Abstract
Objective:
To assess the effects of exposure time and gauge of Foley catheters in biofilm formation.
Method:
In vitro study with samples of Foley catheter fragments made of siliconized latex of different gauges (#14 and #16 French gauge). Artificial urine was produced, which was inoculated with Staphylococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853) standard bacteria, incubated at 37 °C for 24 hours and 72 hours. The material was analyzed by means of culture (bacterial load) and scanning electron microscopy.
Results:
There was no difference in bacterial load of biofilms formed in Foley catheter surfaces with regard to different gauges (p > 0.05). On the other hand, exposure time (24 hours and 72 hours) was a determining factor for P. aeruginosa biofilm formation in Foley catheters (p < 0.05).
Conclusion:
Exposure time had an effect on P. aeruginosa biofilm formation in Foley catheters, regardless of gauges.
Keywords
Biofilm; Urinary catheters; Urinary tract infections; Staphylococcus aureus; Pseudomonas aeruginosa
Resumo
Objetivo:
Avaliar a influência do tempo de exposição e calibre na formação de biofilme em cateteres urinários de Foley (CUFs).
Método:
Pesquisa in vitro com amostras de fragmentos de CUFs em látex siliconizado de diferentes calibres (n° 14 e n° 16 Frenchs). A urina artificial foi confeccionada, inoculada com bactérias-padrão Staphylococcus aureus (ATCC 25923) e Pseudomonas aeruginosa (ATCC 27853) e incubada a 37 °C por 24 horas e 72 horas. As análises foram realizadas por meio de cultura (carga bacteriana) e microscopia eletrônica de varredura.
Resultados:
Não houve diferença na carga bacteriana dos biofilmes formados nas superfícies dos CUFs com relação aos diferentes calibres (p > 0,05). Por outro lado, o tempo de exposição (24 horas e 72 horas) foi o fator determinante para formação do biofilme de P. aeruginosa nos CUFs (p < 0,05).
Conclusão:
O tempo de exposição influenciou a formação do biofilme de P. aeruginosa nos CUFs, independentemente dos calibres.
Descritores
Biofilmes; Cateteres urinários; Infecções urinárias; Staphylococcus aureus; Pseudomonas aeruginosa
Resumen
Objetivo:
Evaluar la influencia del tiempo de exposición y calibre en la formación de biofilm en catéteres urinarios de Foley (CUFs).
Método:
Investigación in vitro con muestras de fragmentos de CUFs en látex siliconizado de diferentes calibres (n ° 14 y n° 16 Frenchs). La orina artificial fue confeccionada, inoculada con bacterias estándar Staphylococcus aureus (ATCC 25923) y Pseudomonas aeruginosa (ATCC 27853) e incubada a 37 °C durante 24 horas y 72 horas. Los análisis se realizaron por medio de cultivo (carga bacteriana) y microscopía electrónica de exploración.
Resultados:
No hubo diferencia en la carga bacteriana de los biofilmes formados en las superficies de los CUFs en relación con los diferentes calibres (p> 0,05). Por otro lado, el tiempo de exposición (24 horas y 72 horas) fue el factor determinante para la formación del biofilm de P. aeruginosa en los CUFs (p <0,05).
Conclusión:
El tiempo de exposición influenció la formación del biofilm de P. aeruginosa en los CUFs, independientemente de los calibres.
Descriptores
Biopelículas; Catéteres urinarios; Infecciones urinarias; Staphylococcus aureus; Pseudomonas aeruginosa
Introduction
Healthcare-associated infections (HAI) are a global health issue, with significant effects, especially when they take place in hospitals and with immunocompromised individuals. In different surveys, urinary tract infection (UTI) is one of the most prevalent and worrying infections in elderly people due to its high morbidity rate.(11. Sousa ÁF, Queiroz AA, Oliveira LB, Moura LK, Andrade D, Watanabe E, et al. Deaths among the elderly with ICU infections. Rev Bras Enferm. 2017;70(4):733–9.)
Catheter-associated urinary tract infections (CAUTI) is defined as any UTI affecting patients who have been using urinary catheters for more than two days and who had a catheter inserted on the day the infection occurred or who had it removed the day before.(22. Agência Nacional de Vigilância Sanitária (ANVISA). Medidas de prevenção de infecção relacionada à assistência à saúde [Internet]. 2a ed. Brasília (DF): ANVISA; 2017. [citado 2018 Set 12]. Disponível em: https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/caderno-5
https://www20.anvisa.gov.br/segurancadop...
