 262:776-781, 1988.
12. HUNT, C.; MORIMOTO, R.I. - Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc. Natl. Acad. Sci. U.S.A.; 82:6455-6459, 1985.
13. JÄÄTTELÄ, M.; WISSING, D. - Emerging role of heat shock proteins in biology and medicine. Ann. Med.; 24:249-258, 1992.
14. JÄÄTTELÄ, M. - Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J. Immunol.; 151:4286-4294, 1993.
15. JACQUIER-SARLIN, M.R.; FULLER, K.; DINH-XUAN, A.T.; RICHARD, M.J.; POLLA, B.S. - Protective effects of hsp70 in inflammation. Experientia ; 50:1031-1038, 1994.
16. KARMAZYN, M.; MAILER, K.; CURRIE, R.W. - Acquisition and decay of heat-shock enhanced post-ischemic ventricular recovery. Am. J. Physiol.; 259:H424-H431, 1990.
17. KLOSTERHALFEN, B.; HAUPTMANN, S.; OFFNER, F.A.; AMO-TAKYI, B.; TÖNS, C.; WINKELTAU, G.; AFFIFY, M.; KÜPPER, W.; KIRKPATRICK, C.J.; MITTERMAYER, C. - Induction of heat shock protein 70 by zinc-bis- (DL-hydrogenaspartate) reduces cytokine liberation, apoptosis, and mortality rate in a rat model of LD100 endotoxemia. Shock ; 7:254-262, 1997.
18. KLOSTERHALFEN, B.; HAUPTMANN, S.; TIETZE, L.; TÖNS, C.; WINKELTAU, G.; KÜPPER, W.; KIRKPATRICK, C.J. - The influence of heat shock protein 70 on hemodynamic variables in a porcine model of recurrent endotoxemia. Shock ; 7:358-363, 1997.
19. KOENIG, W.J.; LOHNER, R.A.; PERDRIZET, G.A. - Improving acute skin-flap survival through stress conditioning using heat shock and recovery. Plast. Reconstr. Surg.; 90:659-664, 1992.
20. LEE, B.S.; CHEN, J.; ANGELIDIS, C.; JURIVICH, D.A.; MORIMOTO, R.I. Pharmacological modulation of heat shock factor 1 by antiinflammatory drugs results in protection against stress-induced cell damage. Proc. Natl. Acad. Sci. U.S.A.; 92:7207-7211, 1995.
21. MEYER, T.N. Resposta ao trauma no idoso. In: PETROIANU, A. & PIMENTA, L.G. Cirurgia Geriátrica. Rio de Janeiro, MEDSI, 1998, pp.187-204.
22. MEYER, T.N. Redução da mortalidade, após queimaduras graves em ratos, pela indução prévia da resposta ao choque térmico, Belo Horizonte, 1998. 115 pp. [Tese Mestrado Faculdade de Medicina da Universidade Federal de Minas Gerais].
23. MORRIS, S.D.; CUMMING, D.V.E.; LATCHMAN, D.S.; YELLON, D.M. Specific induction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. J.Clin. Invest.; 97:706-712, 1996.
24. NAKAMURA, K.; ROKUTAN, K.; MARUI, N.; AOIKE, A.; KAWAI, K. Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology; 101:161-166 1991.
25. NAYEEM, M.A.; HESS, M.L.; QIAN, Y.Z.; LOESSER, K.E.; KUKREJA, R.C. Delayed preconditioning of cultured adult rat cardiac myocytes: role of 70- and 90-kDa heat stress proteins. Am. J. Physiol.; 273:H861-H868, 1997.
26. OTAKA, M.; ITOH, H.; KUWABARA, T.; ZENIYA, A.; FUJIMORI, S.; OTANI, S.; TASHIMA, Y.; MASAMUNE, O. - Induction of heat shock protein and prevention of caerulein-induced pancreatitis by water-immersion stress in rats. Int. J. Biochem.; 26:805-811, 1994.
27. RIBEIRO, S.P.; VILLAR, G.; DOWNEY, G.P.; EDELSON, J.D.; SLUTSKY, A.D. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit.Care Med.; 22:922-929, 1994.
28. RIBEIRO, S.P.; VILLAR, J.; DOWNEY, G.P.; EDELSON, J.D.; SLUTSKY, A.D.- Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: evidence for TNF-a posttranslational regulation. Am. J. Respir. Crit. Care Med.; 154:1843-1850, 1996.
