Abstracts
PURPOSE: To evaluate in vitro the antibacterial effect of diode laser light of wavelength 650 nm, in association with the photosensitive substance toluidine blue, on the bacteria in infected skin ulcers. METHODS: Samples were collected by means of swabs containing a medium for transporting infected material from skin ulcers. The material was inoculated into culturing medium containing azide blood agar for the growth of Gram-positive bacteria, and MacConkey agar for Gram-negative bacteria, and incubated for 48 hours. The results obtained from counting the colony-forming units were correlated and subjected to statistical analysis, adopting the significance level of p > or = 0.05. RESULTS: From analysis of variance (ANOVA), the result for the general mean was p = 0.0215. Using the t test with post-hoc test, the result for TBO vs. Control was p = 0.0186, and for TBO + Laser vs. Control it was p = 0.0039. CONCLUSION: There was a significant reduction in colony-forming units when the cultures were subjected to photodynamic therapy.
Photosensitizing Agents; Anti-Bacterial Agents; Skin Ulcers
OBJETIVO: Avaliar in vitro o efeito antibacteriano do laser diodo com comprimento de onda de 650nn, associado a substancia fotossensível azul de toluidina sobre as bactérias de ulceras cutâneas infectadas. MÉTODOS: Foram coletadas amostras através de um swab com meio de transporte, de material infectado de úlceras cutâneas. Os materiais foram inoculadas em meios de cultura contendo ágar sangue azida para o crescimento de bactérias gram-positivas e agar Mac Conkey para as gram-negativas, e incubadas por 48 horas. Os resultados obtidos da contagem das unidades formadoras de colônias foram relacionados e submetidos a analise estatística adotando como nível de significância p > ou = 0.05. RESULTADOS: Os resultados da análise de variância ANOVA para a media geral foi p= 0,0215 e para o post hoc test teste t. TBO x Controle p=0,0186, TBO + Laser x Controle p=0,0039. CONCLUSÃO: Houve redução, significativa das unidades formadoras de colônias quando submetidas à terapia fotodinâmica.
Agentes Fotossensibilizantes; Agentes Antibacterianos; Úlceras Cutâneas
ORIGINAL ARTICLE
Photodynamic inactivation of in vitro bacterial cultures from pressure ulcers1 1 Department of Physiotherapy at the University for Development of the State and the Pantanal Region (UNIDERP), Campo Grande, Mato Grosso do Sul, Brazil
Inativação fotodinâmica de culturas de bactérias in vitro provenientes de úlceras de pressão
Paulo de Tarso Camillo de CarvalhoI; Ana Paula da Costa MarquesII; Felipe Abdalla dos ReisIII; Ana Carulina Guimarães BelchiorIII; Iandara Schettert SilvaIV; Carlos Alexandre HabitanteV; Daniela Aparecida SussaiVI
IPhD, Associate Professor, Department of Physiotherapy, UNIDERP, Brazil
IIMaster , Assistant Professor, Department of Physiotherapy, UNIDERP, Brazil
IIIAssistant Professor, Department of Physiotherapy, UNIDERP, Brazil
IVPhD, Associate Professor, Department of Veterinary, UNIDERP, Brazil
VPhD, Associate Professor, Department of Ed. Physical, UNIDERP, Brazil
VIFellow Master degree in Postgraduate Program in Health and Development in the West Central Region, Federal University of Mato Grosso do Sul, Brazil
Correspondence Correspondence:
ABSTRACT
PURPOSE:To evaluate in vitro the antibacterial effect of diode laser light of wavelength 650 nm, in association with the photosensitive substance toluidine blue, on the bacteria in infected skin ulcers.
METHODS: Samples were collected by means of swabs containing a medium for transporting infected material from skin ulcers. The material was inoculated into culturing medium containing azide blood agar for the growth of Gram-positive bacteria, and MacConkey agar for Gram-negative bacteria, and incubated for 48 hours. The results obtained from counting the colony-forming units were correlated and subjected to statistical analysis, adopting the significance level of p³ 0.05.
RESULTS: From analysis of variance (ANOVA), the result for the general mean was p = 0.0215. Using the t test with post-hoc test, the result for TBO vs. Control was p = 0.0186, and for TBO + Laser vs. Control it was p = 0.0039.
CONCLUSION: There was a significant reduction in colony-forming units when the cultures were subjected to photodynamic therapy.
Key words: Photosensitizing Agents . Anti-Bacterial Agents Skin Ulcers.