)
The situation is alarming since 15% to 25% of admitted patients are submitted to urinary catheterization, in most cases without proper indication.(33. Barbadoro P, Labricciosa FM, Recanatini C, Gori G, Tirabassi F, Martini E, et al. Catheter-associated urinary tract infection: role of the setting of catheter insertion. Am J Infect Control. 2015;43(7):707–10.)
With regard to urinary catheters made of latex or silicone, CAUTI risk frequency in hospitalized patients was similar; however, the use of devices coated with silver or nitrofurazone showed a decrease in rates of this kind of infection.(44. Pickard R, Lam T, Maclennan G, Starr K, Kilonzo M, McPherson G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16(47):1–197.)
The etiological agents that are more often related to CAUTI are: Pseudomonas aeruginosa, Proteus mirabilis, Candida spp., Escherichia coli and Klebsiella pneumoniae,(55. Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016 Sep;57(9):485–90.,66. Schulz L, Hoffman RJ, Pothof J, Fox B. Top ten myths regarding the diagnosis and treatment of urinary tract infections. J Emerg Med. 2016;51(1):25–30.) which can be present in the form of a “community” embedded in an extracellular polymeric material matrix composed of carbohydrates, proteins, and nucleic acids, called biofilm.
The impact of biofilms on HAIs is an object of research around the world, especially in prolonged use products. Hence, strategies to control biofilm formation in these devices are still a challenge.(77. Sousa AF, Marques DM, Monteiro RM, Queiroz AA, Andrade D, Watanabe E. Prevention of biofilm formation on artificial pacemakers: is it feasible? Acta Paul Enferm. 2017;30(6):644–50.) In urology, the treatment of microbial colonization and the resulting infection from biofilms is complex and often requires the removal of urinary catheters.(88. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–84.,99. Melzer M, Welch C. Outcomes in UK patients with hospital-acquired bacteraemia and the risk of catheter-associated urinary tract infections. Postgrad Med J. 2013;89(1052):329–34.)
The scientific literature reports the importance of the correct choice of the Foley catheter gauge mainly for the prevention of urethral traumas,(1010. Lo E, Nicolle LE, Coffin SE, Gould C, Maragakis LL, Meddings J, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464–79.,1111. Azar R, Shadpour P. In Vivo Trial of a Novel Atraumatic Urinary Catheter Design for Prevention of Catheter-Induced Trauma. J Endourol. 2016;30(7):822–7.) but there are no reports of studies that address the correlation between exposure time in biofilm formation and the gauge of these devices.
Therefore, our study has the following research questions: How exposure time and the gauge of Foley catheters in artificial urine (AU) contaminated with Staphylococcus aureus and Pseudomonas aeruginosa can have an influence on biofilm formation? What are the bacterial load and microscopic morphology of biofilms formed in catheters coming into contact with AU, in different experimental conditions (bacterial inoculum type, exposure time, and catheter gauge)?
In order to answer these questions, this study tried to assess the influence of exposure time and catheter gauge on biofilm formation in Foley catheters.
Methods
This experimental/laboratory study was conducted in vitro with the use of two-way Foley catheters (Teleflex, Kamunting, Perak, Malaysia) made of latex and silicone (siliconized), #14 and #16 French gauge (Fr). It is worth highlighting that the choice for catheters of different gauges was based on clinical practice, as a result of a more frequent use in adult patients. In order to simulate an actual Foley catheter in the urinary tract, as well as the chemical and nutritional conditions for the development of biofilms, an artificial urine (AU) with pH = 6.1 was produced according to Levering,(1212. Potter P, Perry A, Stockert P, Hall A, Ochs A. Fundamentals of nursing - text and study guide package. 9th ed. Saint Louis: Elsevier Health Sciences; 2017.) in which the sterilization process was changed and performed with the help of 0.22 μm filters (urea) and vertical autoclave (Phoenix, Araraquara, SP, Brazil) at 120 °C for 20 minutes (jelly and other AU components).