29. RITOSSA F. - A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia; 18:571-573, 1962.
30. RÖSKEN, F.; RÜCKER, M.; BAUER, I.; AMON, M.; MENGER, M.D. "Heat shock priming" reduces microvascular perfusion failure of critically perfused skin flaps a possible mechanism of tissue protection in acute ischemia. Langenbecks Arch. Chir.; 114:527-530, 1997.
31. RYAN, A.J.; FLANAGAN, S.W.; MOSELEY, P.L.; GISOLFI, C.V. - Acute heat stress protects rats against endotoxin shock. J. Appl. Physiol.; 73:1517-1522, 1992.
32. SATO, H.; YAMAGUCHI, M.; SHIBASAKI, T.; ISHII, T.; BANNAI, S. Induction of stress proteins in mouse peritoneal macrophages by the anti-rheumatic agents gold sodium thiomalate and auranofin. Biochem. Pharm.; 49:1453-1457, 1995.
33. STOJADINOVIC, A.; KIANG, J.; GOLDHILL, J.; MATIN, D.; SMALLRIDGE, R.; GALLOWAY, R.; SHEA-DONOHUE, T. - Induction of heat shock response prevents tissue injury during acute inflammation of the rat ileum. Crit. Care Med.; 25:309-317, 1997.
34. TÖNS, C.; KLOSTERHALFEN, B.; KLEIN, H.M.; RAU, H.M.; ANUROV, M.; OETTINGER, A.; SCHUMPELICK, V. - Induction of heat shock protein 70 (HSP70) by zinc-bis-(DL-hydrogenaspartate) reduces ischemic small-bowel tissue damage in rats. Langenbecks Arch. Chir.; 382:43-48, 1997.
35. UDELSMAN, R.; HOLBROOK, N.J. - Endocrine and molecular responses to surgical stress. Curr. Probl. Surg.; 31:658-720, 1994.
36. VILLAR, J.; EDELSON, J.D.; POST, M.; MULLEN, B.M.; SLUTSKY, A.S. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am. Rev. Respir. Dis.; 147:177-181, 1993.
37. VILLAR, J.; RIBEIRO, S.P.; MULLEN, B.M.; KULISZEWSKI, M.; POST, M.; SLUTSKY, A.S. - Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit. Care Med.; 22:914-921, 1994.
38. WANG, B.H.; CAIYING, Y.; STAGG, C.A.; LIN, M.; FAWCETT, T.; VANDERKOLK, C.A.; UDELSMAN, R. - Improved free musculocutaneous flap survival with induction of heat shock protein. Plast. Reconstr. Surg.; 101:776-784, 1998.
39. WANG, J.H.; REDMOND, H.P.; WATSON, R.W.G.; CONDRON, C.; BOUCHIER-HAYES, D. - Induction of heat shock protein 72 prevents neutrophil-mediated human endothelial cell necrosis. Arch. Surg.; 130:1260-1265, 1995.
40. WELCH WJ.- Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol. Rev.; 72:1063-1081, 1992.
MEYER, T.N. & LÁZARO da SILVA, A. Um modelo experimental simples de resposta ao choque térmico em ratos. Acta Cir. Bras., 13(4):217-21, 1998.
RESUMO:OBJETIVO: Obter um modelo simples para tentar esclarecer a resposta ao choque térmico em ratos. LOCAL: Laboratório de pesquisa da Universidade. MÉTODO: Amostra: 79 ratos albinos, adultos, entre 200g a 570g. Procedimentos: Exposição ao calor, em banho quente, por 5 minutos, após a temperatura retal chegar a 42 graus centigrados. Biópsias de fígado e pulmão foram obtidas para detectar a proteina 70 (HSP 70), pelo "Western blot". RESULTADOS: As análises foram positivas nos animais aquecidos, com uma correlação entre aquecimento e constatação da HSP 70. Foi mais elevada no primeiro dia e não houve óbitos nos animais aquecidos. CONCLUSÃO: Os ratos aquecidos a 45 graus centígrados respondem eficientemente ao choque térmico.
DESCRITORES: Resposta a choque térmico. Proteinas. Expressão gênica. Ratos.
Address for correspondence :
Tufi Neder Meyer, MD
Rua Desembargador Alberto Luz, 129 .
37410-000 Três Corações MG Brazil
E-mail: tufi@unincor.br
Accepted for publication on August 1998
2 MD, FACS, Full Professor (Anatomy), INCIS (Health Sciences Institute), UNINCOR (Vale do Rio Verde University), Três Corações, MG, Brazil
3 MD, FACS, Full Professor (Surgery), School of Medicine, UFMG (Federal University of Minas Gerais), Belo Horizonte, MG, Brazil
- 1. BARBE, M.F.; TYTELL, M.; GOWER, D.J.; WELCH, W.J.- Hyperthermia protects against light damage in the rat retina. Science (Wash DC); 241:1817- 1820, 1988.
