ABSTRACT.
Poincianella pyramidalis (catingueira) is a endemic plant of the Caatinga, selected by animals grazing on native pasture. With the aim of evaluating characteristics indicative of its nutritional quality, 10 plants were selected and identified, sampled at five different ages, were used to determine dry matter (DM), crude protein (CP), neutral detergent fibre (NDF), mineral matter (MM), DM degradability (Deg DM), NDF degradability (Deg NDF) and in situ and in vitro leaf-tissue degradability. Phytochemical prospection was performed, and 1H and 13C nuclear magnetic resonance applied to detect the presence of secondary compounds. The data were submitted to analysis of variance and Tukey’s test at 5%, and correlation analysis was carried out on the variables for leaf maturity in days. The levels of CP, NDF and Deg NDF showed a negative correlation with the increases in leaf age. Leaf-tissue degradation was restricted due to a physical barrier developed in the leaf fragments, which can be attributed to plant defence mechanisms. The in situ degradability of the cell wall components decreased with the increase in leaf age. The high levels of tannins and lignin, and the strong presence of flavonoids, should be considered for their anti-nutritional and pharmacological potential.
Keywords:
degradability; native forage; nutrients; plant defence; tannins
Introduction
Caatinga, a type of vegetation found in the semi-arid region of Brazil, includes a diversity of native plants with forage potential, whose nutritive value is mainly utilised by small ruminants (Oliveira et al., 2016bOliveira, O. F., Santos, M. V. F., Cunha, M. V., Dubeux Júnior, J. C. B., Muir, J. P., Mello, A. C. L., ... Barros, G. F. N. P. (2016b). Botanical composition of Caatinga rangeland and diets selected by grazing sheep. Tropical Grasslands, 4(2), 71-81. doi: 10.1016/j.phytol.2016.02.017.
https://doi.org/10.1016/j.phytol.2016.02...
; Santos et al., 2008Santos, G. R. A., Batista, A. M. V., Guim, A., Santos, M. V. F., Silva, M. J. A., & Pereira, V. L. A. (2008). Determinação da composição botânica da dieta de ovinos em pastejo na Caatinga. Revista Brasileira de Zootecnia , 37(10), 1876-1883. doi: 10.1590/S1516-35982008001000023
https://doi.org/10.1590/S1516-3598200800...
). The species Poincianella pyramidalis, endemic to the Caatinga and popularly known as catingueira, is a deciduous legume shrub, widely used both in popular medicine, and as wood and forage, and preferred by ruminants in this environment during the last phase of its phenological cycle. It is a medium-sized, thornless tree, 4-6 m in height, sometimes reaching 12 m. The frequency and extent of the rainfall pulses influence the intensity and duration of the phenophases; leaf senescence takes place when the rainfall interpulses intensify as the dry season progresses, characterising deciduousness (Lima et al., 2018Lima, C. R., Bruno, R. L. A., Andrade, A. P., Pacheco, M. V., Quirino, Z. G. M., Silva, K. d. R. G., & Belarmino, K. d. S. (2018). Phenology of Poincianella pyramidalis (Tul.) L. P. Queiroz and its relationship with the temporal distribution of rainfall in the brazilian semi-arid region. Ciência Florestal, 28(3), 1035-1048. doi: 10.5902/1980509833387
https://doi.org/10.5902/1980509833387...
).
Studies relating to rainfall input and the nutritional aspects of forage plants could result in tools that would help in the better management and use of these plants by the animals (Godde et al., 2019Godde, C., Dizyee, K., Ash, A., Thornton, P., Sloat, L., Roura, E., ... Herrero, M. (2019). Climate change and variability impacts on grazing herds: Insights from a system dynamics approach for semi‐arid Australian rangelands. Global Change Biology, in press. doi: 10.1111/gcb.14669.
https://doi.org/10.1111/gcb.14669...
; Habermann et al., 2019Habermann, E., Oliveira, E. A. D., Contin, D. R., Delvecchio, G., Viciedo, D. O., Moraes, M. A., ... Martinez, C. A. (2019). Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiologia Plantarum, 165(2), 383-402. doi: 10.1111/ppl.12891
https://doi.org/10.1111/ppl.12891...
; Hui et al., 2018Hui, D., Yu, C.-L., Deng, Q., Dzantor, E. K., Zhou, S., Dennis, S., . . . Shen, W. (2018). Effects of precipitation changes on switchgrass photosynthesis, growth, and biomass: A mesocosm experiment. PloS One, 13(2), e0192555. doi: 10.1371/journal.pone.0192555
https://doi.org/10.1371/journal.pone.019...
). Aspects of the nutritional potential of the plant were studied by Gonzaga Neto et al. (2001Gonzaga Neto, S., Batista, Â. M. V., Carvalho, F. F. R., Martínez, R. L. V., Barbosa, J. E. A. S., & Silva, E. O. (2001). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea bracteosa), fornecidas para ovinos Morada Nova. Revista Brasileira de Zootecnia , 30(2), 553-562. doi: 10.1590/S1516-35982001000200035
https://doi.org/10.1590/S1516-3598200100...
), and Mendonça Júnior, Braga, and Galvão (2008Mendonça Júnior, A. F., Braga, A. P., & Galvão, R. J. D. (2008). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea pyramidalis Tul), fornecidas para ovinos SRD. Revista de Biologia e Ciências da Terra, 8(1), 190-197. doi: 10.1590/S1516-35982001000200035
https://doi.org/10.1590/S1516-3598200100...
) and there are many studies of the pharmacological and medicinal potential of the species due to the presence of biflavanoids and other compounds (Oliveira, David, & David, 2016aOliveira, J. C. S., David, J. P., & David, J. M. (2016a). Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochemistry Letters, 16, 18-22. doi: 10.1016/j.phytol.2016.02.017
https://doi.org/10.1016/j.phytol.2016.02...
).
Plant maturation during development of the phenological cycle causes variations in nutrient content due to the mobilisation of nutrients between the plant organs; in addition, environmental conditions such as the precipitation of water, temperature and soil fertility may interfere with the metabolic processes of the plant, altering its chemical composition (Arzani et al., 2004Arzani, H., Zohdi, M., Fish, E., Amiri, G. H. Z., Nikkhah, A., & Wester, D. (2004). Phenological effects on forage quality of five grass species. Rangeland Ecology and Management, 57(6), 624-630. doi: 10.2458/azu_jrm_v57i6_arzani
https://doi.org/10.2458/azu_jrm_v57i6_ar...
; Buxton, Mertens, & Fisher, 1996Buxton, D. R., Mertens, D. R., & Fisher, D. S. (1996). Forage quality and ruminant utilization. Cool-Season Forage Grasses, 1, 229-266. doi: 10.1016/0377-8401(95)00885-3
https://doi.org/10.1016/0377-8401(95)008...
; Habermann et al., 2019Habermann, E., Oliveira, E. A. D., Contin, D. R., Delvecchio, G., Viciedo, D. O., Moraes, M. A., ... Martinez, C. A. (2019). Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiologia Plantarum, 165(2), 383-402. doi: 10.1111/ppl.12891
https://doi.org/10.1111/ppl.12891...
