Abstracts
Male goats of mating age serologically negative for Toxoplasma gondii were divided into three groups: GI - controls (placebo) (n = 2); GII - infected with 1 × 10(6) tachyzoites (RH strains) (n = 2); and GIII - infected with 2 × 10(5) oocysts (P strains) (n = 2). Clinical, hematology, parasite and serology tests and studies of parasites in the semen through bioassay and polymerase chain reaction (PCR), and in reproductive organs (bioassay) were performed to assess toxoplasma infection. Serological titers peaked at 4096 in two animal groups infected with the protozoan. The bioassays allowed an early detection of protozoa in semen samples of tachyzoite-inoculated animals. T. gondii DNA was identified through PCR in the semen in five (Days 5, 7, 28, 49, and 70) and two (both at day 56) different days post-inoculation in GII and GIII animals, respectively. It was also possible to detect T. gondii DNA in reproductive organs (prostate pool, testicles, seminal vesicle and epididymis) of goats inoculated with either tachyzoites or oocysts. The present study suggests the possibility of venereal transmission of T. gondii among goats and it should be further assessed.
Toxoplasma gondii; goats; reproductive systems; PCR and bioassay
Caprinos machos, em idade reprodutiva, sorologicamente negativos para Toxoplasma gondii foram distribuídos em três grupos de animais: GI (n = 2) controle (placebo), GII (n = 2) - infectado com 1 × 10(6) taquizoítos (cepa RH) e GIII (n = 2) infectado com 2 × 10(5) oocistos (cepa P). Exames clínicos, hematológicos, parasitêmicos, sorológicos, pesquisa no sêmen e em tecidos do sistema reprodutor, por meio da bioprova, e da Reação em Cadeia pela Polimerase (PCR), foram conduzidas para avaliar a infecção toxoplásmica. Os títulos sorológicos alcançaram valores máximos de 4096 nos dois grupos de animais infectados. Pela técnica da bioprova, foi possível revelar precocemente a presença do coccídio nas amostras seminais dos animais inoculados com taquizoítos. Pela PCR, foi possível identificar, no sêmen, material genético de T. gondii, em cinco (5º, 7º, 28º, 49º e 70º) e em duas (ambos ao 56º) datas experimentais pós-inoculação dos animais pertencentes aos grupos GII e GIII, respectivamente.Por esta mesma técnica, foi possível ainda isolar material genético deste protozoário, também em amostras teciduais (pool de próstata, testículo, vesícula seminal e epidídimo) dos caprinos inoculados com taquizoítos e oocistos. A presente pesquisa sugere a possibilidade da ocorrência da transmissão sexual do T. gondii na espécie caprina.
Toxoplasma gondii; caprinos; sistema reprodutor; PCR e bioprova
FULL ARTICLE
Detection of Toxoplasma gondii in the reproductive system of male goats
Detecção de Toxoplasma gondii no sistema reprodutor de caprinos machos
Luís Fernando Santana; Alvimar José da Costa; Juliana Pieroni; Welber Daniel Zanetti Lopes; Ricardo Silva Santos; Gilson Pereira de Oliveira; Rafael Paranhos de Mendonça; Cláudio Alessandro Massamitsu Sakamoto
Departamento de Patologia Veterinária, Centro de Pesquisas em Sanidade Animal, Universidade Estadual Paulista - UNESP
Corresponding author Corresponding author: Luís Fernando Santana Departamento de Patologia Veterinária, Centro de Pesquisas em Sanidade Animal, Universidade Estadual Paulista - UNESP Via de acesso Prof. Paulo Donatto Castellani, s/n CEP 14884-900, Jaboticabal - SP, Brazil e-mail: lfsantana_2000@yahoo.com.br
ABSTRACT
Male goats of mating age serologically negative for Toxoplasma gondii were divided into three groups: GI - controls (placebo) (n = 2); GII - infected with 1 × 106 tachyzoites (RH strains) (n = 2); and GIII - infected with 2 × 105 oocysts (P strains) (n = 2). Clinical, hematology, parasite and serology tests and studies of parasites in the semen through bioassay and polymerase chain reaction (PCR), and in reproductive organs (bioassay) were performed to assess toxoplasma infection. Serological titers peaked at 4096 in two animal groups infected with the protozoan. The bioassays allowed an early detection of protozoa in semen samples of tachyzoite-inoculated animals. T. gondii DNA was identified through PCR in the semen in five (Days 5, 7, 28, 49, and 70) and two (both at day 56) different days post-inoculation in GII and GIII animals, respectively. It was also possible to detect T. gondii DNA in reproductive organs (prostate pool, testicles, seminal vesicle and epididymis) of goats inoculated with either tachyzoites or oocysts. The present study suggests the possibility of venereal transmission of T. gondii among goats and it should be further assessed.