The whole microbiological experiment was conducted with five samples using aseptic biosafety techniques in a class II type A1 biosafety cabinet (VECO Group, Campinas, SP, Brazil), in the laboratory of the Study Center for Infection Prevention and Control in Health Services (NEPECISS, as per its acronym in Portuguese) of the Ribeirão Preto College of Nursing at the University of São Paulo.
Catheter fragments (CF) cut across (3 cm) and lengthwise were transferred to Falcon tubes (15 ml) containing 7 ml of AU and 1% of standard bacterial inoculum (~108 CFU/ml – colony forming units per milliliter) of Staphylococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853), separately, by means of a spectrophotometer (Spectrumlab, China; λ = 625 nm and absorbance between 0.08 and 0.100). Incubation was carried out in an orbital shaking incubator (Quimis, Diadema, SP, Brazil) at 37 °C for 24 hours and 72 hours, in which CFs were transferred to Falcon tubes (15 ml) with 7 ml of AU every 24 hours, but without bacterial inoculum so as to allow proper development of biofilms.
Once the incubation period was elapsed, CFs were rinsed three times with 5 ml of a saline solution at 0.85% (SF) and sterilized before they were transferred to Falcon tubes containing 7 ml of SF. Afterwards, CFs were homogenized with glass pearls in tube shakers (Phoenix Luferco, Araraquara, SP, Brazil) for two minutes, submitted to serial decimal dilutions in microtubes with 450 μl of SF up to 10-5 (24 hours) and 10-7 (72 hours); and diluted portions of 50 μl of in natura samples were spread onto Petri dishes with Mannitol Salt Agar (BD Difco™, Sparks, MD, USA) for S. aureus and Cetrimide Agar (BD Difco™, Sparks, MD, USA) for P. aeruginosa. After incubation at 37 °C for 48 hours in an incubator (Quimis, Diadema, SP, Brazil), the bacterial load (CFU/CF) was determined with the help of a stereomicroscope under reflected light. Furthermore, negative controls (without bacterial inocula) were used to assess the sterility of culture media, AU, materials and reagents after incubation at 37 °C for 14 days.
Measurement of AU pH before and after 24 hours and 72 hours of incubation with bacteria was performed with pH indicator strips (Kasvi, Curitiba, PR, Brazil). For the analysis of biofilms formed in CFs by means of scanning electron microscopy (SEM), the samples of biological material (biofilm) were fixed with glutaraldehyde at 2.5% for at least 12 hours, dehydrated in series of alcohols (15%, 30%, 50%, 70%, 95%, and 100%) for 15 minutes for each concentration, impregnated with gold and submitted to analysis by SEM Zeiss EVO 50 in the Scanning Electron Microscopy Laboratory of the Chemistry Department of the Philosophy, Arts, and Science Faculty - USP, which belongs to the Multiuser Equipment Program of the São Paulo Research Foundation (FAPESP) - File number 04/09320-9.
Data collected were submitted to appropriate encoding, double typing validation, exported to BioEstat® (version 5.3) software and analyzed by descriptive statistics (mean, standard deviation, minimum and maximum values) and Kruskal-Wallis non-parametric test followed by Student–Newman–Keuls method. A significance level of 5% was defined.