; Hui et al., 2018Hui, D., Yu, C.-L., Deng, Q., Dzantor, E. K., Zhou, S., Dennis, S., . . . Shen, W. (2018). Effects of precipitation changes on switchgrass photosynthesis, growth, and biomass: A mesocosm experiment. PloS One, 13(2), e0192555. doi: 10.1371/journal.pone.0192555
https://doi.org/10.1371/journal.pone.019...
). These aspects are of great relevance to native Caatinga pasture, as the peculiarities of the semi-arid climate are important in triggering the phenological periodicity of the plants (Karnieli, 2003Karnieli, A. (2003). Natural vegetation phenology assessment by ground spectral measurements in two semi-arid environments. International Journal of Biometeorology, 47(4), 179-187. doi: 10.1007/s00484-003-0169-z.
https://doi.org/10.1007/s00484-003-0169-...
), as well as the development of physiological mechanisms of adaptation and defence, which compromise intake and use of the forage by the animals. In the production of secondary metabolites, phenolic compounds, such as lignin and tannins, are present in large quantities in the plants, and flavonoids and biflavanoids can be significantly present in plants of the catingueira (Bahia, David, & David, 2010Bahia, M. V., David, J. P., & David, J. M. (2010). Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nova, 33(6), 1297-1300. doi: 10.1590/S0100-40422010000600015
https://doi.org/10.1590/S0100-4042201000...
; Bahia, Santos, David, & David, 2005Bahia, M. V., Santos, J. B., David, J. P., & David, J. M. (2005). Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae). Journal of the Brazilian Chemical Society, 16(6B), 1402-1405. doi: 10.1590/S0103-50532005000800017
https://doi.org/10.1590/S0103-5053200500...
; Oliveira et al., 2016aOliveira, J. C. S., David, J. P., & David, J. M. (2016a). Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochemistry Letters, 16, 18-22. doi: 10.1016/j.phytol.2016.02.017
https://doi.org/10.1016/j.phytol.2016.02...
; Oliveira et al., 2016bOliveira, O. F., Santos, M. V. F., Cunha, M. V., Dubeux Júnior, J. C. B., Muir, J. P., Mello, A. C. L., ... Barros, G. F. N. P. (2016b). Botanical composition of Caatinga rangeland and diets selected by grazing sheep. Tropical Grasslands, 4(2), 71-81. doi: 10.1016/j.phytol.2016.02.017.
https://doi.org/10.1016/j.phytol.2016.02...
). Phenolic compounds in general may self-oxidise and limit digestion inside the rumen, aided by other plant defence mechanisms (Barbehenn & Constabel, 2011Barbehenn, R. V., & Constabel, C. P. (2011). Tannins in plant-herbivore interactions. Phytochemistry, 72(13), 1551-1565. doi: /10.1016/j.phytochem.2011.01.040
https://doi.org//10.1016/j.phytochem.201...
). Lignin exerts its secondary function in plants by interfering with the degradation of other cell wall compounds by rumen microorganisms (Grabber, 2005Grabber, J. H. (2005). How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Science, 45(3), 820-831. doi: 10.2135/cropsci2004.0191
https://doi.org/10.2135/cropsci2004.0191...
; Jung & Vogel, 1986Jung, H. G., & Vogel, K. P. (1986). Influence of lignin on digestibility of forage cell wall material. Journal of Animal Science, 62(6), 1703-1712. doi: 10.2527/jas1986.6261703x
https://doi.org/10.2527/jas1986.6261703x...
); while the tannins, phenolic polymers of high molecular weight, are complexed with proteins and other macromolecules, having positive or negative effects on the ruminants according to their amount in the animal diet (Muir, 2011Muir, J. P. (2011). The multi-faceted role of condensed tannins in the goat ecosystem. Small Ruminant Research, 98(1-3), 115-120. doi: 10.1016/j.smallrumres.2011.03.028
https://doi.org/10.1016/j.smallrumres.20...
; Silva, Guim, Ferreira, & Soares, 2016Silva, J. L., Guim, A., Ferreira, M. A., & Soares, L. F. P. (2016). Forragens taniníferas na produção de caprinos e ovinos. Archivos de zootecnia, 65(252), 605-614. doi: 10.21071/az.v65i252.1933
https://doi.org/10.21071/az.v65i252.1933...
).
Maximum rumen degradability of roughage remains constant after 48 h of incubation (Goes et al., 2012Goes, B. T. R. H., Tramontini, R. C. M., Cardim, S. T., Almeida, G. D., Ribeiro, J., Morotti, F., ... Brabes, K. C. S. (2012). Ruminal degradability of dry matter and crude protein of roughages for cattle. Revista Acadêmica Ciências Agrárias e Ambientais, 10(3), 285-291. doi: 10.7213/academica.7709
https://doi.org/10.7213/academica.7709...
; Pires et al., 2006Pires, A. J. V., Reis, R. A., Carvalho, G. G. P., Siqueira, G. R., Bernardes, T. F., Ruggieri, A. C., ... Roth, M. T. P. (2006). Degradabilidade ruminal da matéria seca, da fração fibrosa e da proteína bruta de forrageiras. Pesquisa Agropecuária Brasileira, 41(4), 643-648. doi: 10.1590/S0100-204X2006000400014
https://doi.org/10.1590/S0100-204X200600...
); 48 h incubation is sufficient to partially degrade even the thicker-walled tissue, such as sclerenchyma and gelatinous fibre (Akin, 1989Akin, D. E. (1989). Histological and physical factors affecting digestibility of forages. Agronomy Journal, 81(1), 17-25. doi: 10.2134/agronj1989.00021962008100010004x
https://doi.org/10.2134/agronj1989.00021...
; França et al., 2010França, A. A., Guim, A., Batista, A. M. V., Pimentel, R. M. M., Ferreira, G. D. G., & Martins, I. D. S. L. (2010). Anatomia e cinética de degradação do feno de Manihot glaziovii. Acta Scientiarum. Animal Sciences, 32(2), 131-138. doi: 10.4025/actascianimsci.v32i2.8800
https://doi.org/10.4025/actascianimsci.v...
; Lima, Alquini, Brito, & Deschamps, 2001Lima, L. M. S., Alquini, Y., Brito, C. J. F. A., & Deschamps, F. C. (2001). Degradação ruminal dos tecidos vegetais e composição bromatológica de cultivares de Axonopus scoparius (Flüegge) Kuhlm. e Axonopus fissifolius (Raddi) Kuhlm. Ciência Rural, 31, 509-515. doi: 10.1590/S0103-84782001000300025
https://doi.org/10.1590/S0103-8478200100...
; Wilson & Mertens, 1995Wilson, J. R., & Mertens, D. R. (1995). Cell wall accessibility and cell structure limitations to microbial digestion of forage. Crop Science , 35(1), 251-259. doi: 10.2135/cropsci1995.0011183X003500010046x
https://doi.org/10.2135/cropsci1995.0011...
).
The aim of this study was to evaluate parameters of nutritional quality in Poincianella pyramidalis at five different ages during the rainy season, by the use of two methods: the in situ degradability of dry matter and cell wall components, and in situ and in vitro tissue degradability.