Keywords: Toxoplasma gondii, goats, reproductive systems, PCR and bioassay.
RESUMO
Caprinos machos, em idade reprodutiva, sorologicamente negativos para Toxoplasma gondii foram distribuídos em três grupos de animais: GI (n = 2) controle (placebo), GII (n = 2) - infectado com 1 × 106 taquizoítos (cepa RH) e GIII (n = 2) infectado com 2 × 105 oocistos (cepa P). Exames clínicos, hematológicos, parasitêmicos, sorológicos, pesquisa no sêmen e em tecidos do sistema reprodutor, por meio da bioprova, e da Reação em Cadeia pela Polimerase (PCR), foram conduzidas para avaliar a infecção toxoplásmica. Os títulos sorológicos alcançaram valores máximos de 4096 nos dois grupos de animais infectados. Pela técnica da bioprova, foi possível revelar precocemente a presença do coccídio nas amostras seminais dos animais inoculados com taquizoítos. Pela PCR, foi possível identificar, no sêmen, material genético de T. gondii, em cinco (5º, 7º, 28º, 49º e 70º) e em duas (ambos ao 56º) datas experimentais pós-inoculação dos animais pertencentes aos grupos GII e GIII, respectivamente.Por esta mesma técnica, foi possível ainda isolar material genético deste protozoário, também em amostras teciduais (pool de próstata, testículo, vesícula seminal e epidídimo) dos caprinos inoculados com taquizoítos e oocistos. A presente pesquisa sugere a possibilidade da ocorrência da transmissão sexual do T. gondii na espécie caprina.
Palavras-chave: Toxoplasma gondii, caprinos, sistema reprodutor, PCR e bioprova.
Introduction
Toxoplasma gondii is the causative agent of toxoplasmosis, a cosmopolitan zoonosis of medical and veterinary relevance leading to miscarriage and a congenital disorder in intermediary hosts (TENTER et al., 2000; MASSALA et al., 2003).
Brazil has a goat herd of over 13 million animals but little is known about the effects of toxoplasmosis among goats. Munday and Mason (1979) were the first to describe toxoplasmosis as an important cause of reproductive losses in goats. Although often unnoticed, this infection can cause significant damage in both young and adult animals (DUBEY, 1987). The main route of infection is ingestion of the parasite's sporulated oocysts present in the environment (DUBEY; BEVERLEY, 1988). Risk factors for T. gondii infection in goats include age, number of cats in the farm, and either no use of feeding troughs or use of wooden feeding troughs (CAVALCANTE et al., 2008).
The objective of this study was to detect the presence of T. gondii in semen samples of experimentally infect goats using bioassay as well as molecular and histopathology techniques.
Material and Methods
1. Experimental infection of goats with T. gondii
This study used "P" (JAMRA; VIEIRA 1991) and "RH" (SABIN, 1941) T. gondii strains kept at the Animal Health Research Center (CPPAR), School of Agrarian and Veterinary Sciences, Universidade Estadual de São Paulo, Jaboticabal campus, São Paulo, Brazil. "P" strains were genotypically characterized by using PCR-RFLP segment of locus SAG2 located in chromosome VIII as a genetic marker and classified as Type III. (BRESCIANI et al., 2009). "RH" strains were previously characterized as Type I by using the same analysis of loci SAG1, SAG2, new SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico (PENA et al., 2008; HERRMANN et al., 2010).