RESUMO
OBJETIVO: Avaliar in vitro o efeito antibacteriano do laser diodo com comprimento de onda de 650nn, associado a substancia fotossensível azul de toluidina sobre as bactérias de ulceras cutâneas infectadas.
MÉTODOS: Foram coletadas amostras através de um swab com meio de transporte, de material infectado de úlceras cutâneas. Os materiais foram inoculadas em meios de cultura contendo ágar sangue azida para o crescimento de bactérias gram-positivas e agar Mac Conkey para as gram-negativas, e incubadas por 48 horas. Os resultados obtidos da contagem das unidades formadoras de colônias foram relacionados e submetidos a analise estatística adotando como nível de significância p ³ 0.05.
RESULTADOS: Os resultados da análise de variância ANOVA para a media geral foi p= 0,0215 e para o post hoc test teste t. TBO x Controle p=0,0186, TBO + Laser x Controle p=0,0039.
CONCLUSÃO: Houve redução, significativa das unidades formadoras de colônias quando submetidas à terapia fotodinâmica.
Descritores: Agentes Fotossensibilizantes . Agentes Antibacterianos . Úlceras Cutâneas.
Introduction
Pressure ulcers are one of the principal examples of injury to skin integrity. They represent a direct threat to the individual, through causing discomfort, disease prolongation and delays in rehabilitation and hospital discharge. They may even lead to death due to septicemia1,2. Ulcers result from a multiplicity of physiopathological mechanisms. Neuropathy is by far the most common reason for the formation of ulceration on the foot that leads to infection. Diabetic foot infections may be monomicrobial or polymicrobial: the latter occurs in around 60 to 80% of such patients. Staphylococcus aureus and S. epidermidis are isolated from around 60% of all infected ulcers. Enterococci, streptococci and enterobacteria are found less frequently, and 15% of infected ulcers have participation by strictly anaerobic bacteria3. In Latin America, bacterial resistance to antimicrobial agents has been reaching very high levels. It is a threat to favorable evolution in anti-infection therapy, both among patients living within the community and among hospitalized patients. Empirical treatment can no longer be utilized, and every effort must be made to supply the clinic with any results relating to bacterial resistance to antimicrobial agents4. Among the various methods for controlling bacterial infection in pressure ulcers, the utilization of low-power laser operating at given wavelengths, in conjunction with a photosensitive drug, has presented a great revolution. Photodynamic therapy (PDT) starts from the principle that the interaction between light of appropriate wavelength and a nontoxic compound (photosensitizer) and oxygen results in reactive species that are capable of inducing a lack of cell viability, which leads to the death of the microorganisms5. Since most bacteria do not absorb visible light, the utilization of a nontoxic photosensitizer that attaches to the bacterial wall and attracts the laser light to it at the time of irradiation is essential for low-power lasers to have antimicrobial action on bacteria6,7. Thus, when the bacteria are irradiated with a laser light of complementary wavelength, photons are absorbed by the photosensitizer, which is converted into an excited state. Following this, the energy transferred to the neighboring molecules may result in the formation of reactive molecules such as singleton oxygen, superoxide ions, hydroxyls and other free radicals, which may damage and ultimately kill the bacterial cells 7-10. The use of diode laser light of wavelength 650 nm in association with toluidine blue produces a bactericide effect on microorganisms that has been proven by several authors7,9,11-13. Although other studies would need to be carried out before making photodynamic therapy applicable in vivo, these preliminary results suggest that this therapy may have clinical applications. Photodynamic therapy may present unexplored therapeutic possibilities as a reducing agent acting on bacterial colonies coming from skin wounds. Starting from this premise, and bearing in mind that bacterial infection in such cases is responsible for increased morbidity, delayed healing and even increased mortality, the importance of the present study is highlighted.
Methods
Sample composition
The microorganisms utilized in this study came from bedsores (pressure ulcers) on patients undergoing treatment at the UNIDERP Rehabilitation Center in Campo Grande, State of Mato Grosso do Sul. The study had been approved by the Ethics Committee for Research on Human Beings of UNIDERP under protocol Nº. 22874, and all the patients had signed a free and informed consent statement in order to be accepted as donors.
Sample collection
Four samples were collected on two sterile swabs containing Stuart transport medium and were sent to the microbiology laboratory.
Laser equipment
A GaAlAs (gallium aluminum arsenide) laser emitter made by Laserline® (Inova model) was utilized, with power of 50 mW, beam area of 0.1 cm2 and wavelength (l) of 650 nm.