Material and methods
The research was developed in an area located at 7º22'45.1" S and 36º31'47.2" W. According to the Köppen and Geiger (1928Köppen, W., & Geiger, R. (1928). Klimate der Erde. Gotha: Verlag Justus Perthes. Wall-map 150cmx200cm. ) classification, the local climate is type BSh, hot semi-arid, characterised by irregular rainfall. Daily rainfall indices were obtained from the Experimental Station at the local Teaching Watershed.
Ten adult plants of the native species Poincianella pyramidalis were randomly selected and identified for observation of their phenological development and the collection of samples. The plants were approximately 3 m high, with branched stems, dense crowns and odd bipinnate leaves with five pinnas of alternating leaflets. The evaluation periods were determined by the phenological phase of the plant (observed in more than 70% of the marked plants) and leaf age (in days from emergence). Five phenological phases were defined: vegetative, start of flowering, full flowering, full fruiting and full senescence, whose corresponding leaf ages were 44, 70, 103, 148 and 231 days. The respective rainfall totals were 104.5, 46.7, 71.7, 160.4 and 131.9 mm.
In each of the 10 plants, three leaves were marked, completely expanded, free from shading, at an average height of 1.5 m from the ground, for the collection of leaflets and petioles to evaluate in situ and in vitro tissue degradation.
Among the 10 plants, three were selected for the collection of samples comprising leaves and branches up to 0.5 cm in diameter to determine bromatological composition, secondary compounds and dry matter (DM) and neutral detergent fibre (NDF) degradability. Dry matter (DM), mineral matter (MM), crude protein (CP), neutral detergent fibre (NDF) with and without the use of amylase, acid detergent fibre (ADF), cellulose, hemicellulose and lignin were analysed, in accordance with the Instituto Nacional de Ciência e Tecnologia de Ciência Animal.
In order to evaluate in situ and in vitro degradability, two samples (one leaflet and one petiole) from each plant were collected in the morning, between 05:30 and 06:30, and prepared to allow access of rumen microorganisms to the interior of the cells; the petioles were cut just above the pulvinus up to 1.2 cm from the leaf axis and the base and apex of the leaflets were cut with a scissors and removed, resulting in fragments of approximately 1 cm², both of which were stored together in plastic histology cassettes, so as to avoid mechanical action of the rumen, and immediately incubated in the rumen of an adult goat for 48 h following a method adapted from França et al. (2010França, A. A., Guim, A., Batista, A. M. V., Pimentel, R. M. M., Ferreira, G. D. G., & Martins, I. D. S. L. (2010). Anatomia e cinética de degradação do feno de Manihot glaziovii. Acta Scientiarum. Animal Sciences, 32(2), 131-138. doi: 10.4025/actascianimsci.v32i2.8800
https://doi.org/10.4025/actascianimsci.v...
).
The in situ degradability of DM and NDF was carried out using an adult rumen-fistulated goat. For each leaf age, three replications of 0.5 g of 2-mm ground samples were used, packed in non-woven fabric (NWF) bags (100 g m-2), which after 48 h incubation were removed, washed and dried in a non-vented oven at 105°C to constant weight, weighed to determine DM disappearance, and then washed in neutral detergent, as per Casali et al. (2009Casali, A. O., Detmann, E., Valadares Filho, S., Pereira, J. C., Cunha, M., Detmann, K. d. S. C., & Paulino, M. F. (2009). Estimação de teores de componentes fibrosos em alimentos para ruminantes em sacos de diferentes tecidos. Revista Brasileira de Zootecnia, 38(1), 130-138. doi: 10.1590/S1516-35982009000100017
https://doi.org/10.1590/S1516-3598200900...
); these were then dried in a similar manner as described above, and weighed to determine the iNDF content.
For in vitro evaluation, the samples were similarly prepared, and the histological cassettes incubated for 48 h in a Daisy II Ankon® artificial rumen, with ruminal fluid from an adult goat and A and B buffer solutions. In both tests, after the incubation period, the cassettes were washed and the material preserved in a formalin:acetic acid:alcohol 50 (FAA 50) solution for further analysis of the fragments after degradation.
For leaf tissue analysis after ruminal incubation, images were obtained by digital camera and Scanning Electron Microscope (Phillips XL-30 ESEM) at the Centre for Study and Research in Natural Gas and Petroleum (NEPGN) of the Federal University of Rio Grande do Norte (UFRN), where the samples preserved in FAA 50 were subjected to alcohol dehydration, and after drying in the open air, were metallised in a BAL-TEC SCD-005 Sputter Coater.
Analysis of the total phenol content was performed following the Folin-Denis method, using Polyvinylpyrrolidone (PVP). Evaluation of the presence of secondary compounds was carried out at the Laboratory of Pharmaceutical Technology CCS/UFPB, using pooled samples from all the phenological phases of the catingueira. Phytochemical prospection was carried out to detect the presence (+) or absence (-) of alkaloids, terpenes, tannins and flavonoids, with abundance expressed by the number of respective symbols. 1H and 13C nuclear magnetic resonance (NMR) analysis, using the Varian Mercury 200 apparatus [200 MHz (1H) and 50 MHz (13C)], was performed on the crude extract of the pooled catingueira samples to detect flavonoids, for which 30 mg were diluted in 0.6 mL deuterated methanol, with tetramethylsilane (TMS) used as internal reference.
The data for chemical composition and degradability were analysed for variance between five periods (ages) and at a significance level of 5% by Tukey’s test, using the Statistical Analysis Software (SAS, 2004Statistical Analysis Software [SAS]. (2004). SAS/STAT User guide, Version 9.1.2. Cary, NC: SAS Institute Inc.). Correlation analysis was performed between the values for chemical composition and the in situ degradability of DM and NDF, with leaf age for each period being evaluated.
Results and discussion
The levels of dry matter, crude protein, neutral detergent fibre, mineral matter and NDF degradability (Deg NDF) varied in relation to leaf maturity. The CP and Deg NDF content showed a high negative correlation with the increase in leaf age, both with r = -0.8 and p > 0.05, and the NDF with r = - 0.9 and p > 0.01. The ADF components, DM degradability and tannin content showed no variation in relation to leaf age (Table 1).
The DM content of the leaves of the catingueira increases with age; however, no significant correlation was seen for this content with age or precipitation in any collection period. The DM content of forages is a function of the amount of water contained in the different tissues, which mainly varies with soil moisture and air humidity, temperature, and the photosynthetic and respiration rates of the plant, but also varies with plant development and metabolic activity (Etienne et al., 2018Etienne, P., Diquelou, S., Prudent, M., Salon, C., Maillard, A., & Ourry, A. (2018). Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture, 8(1), 14. doi: 10.3390/agriculture8010014
https://doi.org/10.3390/agriculture80100...
; Fahad et al., 2017Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., ... Saud, S. (2017). Crop production under drought and heat stress: plant responses and management options. Frontiers in Plant Science, 8(1147), 1-16. doi: 10.3389/fpls.2017.01147
https://doi.org/10.3389/fpls.2017.01147...