- 2. BUCHMAN, T.G & CABIN, D.E. - Molecular biology of circulatory shock III. HepG2 cells demonstrate two patterns of shock-induced gene expression which are independent, exclusive and prioritized. Surgery; 108:902-911, 1990.
- 3. CHU, E.K.; RIBEIRO, S.P.; SLUTSKY, A.S.- Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit. Care Med.; 25:1727-1732, 1997.
- 4. CURRIE, R.W.; TANGUAY, R.M.; KINGMA, J.G.- Heat shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation; 87:963-971, 1993.
- 5. ELLIS, R.J.- Proteins as molecular chaperones. Nature; 328:378-379, 1987.
- 6. EMAMI, A.; SCHWARTZ, J.H.; BORKAN, S. -Transient ischemia or heat stress induced a cytoprotectant protein in rat kidney. Am. J. Physiol.; 260:479-485, 1991.
- 8. GARRAMONE, R.R.; WINTERS, R.M.; DAS, D.K.; DECKERS, P.J.- Reduction of skeletal muscle injury through stress conditioning using the heat-shock response. Plast. Reconstr. Surg.; 93:1242-1247, 1994.
- 9. HAHM, K.B.; PARK, I.S.; KIM, Y.S.; KIM, J.H.; CHO, S.W.; LEE, S.I.; YOUN, J.K. - Role of rebapimide on induction of heat-shock proteins and protection against reactive oxygen metabolites-mediated cell damage in cultured gastric mucosal cells. Free Radic. Biol. Med.; 22:711-716, 1997.
- 10. HALL, T.J. - Role of hsp70 in cytokine production. Experientia; 50:1048-1053, 1994.
- 11. HASS, M.A.; MASSARO, D. - Regulation of the synthesis of superoxide dismutase in ratlings during oxidant and hyperthermic stresses. J. Biol. Chem.; 262:776-781, 1988.
- 12. HUNT, C.; MORIMOTO, R.I. - Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc. Natl. Acad. Sci. U.S.A.; 82:6455-6459, 1985.
- 13. JÄÄTTELÄ, M.; WISSING, D. - Emerging role of heat shock proteins in biology and medicine. Ann. Med.; 24:249-258, 1992.
- 14. JÄÄTTELÄ, M. - Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J. Immunol.; 151:4286-4294, 1993.
- 15. JACQUIER-SARLIN, M.R.; FULLER, K.; DINH-XUAN, A.T.; RICHARD, M.J.; POLLA, B.S. - Protective effects of hsp70 in inflammation. Experientia ; 50:1031-1038, 1994.
- 16. KARMAZYN, M.; MAILER, K.; CURRIE, R.W. - Acquisition and decay of heat-shock enhanced post-ischemic ventricular recovery. Am. J. Physiol.; 259:H424-H431, 1990.
- 17. KLOSTERHALFEN, B.; HAUPTMANN, S.; OFFNER, F.A.; AMO-TAKYI, B.; TÖNS, C.; WINKELTAU, G.; AFFIFY, M.; KÜPPER, W.; KIRKPATRICK, C.J.; MITTERMAYER, C. - Induction of heat shock protein 70 by zinc-bis- (DL-hydrogenaspartate) reduces cytokine liberation, apoptosis, and mortality rate in a rat model of LD100 endotoxemia. Shock ; 7:254-262, 1997.
- 18. KLOSTERHALFEN, B.; HAUPTMANN, S.; TIETZE, L.; TÖNS, C.; WINKELTAU, G.; KÜPPER, W.; KIRKPATRICK, C.J. - The influence of heat shock protein 70 on hemodynamic variables in a porcine model of recurrent endotoxemia. Shock ; 7:358-363, 1997.
- 19. KOENIG, W.J.; LOHNER, R.A.; PERDRIZET, G.A. - Improving acute skin-flap survival through stress conditioning using heat shock and recovery. Plast. Reconstr. Surg.; 90:659-664, 1992.
- 20. LEE, B.S.; CHEN, J.; ANGELIDIS, C.; JURIVICH, D.A.; MORIMOTO, R.I. Pharmacological modulation of heat shock factor 1 by antiinflammatory drugs results in protection against stress-induced cell damage. Proc. Natl. Acad. Sci. U.S.A.; 92:7207-7211, 1995.