Six male goats of undefined breed serologically negative for T. gondii aged between one and two years were selected. Serology titers obtained by indirect immunofluorescence (IFI) were considered positive starting at a 1:16 dilution. The animals were identified, sorted and randomized into three groups: GI - non-inoculated controls (n = 2); GII - subcutaneously infected with 1 × 106 RH strain tachyzoites per animal (n = 2); and GIII -orally infected with 2 × 105 P strain oocysts per animal (n = 2).
All goats were kept in proper individual stalls with water and food ad libitum. Serological tests were carried out during the experimental period as follows: acidified buffered antigen test (ALTON et al., 1988) for brucellosis; and microscopic agglutination test (COLE et al., 1973) for leptospirosis.
2. Clinical, hematology, serology and semen follow-up
The study animals were clinically evaluated (respiratory and heart frequency and rectal temperature) two days before inoculation and on Days 3, 5, 7, 11, 14, 21, 28, 35, 42, 49, 56, 63 and 70 post-inoculation (PI). Parasitemia was assessed using white mice according to Costa et al. (1977). Sera from blood samples collected two days before inoculation and on Days 3, 5, 7, 11 and 14 PI and then weekly until the end of the experiment from all experimentally infected goats were tested for T. gondii antibodies* * Monoclonal anti-goat/sheep IgG-FITC antibody produced in mouse - GT-34- purified immunoglobulin, buffered aqueous solution (Sigma). using IFI (CAMARGO, 1964). For nearly two months, all animals were submitted to semen collection with an electroejaculator. Ejaculate samples were obtained two days before inoculation and on Days 3, 5, 7, 11 and 14 PI and then weekly until the end of the experiment. T. gondii presence was assessed by bioassays in mice (DUBEY; SHARMA, 1980) and positive samples were tested through PCR.
3. Molecular detection of T. gondii DNA
The methods as proposed by Fuentes et al. (1996) were used for semen and tissue sample standardization. RH strain T. gondii tachyzoites were counted on a modified Neubauer chamber in dark field microscopy (400×) with at a concentration of 1010 parasites.mL-1. From this first dilution, 500 µL were diluted in 9.5 mL of sterile saline solution (SST), and dilutions of 109, 108, 107, 106, 105, 104, 103, 102, 101 and 100 parasites.mL-1 of the sample were successively obtained. Dilutions were adjusted in 700 µL volume aliquots and DNA extracted from each sample. T. gondii DNA of the scales of dilution (positive control) and from semen samples for DNA detection were extracted according to the modified method as described by Teale et al. (1982). At the end of the experiment, goats were euthanized** ** Procedures approved by the Animal Welfare and Ethics Commission (protocol number 010850-08) of School of Agrarian and Veterinary Sciences, Universidade Estadual de São Paulo (FCAV/UNESP), Jaboticabal campus, São Paulo, Brazil. and specimens from testes, seminal vesicles, epididymides and prostates were collected for histopathology tests and for T. gondii isolation by bioassay (DUBEY, 1980).
Results and Discussion
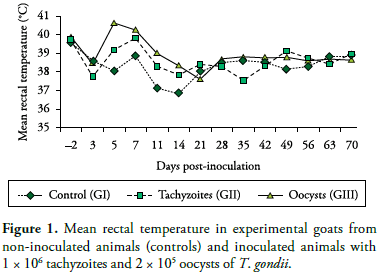
Following inoculation with T. gondii tachyzoites or oocysts, toxoplasma infection of goats was confirmed by parasitemia as well as seroconversion of the inoculated animals. Hyperthermia (40.65 ºC) on Day 5 PI was the most remarkable clinical sign seen in the group of animals inoculated with oocysts (Figure 1). Likewise, Nishi et al. (2001) reported that temperature increase is one of the most evident clinical signs in goats orally inoculated with 105 oocysts of T. gondii. Additionally, anorexia and lethargy were seen from Day 3 to 7 PI in all inoculated animals.
Thirteen parasitemic outbreaks were detected during the experimental period: one on Days 11, 21, 28, 49, 63, two on Day 56 and 70 and four on Day 14 PI. Parasitemic outbreaks during acute stage of disease have been described from Day 7 to 14 (CHHABRA et al., 1982) and Day 5 to 12 PI (NISHI et al., 2001).