Application of the laser:
The swabs containing the samples were agitated in vortex with 1.5 ml Muller Hynton liquid culturing medium. Each sample was then divided into two aliquots of 500 ml, and 50 mg of toluidine blue was added to one of them. The aliquots were distributed on a microplate in the following manner: the sample containing toluidine blue occupied eight wells in one row of the microplate (50 ml of the solution in each); and the other sample diluted in the liquid medium was similarly distributed in another row of the same microplate. Following this, GaAlAs diode laser light was applied with an energy density of 8 J/cm2, to four wells of each sample. The samples were then collected and separately placed and homogenized in four test tubes containing Muller Hynton culturing medium.
Identification of the bacterial group
The Gram staining technique was carried out using the sample from the second swab, to identify Gram-positive and Gram-negative bacteria.
Observation of microbial growth (colony-forming units):
The samples contained in the four test tubes were diluted in the proportions 1:10 and 1:100 in Muller Hynton liquid medium and seeded using the drainage technique on MacConkey agar and on azide blood agar, to observe the Gram-negative and Gram-positive bacteria, respectively, in accordance with the findings from the slides stained via the Gram technique. The plates were incubated at 37°C for 24h and then the colony-forming units were counted using a digital colony counter. The tubes containing the diluted samples were also incubated under the same conditions as for the plates and, after 24h, they were seeded in new culturing media for confirmation of the results.
Statistical analysis
The results obtained from counting the colony-forming units were correlated and subjected to statistical analysis utilizing the parametric ANOVA test (variance analysis). The nullity hypothesis of p< 0.05 was taken and data were subjected to post-hoc testing using the Student t test. The statistical analyses were done using the SigmaStat 3.1 software.
Results
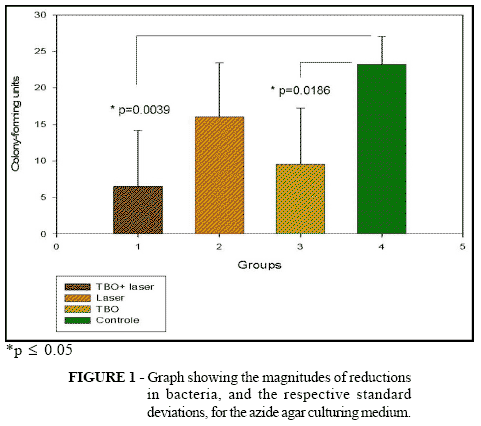
Means were obtained from counting the colony-forming units from the four groups: toluidine blue plus laser (TBO + Laser), laser alone, toluidine blue and control. These data were subjected to statistical analysis, giving a result of p = 0.0215* for the general mean, i.e. there was a statistical difference between the data described. In the analyses between the groups, the following values were obtained between the cross-comparisons: laser vs. TBO, p = 0.1355; TBO + laser vs. laser, p = 0.0630; laser vs. control, p = 0.0664; TBO vs. control, p = 0.0186*; TBO + laser vs. control, p = 0.0039* (Fig. 1). The percentage reduction in the quantity of bacteria was obtained in accordance with the following formula:
___________________________________X 100
Discussion
The utilization of photodynamic therapy for bacterial control has been reported by several authors. Among these, the studies by9,10,13,14 can be cited. In the light of these affirmations, we sought in the present study to conduct an in vitro microbiological study, considering that photodynamic therapy has been utilized in controlling bacteria in periodontal diseases10 and radicular canals3 but no studies are to be found in the literature describing the use of photodynamic therapy in skin ulcers.
In the present study, the actions of laser, staining agent and laser plus staining agent were compared. All of these presented some inhibitory action on the growth of bacterial colonies, in comparison with the control group. Carvalho15 reported that low-power laser therapy using a wavelength of 632.8 nm was capable of reducing and controlling the numbers of colony-forming units in bacterial cultures from pressure ulcers. On the other hand, when laser application was halted, the numbers of bacteria started to grow again. In our study, the samples that were only subjected to laser presented behavior similar to what was described by Carvalho15, when compared with the control group. The first treatments with photodynamic therapy utilized conventional lamp bulbs with a wide spectrum, such as incandescent filament bulbs (tungsten), arc bulbs (xenon or mercury) and slide projector bulbs. These were equipped with red filters emitting light at a wavelength of 630 nm16. In the samples with the toluidine blue staining agent without application of laser, there was also a slight diminution. However, we believe that this may have been due to the impossibility of working in an environment free from white light, which may have photosensitized some samples. However, such diminution has also occurred in studies by other researchers, such as: The results found in our study are corroborated by studies carried out by several researchers11,13,17,18, who described decreases in the numbers of bacteria when they were subjected to the action of laser radiation in the presence of a photosensitive staining agent. The ideal characteristics for a photosensitizing agent, in addition to the wavelength, are: high efficiency, selective accumulation in tissues, rapid elimination and low toxicity. The quantum efficiency depends not only on the photophysical properties, but also on the state of staining agent aggregation, its location at the time of irradiation and its concentration19. We conclude that photodynamic therapy was shown to be capable of reducing the numbers of bacterial colonies present in bedsores (pressure ulcers), under the experimental conditions analyzed.