Results
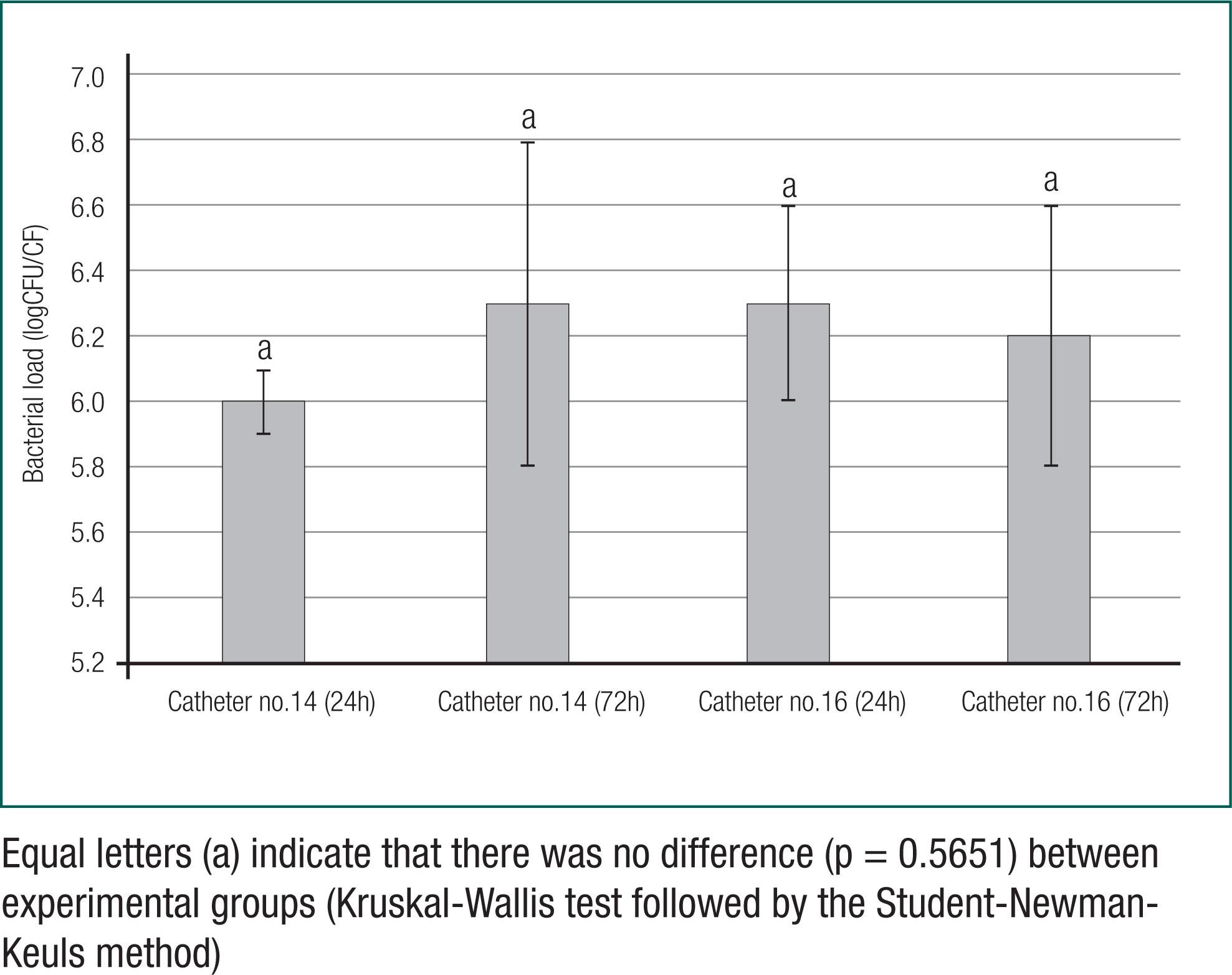
According to figure 1, the assessment of means of bacterial loads (logCFU/CF) revealed that there was no difference in biofilm formation of S. aureus with regard to exposure time and Foley catheter gauge (p = 0.5651).
Comparison of biofilm formation through means of bacterial loads (logCFU/CF) of Staphylococcus aureus in urinary catheter fragments (Foley) - (CF) with different gauges (14 and 16) and exposure times (24 hours and 72 hours)
On the other hand, according to figure 2, there was a difference between the means of bacterial loads (logCFU/CF) of P. aeruginosa in the biofilm with regard to different exposure times (24 hours and 72 hours) for gauges 14 (p = 0.0046) and 16 (p = 0.0162) of CFs. However, CF gauges did not have an influence on biofilm formation of P. aeruginosa in both exposure times, 24 hours (p = 0.5212) and 72 hours (p = 0.8307).