Toxoplasma infection elicited a fast immune response in goats with antibody anti-IgG detection by IFI from Day 11 PI. An early humoral response was also detected by Nishi et al. (2001) on Day 10 PI, demonstrated through IFI (anti-IgG) test.
IgG detection started on Day 11 PI in both tachyzoite-inoculated goats (titers of 256 and 1024) and one oocyst-inoculated goat (256). The peak titer (4096) was detected on Days 21 and 28 PI in all animals of GI and GII, respectively. IgG titer decreased from Day 35 PI on, but remained high (1024) in all inoculated animals until the end of the experiment.
These results are partially inconsistent with that reported by Nishi et al. (2001) who detected serological titer peaks (IFI) at a later stage (after Day 35 PI) in goats infected with a different dose and strain of T. gondii (105 oocysts and AS 28 strain). Corroborating our findings, these authors found that serological titers remained at relatively high levels until Day 56 PI. Persistently high serological (IgG) levels until Day 70 PI found in the present study can be explained by the fact that in chronic infections IgG antibodies can remain "active" for a long time compared to IgM immunoglobulins, notably identified only a few weeks after infection, as described in Dubey and Towle (1986).
Dubey and Sharma (1980) used a bioassay in mice to demonstrate the presence of T. gondii in the semen of three goats orally inoculated with 104 oocysts of GT-1 strain (isolated from goats). Protozoa were detected on Day 7 PI in semen samples from two animals and from Day 12 PI on in the third animal studied. In semen samples of these animals it was possible to identifly parasite excretion on Day 59 PI. Despite differences between strains studied, in this study the protozoan was detected on Days 56 PI in semen samples from oocyst-inoculated animals.
Molecular detection of T. gondii DNA in semen samples from tachyzoite-infected animals in more experimental days (Days 5, 7, 28, 49 and 70) is similar to that found by Dubey and Sharma (1980). This technique also allowed to detecting T. gondii DNA in samples from two sporulated oocysts-infected animals on Day 56 DPI.
Bioassay-positive semen samples from animal 1 on Day 63 PI, animal 11 on Days 28 and 63 PI, and animal 47 on Day 70 PI were not PCR-positive for T. gondii (Figure 2). PCR results supported bioassay findings and confirmed that it is an useful ancillary tool for toxoplasma infection diagnosis in semen samples.
In contrast to Pescador et al. results (2007), histopathology tests did not identify any significant change related to T. gondii parasitism on tissues of the reproductive system. These authors, when evaluating tissues from goat miscarriage, found significant microscopic and macroscopic alterations like enlarged pale mesenteric lymph nodes with lymphoplasma cells infiltrate in brain and lungs.
Nishi et al. (2001) successfully isolated through bioassay T. gondii from brain tissue, lymph nodes, liver, kidneys, skeletal and cardiac muscles but they did not find any macroscopic lesions in these tissues. Similar findings were reported by Cavalcante et al. (2007) regarding protozoan isolation in skeletal muscle samples from goats slaughtered in the State of Ceará, Brazil.
Conclusion
T. gondii was isolated from semen samples of experimentally infected goats associated to tissue parasitism in specimens of the reproductive system diagnosed by bioassay and PCR techniques, suggesting the possibility of venereal transmission of this coccidium in goats.
Acknowledgements
This research study was partially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (04/12296-2) and Centro de Pesquisa em Sanidade Animal (CPPAR), Faculdade de Ciências Agrárias e Veterinárias, UNESP-Jaboticabal, SP, Brazil.
Received May 11, 2010
Accepted July 12, 2010
- ALTON, G. G. et al. Techniques for the brucellosis laboratory Paris: Institute National de la Recherche Agronomique, 1988. 190 p.
- BRESCIANI, K. D. S. et al. Transplacental transmission of Toxoplasma gondii in reinfected pregnant female canines. Parasitology Research, v. 104, n. 5, p. 1213-1217, 2009.
- CAMARGO, M. E. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Revista do Instituto de Medicina Tropical de São Paulo, v. 6, n. 3, p. 117-118, 1964.