Paulo de Tarso Camillo de Carvalho
Rua Abricó do Pará, 146, Carandá Bosque.
79032-423 - Campo Grande - MS - Brazil
e-mail: ptpaulo@terra.com.br
Conflict of interest: None
Financial source: Manoel de Barros Foundation
- 1. Kelly M A. Nursing diagnosis source book: guidelines for clinical application. Norwalk: Appleton-Century-Crofts, 1989.
- 2. Reddy MMP. Prevención y tratamiento de las úlceras de decúbito en el anciano. Sandorama; 1986.
- 3. Carvalho CBM, Neto M, Aragão R, Luciana P. Pé diabético: análise bacteriológica de 141 casos. Arq Bras Endocrinol Metab. 2004;48(3):406-13.
- 4. Guzmám BM, Casellas JM, Sader HS. Bacterial resistance to antimicrobial agents in Latin America: the giant is awakening. Infect Dis Clin North Am. 2000;14:67-81.
- 5. Jori G, Brown SB. Photosensitized inactivation of microorganisms. Photochem Photobiol Sci. 2004;3(5):403-5.
- 6. Wilson M, Dobson J, Harvey W. Sensitization of oral bacteria to killing by low-power laser radiation. Curr Microbiol. 1992;2(25):77-81.
- 7. Konig K, Teschke M, Sigusch B, Glockmann E, Eick S, Pfister W. Red light kills bacteria via photodynamic action. Cell Mol Biol (Noisy-le-Grand). 2000;46(7):1297-303.
- 8. SPIKES JD, JORI G. Photodynamic therapy of tumours and other diseases using porphyrins. Lasers Med Sci. 1987;2:3-15.
- 9. DOBSON J, WILSON M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch Oral Biol. 1992;11(37):883-7.
- 10. Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro Photochem Photobiol. 1997;6(65):1026-31.
- 11. Burns T, Wilson M, Pearson GJ. Killing of cariogenic bacteria by light from gallium arsenide diode laser. J Dent. 1994;5(22):273-8.
- 12. Maisch T, Szeimies RM, Lehn N, Abels C. Antibacterial photodynamic therapy A new treatment for bacterial skin diseases? Hautarzt. 2005;56(11):1048-55.
- 13. Zanin ICJ, Brugnera A, Höfling JF, Gonçalves RB. Antimicrobial activity of low-level laser in presence of photosensitizer. J Dental Res. 2002;81:A-446.
- 14. Malik Z, Hanania J, Nitzan Y.effects of photoactivated porphyrins an alternative approach to antimicrobial drugs. J Photochem Photobiol B. 1990;5(3-4):281-93.
- 15. Carvalho PTC, Silva RR, Silva RJC. Estudo microbiológico in vitro do crescimento após aplicação de laser HeNe em úlceras com infecção bacteriana. Rev Fisioter Bras. 2001; 2(3):183-88.
- 16. Depasse F, Sensebe L, Seghatchian J, Andreu G, Samama MM. The influence of methylene blue light treatment and methylene blue removal filter on fibrinogen activity states and fibrin polymerisation indices. Transfus Apher Sci. 2005;33(1):63-9.
- 17. Visona A, Angelini A, Gobbo S, Bonanome A, Thiene G, Pagnan A, Tonello D, Bonandini E, Jori G. Local photodynamic therapy with Zn(II)-phthalocyanine in an experimental model of intimal hyperplasia. J Photochem Photobiol B: Biol. 2000;1(57):94-101.
- 18. Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines. 2001;5:105-29.
- 19. Sternberg E, Dolphin D. Pyrrolic photosensitizers. Curr Med Chem. 1996;3 (4):239-72.
Publication Dates
-
Publication in this collection
07 Feb 2007 -
Date of issue
2006