). With the increase in age, there was a significant increase in the concentration of minerals, which may be especially related to the accumulation of silica and calcium oxalate over time due to the low mobility of calcium (Currie & Perry, 2007Currie, H. A., & Perry, C. C. (2007). Silica in plants: biological, biochemical and chemical studies. Annals of Botany, 100(7), 1383-1389. doi: 10.1093/aob/mcm247
https://doi.org/10.1093/aob/mcm247...
; Rahman & Kawamura, 2011Rahman, M. M., & Kawamura, O. (2011). Oxalate accumulation in forage plants: some agronomic, climatic and genetic aspects. Asian-Australasian Journal of Animal Sciences, 24(3), 439-448. doi: 10.5713/ajas.2011.10208
https://doi.org/10.5713/ajas.2011.10208...
; Webb, 1999Webb, M. A. (1999). Cell-mediated crystallization of calcium oxalate in plants. The Plant Cell, 11(4), 751-761. doi: 10.1105/tpc.11.4.751
https://doi.org/10.1105/tpc.11.4.751...
).
The proportion of crude protein found was lower than values reported in the literature, ranging from 130 to 86 g kg-1, decreasing by 40% from the vegetative phase to leaf senescence in the catingueira plants, as reported by Avice and Etienne (2014Avice, J.-C., & Etienne, P. (2014). Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). Journal of Experimental Botany, 65(14), 3813-3824. doi: 10.1093/jxb/eru177
https://doi.org/10.1093/jxb/eru177...
) in studies on N fluxes during remobilisation, seen from maturity to senescence in the leaves of Brassica napus. The crude protein content found in the leaves when the plant was in the flowering and fruiting phases was 100 g kg-1; in general, the start of the reproductive period is recommended for cutting forages to be used for conservation, considering that this nutrient may be redistributed to the reproductive organs and also to the storage organs of the plant before leaf-fall (Etienne et al., 2018Godde, C., Dizyee, K., Ash, A., Thornton, P., Sloat, L., Roura, E., ... Herrero, M. (2019). Climate change and variability impacts on grazing herds: Insights from a system dynamics approach for semi‐arid Australian rangelands. Global Change Biology, in press. doi: 10.1111/gcb.14669.
https://doi.org/10.1111/gcb.14669...
). Thus, Gonzaga Neto et al. (2001Gonzaga Neto, S., Batista, Â. M. V., Carvalho, F. F. R., Martínez, R. L. V., Barbosa, J. E. A. S., & Silva, E. O. (2001). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea bracteosa), fornecidas para ovinos Morada Nova. Revista Brasileira de Zootecnia , 30(2), 553-562. doi: 10.1590/S1516-35982001000200035
https://doi.org/10.1590/S1516-3598200100...
) and Mendonça Júnior et al. (2008Mendonça Júnior, A. F., Braga, A. P., & Galvão, R. J. D. (2008). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea pyramidalis Tul), fornecidas para ovinos SRD. Revista de Biologia e Ciências da Terra, 8(1), 190-197. doi: 10.1590/S1516-35982001000200035
https://doi.org/10.1590/S1516-3598200100...
) found CP levels of 112.5 and 123.1 g kg-1 respectively, in hay produced from the catingueira during the fruiting phase.
Contary to expectations, the NDF concentration decreased as maturity increased, showing a strong negative correlation. The highest levels of NDF were found after 70 days of plant development and when the rainfall reached 46mm, decreasing after 148 and 231 days, when the plants received 160.4 mm and 131.9 mm of rainfall respectively. According to Habermann et al. (2019Habermann, E., Oliveira, E. A. D., Contin, D. R., Delvecchio, G., Viciedo, D. O., Moraes, M. A., ... Martinez, C. A. (2019). Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiologia Plantarum, 165(2), 383-402. doi: 10.1111/ppl.12891
https://doi.org/10.1111/ppl.12891...
), the effects of water stress alter the quality of the forage more than when this factor is associated with high temperature; in their paper, cell-wall components of Panicum maximum were greater when the plant was under water stress only, compared to situations with or without irrigation associated with high temperature. The physiological mechanisms involved during the senescence phase, such as the production of ethylene and abscisic acid, have an important effect on the loosening of the cell wall in senescent tissue (Dubois, Van den Broeck, & Inzé, 2018Dubois, M., Van den Broeck, L., & Inzé, D. (2018). The pivotal role of ethylene in plant growth. Trends in Plant Science, 23(4), 311-323. doi: 10.1016/j.tplants.2018.01.003
https://doi.org/10.1016/j.tplants.2018.0...
; Piotrowska & Bajguz, 2011Piotrowska, A., & Bajguz, A. (2011). Conjugates of abscisic acid, brassinosteroids, ethylene, gibberellins, and jasmonates. Phytochemistry , 72(17), 2097-2112. doi: 10.1016/j.phytochem.2011.08.012.
https://doi.org/10.1016/j.phytochem.2011...
), which may involve the mobilisation of these carbohydrates for plant reserves, since there was a reduction in the values of cellulose and hemicellulose. However, lignin values remained constant throughout the development phases, which contributed to a lower degradability of NDF in the last stages of plant development.
Lignin values were high during all the phases under evaluation. The high values of tannin, in addition to the proteins of the of the cell wall, may together with the lignin, overestimate its true value (Marles, Coulman, & Bett, 2008Marles, M. A. S., Coulman, B. E., & Bett, K. E. (2008). Interference of condensed tannin in lignin analyses of dry bean and forage crops. Journal of Agricultural and Food Chemistry, 56(21), 9797-9802. doi: 10.1021/jf800888r
https://doi.org/10.1021/jf800888r...
). The association of lignin with other components of the cell-wall matrix greatly influences the properties of digestion. As such, the degree of cross-linking of phenolics within the cell wall, together with the thickness of the secondary cell wall at maturity, limit forage degradation more than just the lignin content (França et al., 2010França, A. A., Guim, A., Batista, A. M. V., Pimentel, R. M. M., Ferreira, G. D. G., & Martins, I. D. S. L. (2010). Anatomia e cinética de degradação do feno de Manihot glaziovii. Acta Scientiarum. Animal Sciences, 32(2), 131-138. doi: 10.4025/actascianimsci.v32i2.8800
https://doi.org/10.4025/actascianimsci.v...
; Raffrenato et al., 2017Raffrenato, E., Fievisohn, R., Cotanch, K. W., Grant, R. J., Chase, L. E., & Van Amburgh, M. E. (2017). Effect of lignin linkages with other plant cell wall components on in vitro and in vivo neutral detergent fiber digestibility and rate of digestion of grass forages. Journal of Dairy Science, 100(10), 8119-8131. doi: 10.3168/jds.2016-12364
https://doi.org/10.3168/jds.2016-12364...
).
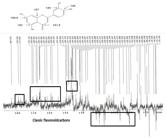
In the test of in situ and in vitro tissue degradability, the leaves of the catingueira presented a pattern of similar behaviour for all the ages under evaluation; this was a physical barrier that prevented the access of microorganisms, allowing the leaf fragments and the petioles to remain intact (Figure 1 A-D).