- CAVALCANTE, A. C. R. et al. Risk factors for infection by Toxoplasma gondii in herds of goats in Ceará, Brazil. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, v. 60, n. 1, p. 36-41, 2008.
- CAVALCANTE, A. C. R. et al. Virulence and molecular characterization of Toxoplasma gondii isolated from goats in Ceará, Brazil. Small Ruminant Research,v. 69, n. 1-3, p. 79-82, 2007.
- CHHABRA, M. B. et al. Experimental toxoplasmosis in pregnant goats. Indian Journal of Animal Sciences, v. 52, p. 661-664, 1982.
- COLE, J. R.; SULZER, C. R.; PULSSELY, P. R. Improved microtechnique for the leptospiral microscopic agglutination test. Applied Microbiology, v. 25, n. 6, p. 976-980, 1973.
- COSTA, A. J. et al. Experimental infection of bovines with oocysts of Toxoplasma gondii Journal of Parasitology, v. 63, n. 2, p. 212-218, 1977.
- DUBEY, J. P. Mouse pathogenecity of Toxoplasma gondii isolated from a goat. American Journal of Veterinary Research, v. 41, n. 3, p. 427-429, 1980.
- DUBEY, J. P. Toxoplasmosis in goats. Agri-practice, v. 8, p. 43-52, 1987.
- DUBEY, J. P.; BEVERLEY, J. K. A. Toxoplasmosis of animals and man Boca Ráton: Boca Ráton Academic, 1988. 315 p.
- DUBEY, J. P.; SHARMA, S. P. Prolonged excretion of Toxoplasma gondii in semen of goats. American Journal of Veterinary Research, v. 41, n. 5, p. 794-795, 1980.
- DUBEY, J. P.; TOWLE, A. Toxoplasmosis in sheep St. Albans: Common Wealt. Institute of Parasitology, 1986.
- FUENTES, I. et al. Urine sample used for congenital toxoplasmosis diagnosis by PCR. Journal of Clinical Microbiology, v. 34, n. 10, p. 2368-2371, 1996.
- HERRMANN, D. C. et al. Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. International Journal for Parasitology, v. 40, n. 3, p. 285-292, 2010.
- JAMRA, L. M. F.; VIEIRA, M. P. L. Isolamento do Toxoplasma gondii de exsudato peritoneal e órgãos de camundongos com infecção experimental. Revista do Instituto de Medicina Tropical de São Paulo, v. 33, n. 6, p. 435-441, 1991.
- MASSALA, G. et al. Survey of ovine and caprine toxoplasmosis by IFAT and PCR assays in Sardinia, Italy. Veterinary Parasitology, v. 117, n. 1-2, p. 15-21, 2003.
- MUNDAY, B. L.; MASON, R. W. Toxoplasmosis as a cause of perinatal death in goats. Australian Veterinary Journal, v. 55, n. 10, p. 485-487, 1979.
- NISHI, S. M.; KASAI, N.; GENNARI, S. M. Antibody levels in goats fed Toxoplasma gondii oocysts. Journal of Parasitology, v. 87, n. 2, p. 445-447, 2001.
- PENA, H. F. J. et al. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. International Journal for Parasitology, v. 38, n. 5, p. 561-569, 2008.
- PESCADOR, C. A. et al. Perdas reprodutivas associadas com infecção por Toxoplasma gondii em caprinos no sul do Brasil. Pesquisa Veterinária Brasileira, v. 27, n. 4, p. 167-171, 2007.
- SABIN, A. B. Toxoplasmic encephalitis in children. The Journal Of the American Medical Association, v. 116, n. 9, p. 801-807, 1941
- TEALE, A. J. et al. Experimentally induced toxoplasmosis in young rams: the clinical syndrome and semen secretion of Toxoplasma The Veterinary Record, v. 111, n. 3, p. 53-55, 1982.
- TENTER, A. M.; HECKEROTH, A. R.; WEISS, L. M. Toxoplasma gondii: from animals to humans. International Journal for Parasitology, v. 30, n. 12-13, p. 1217-1258, 2000.
Corresponding author:
Publication Dates
-
Publication in this collection
20 Oct 2010 -
Date of issue
Sept 2010
History
-
Received
11 May 2010 -
Accepted
12 July 2010