- 21. MEYER, T.N. Resposta ao trauma no idoso. In: PETROIANU, A. & PIMENTA, L.G. Cirurgia Geriátrica Rio de Janeiro, MEDSI, 1998, pp.187-204.
- 22. MEYER, T.N. Reduçăo da mortalidade, após queimaduras graves em ratos, pela induçăo prévia da resposta ao choque térmico, Belo Horizonte, 1998. 115 pp. [Tese Mestrado Faculdade de Medicina da Universidade Federal de Minas Gerais].
- 23. MORRIS, S.D.; CUMMING, D.V.E.; LATCHMAN, D.S.; YELLON, D.M. Specific induction of the 70-kD heat stress proteins by the tyrosine kinase inhibitor herbimycin-A protects rat neonatal cardiomyocytes. J.Clin. Invest.; 97:706-712, 1996.
- 24. NAKAMURA, K.; ROKUTAN, K.; MARUI, N.; AOIKE, A.; KAWAI, K. Induction of heat shock proteins and their implication in protection against ethanol-induced damage in cultured guinea pig gastric mucosal cells. Gastroenterology; 101:161-166 1991.
- 27. RIBEIRO, S.P.; VILLAR, G.; DOWNEY, G.P.; EDELSON, J.D.; SLUTSKY, A.D. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit.Care Med.; 22:922-929, 1994.
- 29. RITOSSA F. - A new puffing pattern induced by temperature shock and DNP in Drosophila Experientia; 18:571-573, 1962.
- 30. RÖSKEN, F.; RÜCKER, M.; BAUER, I.; AMON, M.; MENGER, M.D. "Heat shock priming" reduces microvascular perfusion failure of critically perfused skin flaps a possible mechanism of tissue protection in acute ischemia. Langenbecks Arch. Chir.; 114:527-530, 1997.
- 31. RYAN, A.J.; FLANAGAN, S.W.; MOSELEY, P.L.; GISOLFI, C.V. - Acute heat stress protects rats against endotoxin shock. J. Appl. Physiol.; 73:1517-1522, 1992.
- 32. SATO, H.; YAMAGUCHI, M.; SHIBASAKI, T.; ISHII, T.; BANNAI, S. Induction of stress proteins in mouse peritoneal macrophages by the anti-rheumatic agents gold sodium thiomalate and auranofin. Biochem. Pharm.; 49:1453-1457, 1995.
- 33. STOJADINOVIC, A.; KIANG, J.; GOLDHILL, J.; MATIN, D.; SMALLRIDGE, R.; GALLOWAY, R.; SHEA-DONOHUE, T. - Induction of heat shock response prevents tissue injury during acute inflammation of the rat ileum. Crit. Care Med.; 25:309-317, 1997.
- 34. TÖNS, C.; KLOSTERHALFEN, B.; KLEIN, H.M.; RAU, H.M.; ANUROV, M.; OETTINGER, A.; SCHUMPELICK, V. - Induction of heat shock protein 70 (HSP70) by zinc-bis-(DL-hydrogenaspartate) reduces ischemic small-bowel tissue damage in rats. Langenbecks Arch. Chir.; 382:43-48, 1997.
- 35. UDELSMAN, R.; HOLBROOK, N.J. - Endocrine and molecular responses to surgical stress. Curr. Probl. Surg.; 31:658-720, 1994.
- 36. VILLAR, J.; EDELSON, J.D.; POST, M.; MULLEN, B.M.; SLUTSKY, A.S. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am. Rev. Respir. Dis.; 147:177-181, 1993.
- 37. VILLAR, J.; RIBEIRO, S.P.; MULLEN, B.M.; KULISZEWSKI, M.; POST, M.; SLUTSKY, A.S. - Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit. Care Med.; 22:914-921, 1994.
- 38. WANG, B.H.; CAIYING, Y.; STAGG, C.A.; LIN, M.; FAWCETT, T.; VANDERKOLK, C.A.; UDELSMAN, R. - Improved free musculocutaneous flap survival with induction of heat shock protein. Plast. Reconstr. Surg.; 101:776-784, 1998.
- 39. WANG, J.H.; REDMOND, H.P.; WATSON, R.W.G.; CONDRON, C.; BOUCHIER-HAYES, D. - Induction of heat shock protein 72 prevents neutrophil-mediated human endothelial cell necrosis. Arch. Surg.; 130:1260-1265, 1995.
Publication Dates
-
Publication in this collection
14 Jan 1999 -
Date of issue
Oct 1998