A-D. Leaflet and petiole of Poincianella pyramidalis after 48 h of ruminal incubation. A-B. Aspect of the leaflet and petiole fragments immediately after removal from the rumen, showing the physical barrier (arrows); C-D. Scanning electromyographs of the outer surface of the leaflet (C) and petiole (D) fragments. Bars: A, B = 1 cm; C = 100 μm; D = 500 μm.
Through phytochemical prospection, the presence of two main classes of secondary compounds, terpenes and phenolic compounds, was detected (Table 2). The number of positive signs (+) indicates the intensity of presence of the compound in the sample. No alkaloids or saponins were detected in this species.
The extract under study also proved to be rich in flavonoids by 1H-NMR analysis, showing absorptions between 6.1 and 6.3, characteristic of the flavonoid A-Ring, and between δH 7.06 and 7.50, characteristic of the flavonoid B-Ring (Figure 2).
Presence or absence of secondary compounds in the crude extract of Poincianella pyramidalis.
In the same spectrum, signals between δH 3.0 and 5.3 were seen compatible with osidic units common in Fabaceae. As this was an analysis of a crude extract, a further analysis of carbon-13 (Figure 3) was performed where data compatible with flavonoids and osidic units could be seen: among the signals at δC 84.3 and 60.5 that are compatible with the structure of pinitol; between the chemical shift at δC 183.9 assigned to C-4, a set of signals at δC 168.8 to 157.2, characteristic of the C-2, C-5, C-7 and C-9 carbons of the benzopyran unit of flavonoids; and again between the signals at δC 133.6 and 112.9, characteristic of flavonoid sp2 carbons. Guidance signals confirming the chemical shifts of flavonoids were seen at δC 96.6 and 100, both CH compatible with C-6 and C-8 shifts and the signals at δC 106.5, 105.5 and 101.8, non-hydrogenated carbons that confirm the fusion of flavonoid units.
Hay from the catingueira during fruiting, evaluated by Gonzaga Neto et al. (2001Gonzaga Neto, S., Batista, Â. M. V., Carvalho, F. F. R., Martínez, R. L. V., Barbosa, J. E. A. S., & Silva, E. O. (2001). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea bracteosa), fornecidas para ovinos Morada Nova. Revista Brasileira de Zootecnia , 30(2), 553-562. doi: 10.1590/S1516-35982001000200035
https://doi.org/10.1590/S1516-3598200100...
) presented a DM digestibility of 50.5% and NDF digestibility of 41.7%. However, these values do not reflect the reality of the test for in situ and in vitro degradation of leaflet and petiole tissue in P. pyramidalis during all the phenological phases; this may have occurred due to the strong presence of phenolic compounds in this species (Bahia et al., 2010Bahia, M. V., David, J. P., & David, J. M. (2010). Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nova, 33(6), 1297-1300. doi: 10.1590/S0100-40422010000600015
https://doi.org/10.1590/S0100-4042201000...
; Gomes-Copeland et al., 2018Gomes-Copeland, K. K. P., Lédo, A. S., Almeida, F. T. C., Moreira, B. O., Santos, D. C., Santos, R. A. F., ... David, J. P. (2018). Effect of elicitors in Poincianella pyramidalis callus culture in the biflavonoid biosynthesis. Industrial Crops and Products, 126, 421-425. doi: 10.1016/j.indcrop.2018.10.038
https://doi.org/10.1016/j.indcrop.2018.1...
; Oliveira et al., 2016aOliveira, J. C. S., David, J. P., & David, J. M. (2016a). Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochemistry Letters, 16, 18-22. doi: 10.1016/j.phytol.2016.02.017
https://doi.org/10.1016/j.phytol.2016.02...
; Oliveira et al., 2016b), which may be oxidised after injury to the plant, forming quinones characterised by the brown colouration of the broken extremity, causing cell death and preventing the entry of microorganisms to the interior of the fragments, thereby demonstrating an adaptive aspect of the plant and a mechanism of plant defence (Barbehenn & Constabel, 2011Barbehenn, R. V., & Constabel, C. P. (2011). Tannins in plant-herbivore interactions. Phytochemistry, 72(13), 1551-1565. doi: /10.1016/j.phytochem.2011.01.040
https://doi.org//10.1016/j.phytochem.201...
). Quinones can also react with functional groups of proteins forming protein-bound phenol and altering ruminant nutrition by improving the utilisation of nitrogen (Lee, 2014Lee, M. R. F. (2014). Forage polyphenol oxidase and ruminant livestock nutrition. Frontiers in Plant Science , 5, 1-9. doi: 10.3389/fpls.2014.00694
https://doi.org/10.3389/fpls.2014.00694...
). The type of feed supplied should be monitored so as to afford greater utilisation by the animal. It is important to highlight the importance of this methodology for analysing the study of leaf tissue degradation, keeping in mind the diversity of the botanical and morphological aspects of plants of the caatinga, in order to ascertain their supposed forage potential.
The variation in tannin content between species, individuals and their phenophases, is related to herbivore pressure and the environmental conditions that influence plant investment in the production of these compounds, especially when growing conditions, such as soil moisture and nutrients, are limiting (Barbehenn & Constabel, 2011Barbehenn, R. V., & Constabel, C. P. (2011). Tannins in plant-herbivore interactions. Phytochemistry, 72(13), 1551-1565. doi: /10.1016/j.phytochem.2011.01.040
https://doi.org//10.1016/j.phytochem.201...
; Muir, 2011Muir, J. P. (2011). The multi-faceted role of condensed tannins in the goat ecosystem. Small Ruminant Research, 98(1-3), 115-120. doi: 10.1016/j.smallrumres.2011.03.028
https://doi.org/10.1016/j.smallrumres.20...
). Tannins influence forage quality mainly by astringency that can affect the palatability and consumption of green plants (Muir, 2011), and by limitations on forage usage due to binding with proteins and other macromolecules, such as the components of the cell wall (Barry & Crowell-Davis, 1999Barry, K. J., & Crowell-Davis, S. L. (1999). Gender differences in the social behavior of the neutered indoor-only domestic cat. Applied Animal Behaviour Science, 64(3), 193-211. doi: 10.1016/S0168-1591(99)00030-1
https://doi.org/10.1016/S0168-1591(99)00...
; Reed, 1995Reed, J. D. (1995). Nutritional toxicology of tannins and related polyphenols in forage legumes. Journal of Animal Science , 73(5), 1516-1528. doi: 10.2527/1995.7351516x
https://doi.org/10.2527/1995.7351516x...
; Silva et al., 2016Silva, J. L., Guim, A., Ferreira, M. A., & Soares, L. F. P. (2016). Forragens taniníferas na produção de caprinos e ovinos. Archivos de zootecnia, 65(252), 605-614. doi: 10.21071/az.v65i252.1933
https://doi.org/10.21071/az.v65i252.1933...
), a fact which may have contributed to the low degradability of the NDF fraction. The amount of tannins may have a strong relationship with the amount of protein, protecting it from degradation in the rumen (Silva et al., 2016).