Comparison of biofilm formation by means of means of bacterial loads (logCFU/CF) of Pseudomonas aeruginosa in urinary catheter fragments (Foley) - (CF) with different gauges (14 and 16) and exposure times (24 hours and 72 hours)
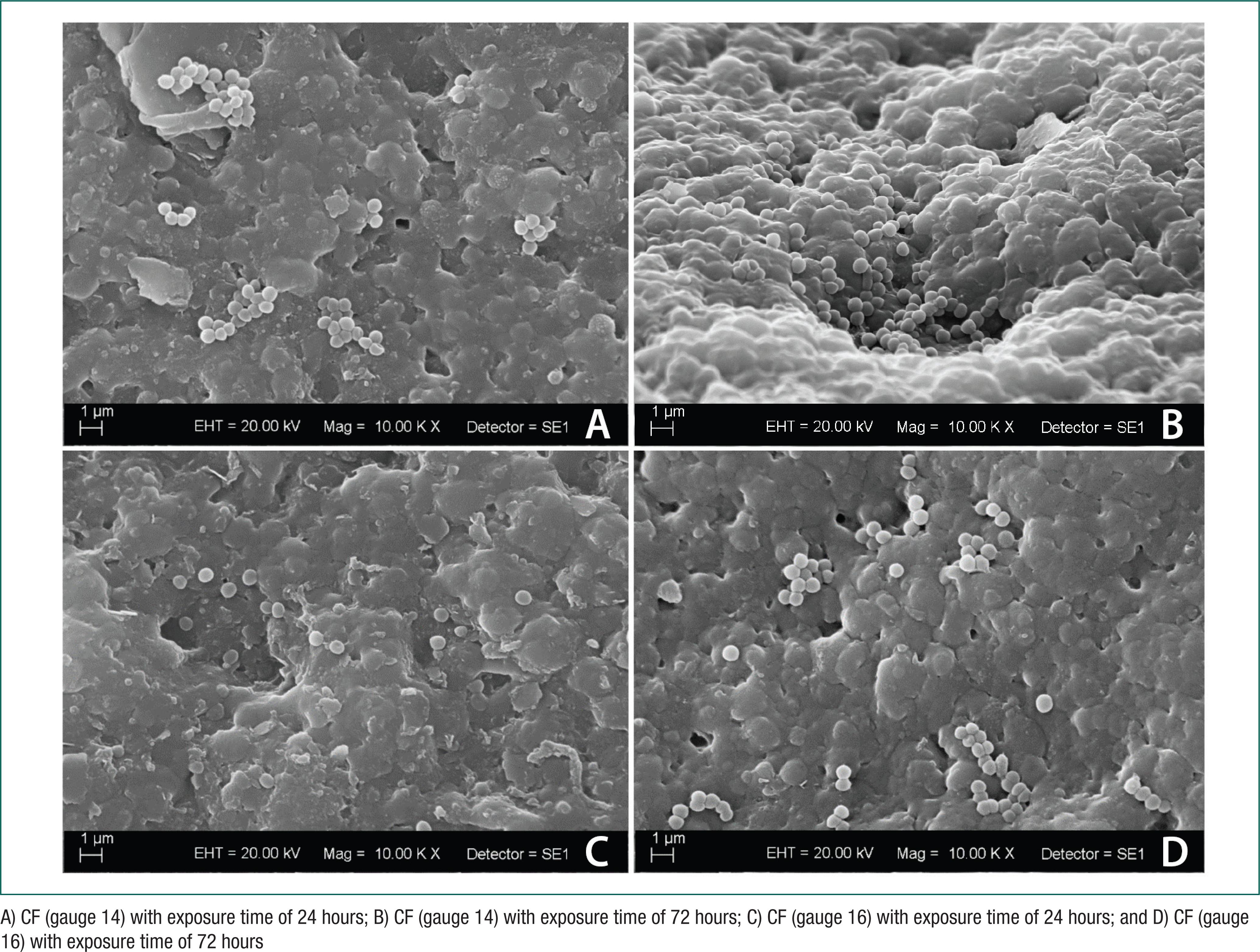
With regard to pH values of AU samples before and after exposure time for biofilm formation in CFs, they remained unchanged throughout the whole experiment (pH = 6.1). Photomicrographs (10.000×) obtained by SEM showed the formation of biofilms composed of dense and large matrices of extracellular polymeric substances and coccus grouped as staphylococcus (S. aureus) (Figure 3), as well as rod bacteria (P. aeruginosas) (Figure 4) in all CF samples. According to figure 3, no difference was seen in biofilm formation (S. Aureus) compared to exposure time (24 hours and 72 hours) and CF gauges (14 and 16).
Photomicrographs by SEM of urinary Foley catheter fragments (CF) with S. aureus biofilm formation
Photomicrographs by SEM of urinary Foley catheter fragments (CF) with P. aeruginosa biofilm formation
In addition, the difference in biofilm formation (P. Aeruginosas) was only observed as to exposure time (24 hours and 72 hours) and not to CF gauges (14 and 16) (Figure 4).