Regarding the presence of steroids, constituents of essential oils, the steroids and saponins may affect the organoleptic characteristics of milk and its derivatives from animals that ingest plants rich in such compounds (Belviso, Giordano, Dolci, & Zeppa, 2011Belviso, S., Giordano, M., Dolci, P., & Zeppa, G. (2011). Degradation and biosynthesis of terpenoids by lactic acid bacteria isolated from cheese: first evidence. Dairy Science & Technology, 91(2), 227-236. doi: 10.1007/s13594-011-0003-z
https://doi.org/10.1007/s13594-011-0003-...
). However, these compounds may act as plant hormones, and are able to act as toxins and inhibit herbivore foraging (Cheng et al., 2007Cheng, A. X., Lou, Y.-G., Mao, Y.-B., Lu, S., Wang, L.-J., & Chen, X. Y. (2007). Plant terpenoids: Biosynthesis and ecological functions. Journal of Integrative Plant Biology, 49(2), 179-186. doi: 10.1111/j.1744-7909.2007.00395.x
https://doi.org/10.1111/j.1744-7909.2007...
). In general, the leaves and bark of this plant are used in popular medicine for the treatment of stomach disorders and as a diuretic - studies point to several antimicrobial antioxidant actions (Chaves et al., 2019Chaves, T. P., Medeiros, F. D., Sousa, J. M. C., Silva, L. A. P., Lima, M. A., Coutinho, H. D. M., & Medeiros, A. C. D. (2019). Phytochemical characterization and mutagenicity, cytotoxicity, antimicrobial and modulatory activities of Poincianella pyramidalis (Tul.) LP Queiroz. Natural Product Research, 1-6. doi: 10.1080/14786419.2019.1566724
https://doi.org/10.1080/14786419.2019.15...
) - and terpenes, phenylpropanoids, flavonoids and, in particular, biflavonoids have been isolated from extracts of the leaves and bark (Oliveira et al., 2016aOliveira, J. C. S., David, J. P., & David, J. M. (2016a). Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochemistry Letters, 16, 18-22. doi: 10.1016/j.phytol.2016.02.017
https://doi.org/10.1016/j.phytol.2016.02...
; Oliveira et al., 2016bOliveira, O. F., Santos, M. V. F., Cunha, M. V., Dubeux Júnior, J. C. B., Muir, J. P., Mello, A. C. L., ... Barros, G. F. N. P. (2016b). Botanical composition of Caatinga rangeland and diets selected by grazing sheep. Tropical Grasslands, 4(2), 71-81. doi: 10.1016/j.phytol.2016.02.017.
https://doi.org/10.1016/j.phytol.2016.02...
). There was a strong presence of flavonoids in the leaves of P. pyramidalis, agreeing with reports by Bahia et al. (2005Bahia, M. V., Santos, J. B., David, J. P., & David, J. M. (2005). Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae). Journal of the Brazilian Chemical Society, 16(6B), 1402-1405. doi: 10.1590/S0103-50532005000800017
https://doi.org/10.1590/S0103-5053200500...
, 2010Bahia, M. V., David, J. P., & David, J. M. (2010). Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nova, 33(6), 1297-1300. doi: 10.1590/S0100-40422010000600015
https://doi.org/10.1590/S0100-4042201000...
). According to Jawla, Kumar, and Khan (2013Jawla, S., Kumar, Y., & Khan, M. S. Y. (2013). Isolation of antidiabetic principle from Bougainvillea spectabilis Willd (Nyctaginaceae) stem bark. Tropical Journal of Pharmaceutical Research, 12(5), 761-765. doi: 10.4314/tjpr.v12i5.15
https://doi.org/10.4314/tjpr.v12i5.15...
) the structure compatible with pinitol, an inositol that has been found in plants which occur under high solar radiance has shown antidiabetic activity. The presence of biflavonoids is rare in legume species (Bahia et. al., 2010); however recent studies by Oliveira et al. (2016aOliveira, J. C. S., David, J. P., & David, J. M. (2016a). Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochemistry Letters, 16, 18-22. doi: 10.1016/j.phytol.2016.02.017
https://doi.org/10.1016/j.phytol.2016.02...
) isolated four new biflavonoids in extracts of root bark, and Gomes-Copeland et al. (2018Gomes-Copeland, K. K. P., Lédo, A. S., Almeida, F. T. C., Moreira, B. O., Santos, D. C., Santos, R. A. F., ... David, J. P. (2018). Effect of elicitors in Poincianella pyramidalis callus culture in the biflavonoid biosynthesis. Industrial Crops and Products, 126, 421-425. doi: 10.1016/j.indcrop.2018.10.038
https://doi.org/10.1016/j.indcrop.2018.1...
) described the production of amentoflavone and agathisflavone in a culture of P. pyramidalis calli, confirming the need for further evaluation of the presence of these compounds and their interaction in a pasture ecosystem, in order to provide greater understanding of their potential for use. Furthermore, the quantity of phenolic compounds, such as flavonoids and tannins, is important, as both can be rapidly oxidised when the plant is injured by chewing or cutting, forming physical barriers at these surfaces that prevent degradation of the leaf tissue (Barbehenn & Constabel, 2011Barbehenn, R. V., & Constabel, C. P. (2011). Tannins in plant-herbivore interactions. Phytochemistry, 72(13), 1551-1565. doi: /10.1016/j.phytochem.2011.01.040
https://doi.org//10.1016/j.phytochem.201...
).
Conclusion
Leaf maturity in Poincianella pyramidalis influences variations in the levels of mineral matter, crude protein and neutral detergent fibre. The presence of phenolic compounds, such as flavonoids, and the high levels of tannins and lignin during the phenophases of the plant, should be considered for their anti-nutritional potential, which may affect the degradability of leaf tissue. The method of evaluating tissue degradability in the rumen is relevant to determine the nutritional potential of this forage plant. Further research should be undertaken regarding the ways in which this plant is used for animal feed.