Discussion
The scientific literature presents a series of recommendations regarding the importance of the correct choice of Foley catheters for prevention, especially urethral traumas, although there are few studies about the approach to the correlation between the gauges of these devices and biofilm formation.(1414. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014 Jul 25;3:23. doi: 10.1186/2047-2994-3-23. eCollection 2014. Review.
https://doi.org/10.1186/2047-2994-3-23...
,1515. Mandakhalikar KD, Rahmat JN, Chiong E, Neoh KG, Shen L, Tambyah PA. Extraction and quantification of biofilm bacteria: method optimized for urinary catheters. Sci Rep. 2018;8(1):8069.) In our study, there was no influence of CF gauges (14 and 16) on the means of bacterial loads (logCFU/CF) for S. aureus (p = 0.5651) (Figure 1) and P. aeruginosa biofilms, exposure times of 24 hours (p = 0.5212) and 72 hours (p = 0.8307) (Figure 2), as well as in the SEM analysis (Figures 3 and 4). Foley catheters used in clinical practice may enable the inlaying of crystals and, consequently, the blocking of lumen of these devices, since they are basically made of latex, a material that is suitable for this issue and for biofilm formation.(1616. Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med. 2014;276(2):120–9.) As an alternative to reduce urethral inflammation, the use of silicone Foley catheter was efficient, but it was not able to minimize biofilm formation.(1717. Verma A, Bhani D, Tomar V, Bachhiwal R, Yadav S. Differences in Bacterial Colonization and Biofilm Formation Property of Uropathogens between the Two most Commonly used Indwelling Urinary Catheters. J Clin Diagn Res. 2016;10(6):PC01–03.) By contrast, other authors reported a decrease in risks of inlaying and biofilm formation in silicone Foley catheters when compared to those made of latex, depending on the type of microorganism.(22. Agência Nacional de Vigilância Sanitária (ANVISA). Medidas de prevenção de infecção relacionada à assistência à saúde [Internet]. 2a ed. Brasília (DF): ANVISA; 2017. [citado 2018 Set 12]. Disponível em: https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/caderno-5
https://www20.anvisa.gov.br/segurancadop...
,1818. Tamura NK, Gasparetto A, Svidzinski TI. Evaluation of the adherence of Candida species to urinary catheters. Mycopathologia. 2003;156(4):269–72.)
Thus, biofilms can be formed by a single type of bacteria or several species, with the possibility of forming structures that are three-dimensional, heterogeneous and highly resistant to physical and chemical agents.(1919. Santos AL, Galdino AC, Mello TP, Ramos LS, Branquinha MH, Bolognese AM, et al. What are the advantages of living in a community? A microbial biofilm perspective! Mem Inst Oswaldo Cruz. 2018;113(9):e180212.,2020. De Souza PR, De Andrade D, Cabral DB, Watanabe E. Endotracheal tube biofilm and ventilator-associated pneumonia with mechanical ventilation. Microsc Res Tech. 2014;77(4):305–12.) In this study, all samples of CF made of latex and silicone formed S. aureus and P. aeruginosa biofilms, separately. The microbial colonization and biofilm formation in Foley catheter can begin right after its insertion, at a rate of 5% to 10% a day, and by four weeks all devices will be colonized. It is estimated that 50% of patients with short-term Foley catheters (up to 7 days) get CAUTI.(2121. Mandakhalikar KD, Chua RR, Tambyah PA. New technologies for prevention of catheter associated urinary tract infection. Curr Treat Options Infect Dis. 2016;8(1):24–41.,2222. Ramanathan R, Duane TM. Urinary tract infections in surgical patients. Surg Clin North Am. 2014;94(6):1351–68.) Biofilm formation in Foley catheters is a process that depends on a series of physical and chemical factors, such as the presence of nutrients and urinary flow, design and length of exposure to the device.(2323. Maeda T, García-Contreras R, Pu M, Sheng L, Garcia LR, Tomás M, et al. Quorum quenching quandary: resistance to antivirulence compounds. ISME J. 2012;6(3):493–501.)
Researchers have shown that Foley catheter exposure time for biofilm formation varies and depends on the type of microbial consortia and material. Therefore, it can occur right after the insertion or up to 24 hours, or from 3 to 7 days.(1616. Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med. 2014;276(2):120–9.,2424. Alves MJ, Barreira JC, Carvalho I, Trinta L, Perreira L, Ferreira IC, et al. Propensity for biofilm formation by clinical isolates from urinary tract infections: developing a multifactorial predictive model to improve antibiotherapy. J Med Microbiol. 2014;63(Pt 3):471–7.
25. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA; Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–26.
26. Tenke P, Mezei T, Bőde I, Köves B. Catheter-associated Urinary Tract Infections. Eur Urol Suppl. 2017;16(4):138–43.-2727. Tenke P, Köves B, Nagy K, Hultgren SJ, Mendling W, Wullt B, et al. Update on biofilm infections in the urinary tract. World J Urol. 2012;30(1):51–7.) In our study, exposure times (24 hours and 72 hours) had an influence only on biofilm formation of P. aeruginosa in both gauges 14 (p = 0.0046) and 16 (p = 0.0162) of CFs. These results suggest that exposure time can affect biofilm formation, depending on the bacterial type.
The urine of a healthy individual has an approximate pH of 6.0 Due to the bacterial metabolism, urine pH can increase and lead to the inlaying of calcium and magnesium crystals, as well as biofilm formation in Foley catheters.(2828. Milo S, Thet NT, Liu D, Nzakizwanayo J, Jones BV, Jenkins AT. An in-situ infection detection sensor coating for urinary catheters. Biosens Bioelectron. 2016;81:166–72.) However, in our study, pH values of AU samples before and after exposure time for biofilm formation in CFs remained unchanged throughout the whole experiment (pH = 6.1). This result might be explained by the constant renewal of AU every 24 hours, with the purpose of properly feeding and developing biofilms. In this way, this type of study is essential due to its contribution to the progress of scientific knowledge about the influence of variables such as time and gauge on biofilm formation in Foley catheters. It is also worth mentioning the pioneering spirit of nursing in the analysis, by means of SEM, of microscopic morphology of biofilms in the most commonly used devices in clinical practice.
Therefore, care in the insertion and maintenance of Foley catheters are essential, in addition to the identification of more prevalent microorganisms, since nursing and healthcare teams can prevent CUTAI. In addition, the influence of Foley catheter exposure time suggested the need for constant monitoring, especially in longer periods, with this device being removed as soon as possible. This study has some limitations that are inherent to an in vitro experiment. Since this is not a research carried out with human beings (artificial urine was used), standard bacterial strains in biofilm formation with a single bacterial species (single-species), exposure times and gauges were defined. Therefore, the results cannot reflect clinical reality accurately.
Conclusion
All Foley catheter fragments, regardless of the gauge (14 and 16 French gauge) and bacterial type (S. aureus e P. aeruginosa), had a biofilm formation in artificial urine with an unchanged pH. However, the gauges did not show any significant difference in biofilm formation, and exposure time had an influence only on P. aeruginosa biofilm.
Referências
-
1Sousa ÁF, Queiroz AA, Oliveira LB, Moura LK, Andrade D, Watanabe E, et al. Deaths among the elderly with ICU infections. Rev Bras Enferm. 2017;70(4):733–9.
-
2Agência Nacional de Vigilância Sanitária (ANVISA). Medidas de prevenção de infecção relacionada à assistência à saúde [Internet]. 2a ed. Brasília (DF): ANVISA; 2017. [citado 2018 Set 12]. Disponível em: https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/caderno-5
» https://www20.anvisa.gov.br/segurancadopaciente/index.php/publicacoes/item/caderno-5 -
3Barbadoro P, Labricciosa FM, Recanatini C, Gori G, Tirabassi F, Martini E, et al. Catheter-associated urinary tract infection: role of the setting of catheter insertion. Am J Infect Control. 2015;43(7):707–10.
-
4Pickard R, Lam T, Maclennan G, Starr K, Kilonzo M, McPherson G, et al. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technol Assess. 2012;16(47):1–197.
-
5Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016 Sep;57(9):485–90.
-
6Schulz L, Hoffman RJ, Pothof J, Fox B. Top ten myths regarding the diagnosis and treatment of urinary tract infections. J Emerg Med. 2016;51(1):25–30.