References
- Akin, D. E. (1989). Histological and physical factors affecting digestibility of forages. Agronomy Journal, 81(1), 17-25. doi: 10.2134/agronj1989.00021962008100010004x
» https://doi.org/10.2134/agronj1989.00021962008100010004x - Arzani, H., Zohdi, M., Fish, E., Amiri, G. H. Z., Nikkhah, A., & Wester, D. (2004). Phenological effects on forage quality of five grass species. Rangeland Ecology and Management, 57(6), 624-630. doi: 10.2458/azu_jrm_v57i6_arzani
» https://doi.org/10.2458/azu_jrm_v57i6_arzani - Avice, J.-C., & Etienne, P. (2014). Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). Journal of Experimental Botany, 65(14), 3813-3824. doi: 10.1093/jxb/eru177
» https://doi.org/10.1093/jxb/eru177 - Bahia, M. V., David, J. P., & David, J. M. (2010). Occurrence of biflavones in leaves of Caesalpinia pyramidalis specimens. Química Nova, 33(6), 1297-1300. doi: 10.1590/S0100-40422010000600015
» https://doi.org/10.1590/S0100-40422010000600015 - Bahia, M. V., Santos, J. B., David, J. P., & David, J. M. (2005). Biflavonoids and other phenolics from Caesalpinia pyramidalis (Fabaceae). Journal of the Brazilian Chemical Society, 16(6B), 1402-1405. doi: 10.1590/S0103-50532005000800017
» https://doi.org/10.1590/S0103-50532005000800017 - Barbehenn, R. V., & Constabel, C. P. (2011). Tannins in plant-herbivore interactions. Phytochemistry, 72(13), 1551-1565. doi: /10.1016/j.phytochem.2011.01.040
» https://doi.org//10.1016/j.phytochem.2011.01.040 - Barry, K. J., & Crowell-Davis, S. L. (1999). Gender differences in the social behavior of the neutered indoor-only domestic cat. Applied Animal Behaviour Science, 64(3), 193-211. doi: 10.1016/S0168-1591(99)00030-1
» https://doi.org/10.1016/S0168-1591(99)00030-1 - Belviso, S., Giordano, M., Dolci, P., & Zeppa, G. (2011). Degradation and biosynthesis of terpenoids by lactic acid bacteria isolated from cheese: first evidence. Dairy Science & Technology, 91(2), 227-236. doi: 10.1007/s13594-011-0003-z
» https://doi.org/10.1007/s13594-011-0003-z - Buxton, D. R., Mertens, D. R., & Fisher, D. S. (1996). Forage quality and ruminant utilization. Cool-Season Forage Grasses, 1, 229-266. doi: 10.1016/0377-8401(95)00885-3
» https://doi.org/10.1016/0377-8401(95)00885-3 - Casali, A. O., Detmann, E., Valadares Filho, S., Pereira, J. C., Cunha, M., Detmann, K. d. S. C., & Paulino, M. F. (2009). Estimação de teores de componentes fibrosos em alimentos para ruminantes em sacos de diferentes tecidos. Revista Brasileira de Zootecnia, 38(1), 130-138. doi: 10.1590/S1516-35982009000100017
» https://doi.org/10.1590/S1516-35982009000100017 - Chaves, T. P., Medeiros, F. D., Sousa, J. M. C., Silva, L. A. P., Lima, M. A., Coutinho, H. D. M., & Medeiros, A. C. D. (2019). Phytochemical characterization and mutagenicity, cytotoxicity, antimicrobial and modulatory activities of Poincianella pyramidalis (Tul.) LP Queiroz. Natural Product Research, 1-6. doi: 10.1080/14786419.2019.1566724
» https://doi.org/10.1080/14786419.2019.1566724 - Cheng, A. X., Lou, Y.-G., Mao, Y.-B., Lu, S., Wang, L.-J., & Chen, X. Y. (2007). Plant terpenoids: Biosynthesis and ecological functions. Journal of Integrative Plant Biology, 49(2), 179-186. doi: 10.1111/j.1744-7909.2007.00395.x
» https://doi.org/10.1111/j.1744-7909.2007.00395.x - Currie, H. A., & Perry, C. C. (2007). Silica in plants: biological, biochemical and chemical studies. Annals of Botany, 100(7), 1383-1389. doi: 10.1093/aob/mcm247
» https://doi.org/10.1093/aob/mcm247 - Dubois, M., Van den Broeck, L., & Inzé, D. (2018). The pivotal role of ethylene in plant growth. Trends in Plant Science, 23(4), 311-323. doi: 10.1016/j.tplants.2018.01.003
» https://doi.org/10.1016/j.tplants.2018.01.003 - Etienne, P., Diquelou, S., Prudent, M., Salon, C., Maillard, A., & Ourry, A. (2018). Macro and micronutrient storage in plants and their remobilization when facing scarcity: The case of drought. Agriculture, 8(1), 14. doi: 10.3390/agriculture8010014
» https://doi.org/10.3390/agriculture8010014 - Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., ... Saud, S. (2017). Crop production under drought and heat stress: plant responses and management options. Frontiers in Plant Science, 8(1147), 1-16. doi: 10.3389/fpls.2017.01147
» https://doi.org/10.3389/fpls.2017.01147 - França, A. A., Guim, A., Batista, A. M. V., Pimentel, R. M. M., Ferreira, G. D. G., & Martins, I. D. S. L. (2010). Anatomia e cinética de degradação do feno de Manihot glaziovii Acta Scientiarum. Animal Sciences, 32(2), 131-138. doi: 10.4025/actascianimsci.v32i2.8800
» https://doi.org/10.4025/actascianimsci.v32i2.8800 - Godde, C., Dizyee, K., Ash, A., Thornton, P., Sloat, L., Roura, E., ... Herrero, M. (2019). Climate change and variability impacts on grazing herds: Insights from a system dynamics approach for semi‐arid Australian rangelands. Global Change Biology, in press. doi: 10.1111/gcb.14669.
» https://doi.org/10.1111/gcb.14669 - Goes, B. T. R. H., Tramontini, R. C. M., Cardim, S. T., Almeida, G. D., Ribeiro, J., Morotti, F., ... Brabes, K. C. S. (2012). Ruminal degradability of dry matter and crude protein of roughages for cattle. Revista Acadêmica Ciências Agrárias e Ambientais, 10(3), 285-291. doi: 10.7213/academica.7709
» https://doi.org/10.7213/academica.7709 - Gomes-Copeland, K. K. P., Lédo, A. S., Almeida, F. T. C., Moreira, B. O., Santos, D. C., Santos, R. A. F., ... David, J. P. (2018). Effect of elicitors in Poincianella pyramidalis callus culture in the biflavonoid biosynthesis. Industrial Crops and Products, 126, 421-425. doi: 10.1016/j.indcrop.2018.10.038
» https://doi.org/10.1016/j.indcrop.2018.10.038 - Gonzaga Neto, S., Batista, Â. M. V., Carvalho, F. F. R., Martínez, R. L. V., Barbosa, J. E. A. S., & Silva, E. O. (2001). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea bracteosa), fornecidas para ovinos Morada Nova. Revista Brasileira de Zootecnia , 30(2), 553-562. doi: 10.1590/S1516-35982001000200035
» https://doi.org/10.1590/S1516-35982001000200035 - Grabber, J. H. (2005). How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Science, 45(3), 820-831. doi: 10.2135/cropsci2004.0191
» https://doi.org/10.2135/cropsci2004.0191 - Habermann, E., Oliveira, E. A. D., Contin, D. R., Delvecchio, G., Viciedo, D. O., Moraes, M. A., ... Martinez, C. A. (2019). Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiologia Plantarum, 165(2), 383-402. doi: 10.1111/ppl.12891
» https://doi.org/10.1111/ppl.12891 - Hui, D., Yu, C.-L., Deng, Q., Dzantor, E. K., Zhou, S., Dennis, S., . . . Shen, W. (2018). Effects of precipitation changes on switchgrass photosynthesis, growth, and biomass: A mesocosm experiment. PloS One, 13(2), e0192555. doi: 10.1371/journal.pone.0192555
» https://doi.org/10.1371/journal.pone.0192555 - Jawla, S., Kumar, Y., & Khan, M. S. Y. (2013). Isolation of antidiabetic principle from Bougainvillea spectabilis Willd (Nyctaginaceae) stem bark. Tropical Journal of Pharmaceutical Research, 12(5), 761-765. doi: 10.4314/tjpr.v12i5.15
» https://doi.org/10.4314/tjpr.v12i5.15 - Jung, H. G., & Vogel, K. P. (1986). Influence of lignin on digestibility of forage cell wall material. Journal of Animal Science, 62(6), 1703-1712. doi: 10.2527/jas1986.6261703x
» https://doi.org/10.2527/jas1986.6261703x - Karnieli, A. (2003). Natural vegetation phenology assessment by ground spectral measurements in two semi-arid environments. International Journal of Biometeorology, 47(4), 179-187. doi: 10.1007/s00484-003-0169-z.