-
7Sousa AF, Marques DM, Monteiro RM, Queiroz AA, Andrade D, Watanabe E. Prevention of biofilm formation on artificial pacemakers: is it feasible? Acta Paul Enferm. 2017;30(6):644–50.
-
8Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–84.
-
9Melzer M, Welch C. Outcomes in UK patients with hospital-acquired bacteraemia and the risk of catheter-associated urinary tract infections. Postgrad Med J. 2013;89(1052):329–34.
-
10Lo E, Nicolle LE, Coffin SE, Gould C, Maragakis LL, Meddings J, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464–79.
-
11Azar R, Shadpour P. In Vivo Trial of a Novel Atraumatic Urinary Catheter Design for Prevention of Catheter-Induced Trauma. J Endourol. 2016;30(7):822–7.
-
12Potter P, Perry A, Stockert P, Hall A, Ochs A. Fundamentals of nursing - text and study guide package. 9th ed. Saint Louis: Elsevier Health Sciences; 2017.
-
13Levering V, Wang Q, Shivapooja P, Zhao X, López GP. Soft robotic concepts in catheter design: an on-demand fouling-release urinary catheter. Adv Healthc Mater. 2014;3(10):1588–96.
-
14Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014 Jul 25;3:23. doi: 10.1186/2047-2994-3-23. eCollection 2014. Review.
» https://doi.org/10.1186/2047-2994-3-23 -
15Mandakhalikar KD, Rahmat JN, Chiong E, Neoh KG, Shen L, Tambyah PA. Extraction and quantification of biofilm bacteria: method optimized for urinary catheters. Sci Rep. 2018;8(1):8069.
-
16Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med. 2014;276(2):120–9.
-
17Verma A, Bhani D, Tomar V, Bachhiwal R, Yadav S. Differences in Bacterial Colonization and Biofilm Formation Property of Uropathogens between the Two most Commonly used Indwelling Urinary Catheters. J Clin Diagn Res. 2016;10(6):PC01–03.
-
18Tamura NK, Gasparetto A, Svidzinski TI. Evaluation of the adherence of Candida species to urinary catheters. Mycopathologia. 2003;156(4):269–72.
-
19Santos AL, Galdino AC, Mello TP, Ramos LS, Branquinha MH, Bolognese AM, et al. What are the advantages of living in a community? A microbial biofilm perspective! Mem Inst Oswaldo Cruz. 2018;113(9):e180212.
-
20De Souza PR, De Andrade D, Cabral DB, Watanabe E. Endotracheal tube biofilm and ventilator-associated pneumonia with mechanical ventilation. Microsc Res Tech. 2014;77(4):305–12.
-
21Mandakhalikar KD, Chua RR, Tambyah PA. New technologies for prevention of catheter associated urinary tract infection. Curr Treat Options Infect Dis. 2016;8(1):24–41.
-
22Ramanathan R, Duane TM. Urinary tract infections in surgical patients. Surg Clin North Am. 2014;94(6):1351–68.
-
23Maeda T, García-Contreras R, Pu M, Sheng L, Garcia LR, Tomás M, et al. Quorum quenching quandary: resistance to antivirulence compounds. ISME J. 2012;6(3):493–501.
-
24Alves MJ, Barreira JC, Carvalho I, Trinta L, Perreira L, Ferreira IC, et al. Propensity for biofilm formation by clinical isolates from urinary tract infections: developing a multifactorial predictive model to improve antibiotherapy. J Med Microbiol. 2014;63(Pt 3):471–7.
-
25Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA; Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–26.
-
26Tenke P, Mezei T, Bőde I, Köves B. Catheter-associated Urinary Tract Infections. Eur Urol Suppl. 2017;16(4):138–43.
-
27Tenke P, Köves B, Nagy K, Hultgren SJ, Mendling W, Wullt B, et al. Update on biofilm infections in the urinary tract. World J Urol. 2012;30(1):51–7.
-
28Milo S, Thet NT, Liu D, Nzakizwanayo J, Jones BV, Jenkins AT. An in-situ infection detection sensor coating for urinary catheters. Biosens Bioelectron. 2016;81:166–72.
Publication Dates
-
Publication in this collection
2018
History
-
Received
19 July 2018 -
Accepted
15 Nov 2018