» https://doi.org/10.1007/s00484-003-0169-z. - Köppen, W., & Geiger, R. (1928). Klimate der Erde. Gotha: Verlag Justus Perthes. Wall-map 150cmx200cm
- Lee, M. R. F. (2014). Forage polyphenol oxidase and ruminant livestock nutrition. Frontiers in Plant Science , 5, 1-9. doi: 10.3389/fpls.2014.00694
» https://doi.org/10.3389/fpls.2014.00694 - Lima, C. R., Bruno, R. L. A., Andrade, A. P., Pacheco, M. V., Quirino, Z. G. M., Silva, K. d. R. G., & Belarmino, K. d. S. (2018). Phenology of Poincianella pyramidalis (Tul.) L. P. Queiroz and its relationship with the temporal distribution of rainfall in the brazilian semi-arid region. Ciência Florestal, 28(3), 1035-1048. doi: 10.5902/1980509833387
» https://doi.org/10.5902/1980509833387 - Lima, L. M. S., Alquini, Y., Brito, C. J. F. A., & Deschamps, F. C. (2001). Degradação ruminal dos tecidos vegetais e composição bromatológica de cultivares de Axonopus scoparius (Flüegge) Kuhlm. e Axonopus fissifolius (Raddi) Kuhlm. Ciência Rural, 31, 509-515. doi: 10.1590/S0103-84782001000300025
» https://doi.org/10.1590/S0103-84782001000300025 - Marles, M. A. S., Coulman, B. E., & Bett, K. E. (2008). Interference of condensed tannin in lignin analyses of dry bean and forage crops. Journal of Agricultural and Food Chemistry, 56(21), 9797-9802. doi: 10.1021/jf800888r
» https://doi.org/10.1021/jf800888r - Mendonça Júnior, A. F., Braga, A. P., & Galvão, R. J. D. (2008). Composição bromatológica, consumo e digestibilidade in vivo de dietas com diferentes níveis de feno de catingueira (Caesalpinea pyramidalis Tul), fornecidas para ovinos SRD. Revista de Biologia e Ciências da Terra, 8(1), 190-197. doi: 10.1590/S1516-35982001000200035
» https://doi.org/10.1590/S1516-35982001000200035 - Muir, J. P. (2011). The multi-faceted role of condensed tannins in the goat ecosystem. Small Ruminant Research, 98(1-3), 115-120. doi: 10.1016/j.smallrumres.2011.03.028
» https://doi.org/10.1016/j.smallrumres.2011.03.028 - Oliveira, J. C. S., David, J. P., & David, J. M. (2016a). Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochemistry Letters, 16, 18-22. doi: 10.1016/j.phytol.2016.02.017
» https://doi.org/10.1016/j.phytol.2016.02.017 - Oliveira, O. F., Santos, M. V. F., Cunha, M. V., Dubeux Júnior, J. C. B., Muir, J. P., Mello, A. C. L., ... Barros, G. F. N. P. (2016b). Botanical composition of Caatinga rangeland and diets selected by grazing sheep. Tropical Grasslands, 4(2), 71-81. doi: 10.1016/j.phytol.2016.02.017.
» https://doi.org/10.1016/j.phytol.2016.02.017. - Piotrowska, A., & Bajguz, A. (2011). Conjugates of abscisic acid, brassinosteroids, ethylene, gibberellins, and jasmonates. Phytochemistry , 72(17), 2097-2112. doi: 10.1016/j.phytochem.2011.08.012.
» https://doi.org/10.1016/j.phytochem.2011.08.012. - Pires, A. J. V., Reis, R. A., Carvalho, G. G. P., Siqueira, G. R., Bernardes, T. F., Ruggieri, A. C., ... Roth, M. T. P. (2006). Degradabilidade ruminal da matéria seca, da fração fibrosa e da proteína bruta de forrageiras. Pesquisa Agropecuária Brasileira, 41(4), 643-648. doi: 10.1590/S0100-204X2006000400014
» https://doi.org/10.1590/S0100-204X2006000400014 - Raffrenato, E., Fievisohn, R., Cotanch, K. W., Grant, R. J., Chase, L. E., & Van Amburgh, M. E. (2017). Effect of lignin linkages with other plant cell wall components on in vitro and in vivo neutral detergent fiber digestibility and rate of digestion of grass forages. Journal of Dairy Science, 100(10), 8119-8131. doi: 10.3168/jds.2016-12364
» https://doi.org/10.3168/jds.2016-12364 - Rahman, M. M., & Kawamura, O. (2011). Oxalate accumulation in forage plants: some agronomic, climatic and genetic aspects. Asian-Australasian Journal of Animal Sciences, 24(3), 439-448. doi: 10.5713/ajas.2011.10208
» https://doi.org/10.5713/ajas.2011.10208 - Reed, J. D. (1995). Nutritional toxicology of tannins and related polyphenols in forage legumes. Journal of Animal Science , 73(5), 1516-1528. doi: 10.2527/1995.7351516x
» https://doi.org/10.2527/1995.7351516x - Santos, G. R. A., Batista, A. M. V., Guim, A., Santos, M. V. F., Silva, M. J. A., & Pereira, V. L. A. (2008). Determinação da composição botânica da dieta de ovinos em pastejo na Caatinga. Revista Brasileira de Zootecnia , 37(10), 1876-1883. doi: 10.1590/S1516-35982008001000023
» https://doi.org/10.1590/S1516-35982008001000023 - Statistical Analysis Software [SAS]. (2004). SAS/STAT User guide, Version 9.1.2 Cary, NC: SAS Institute Inc.
- Silva, J. L., Guim, A., Ferreira, M. A., & Soares, L. F. P. (2016). Forragens taniníferas na produção de caprinos e ovinos. Archivos de zootecnia, 65(252), 605-614. doi: 10.21071/az.v65i252.1933
» https://doi.org/10.21071/az.v65i252.1933 - Webb, M. A. (1999). Cell-mediated crystallization of calcium oxalate in plants. The Plant Cell, 11(4), 751-761. doi: 10.1105/tpc.11.4.751
» https://doi.org/10.1105/tpc.11.4.751 - Wilson, J. R., & Mertens, D. R. (1995). Cell wall accessibility and cell structure limitations to microbial digestion of forage. Crop Science , 35(1), 251-259. doi: 10.2135/cropsci1995.0011183X003500010046x
» https://doi.org/10.2135/cropsci1995.0011183X003500010046x
Publication Dates
-
Publication in this collection
24 Oct 2020 -
Date of issue
2020
History
-
Received
10 Apr 2019 -
Accepted
15 May 2019